Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Asghar Ali | -- | 2035 | 2024-02-06 13:50:39 | | | |
| 2 | Fanny Huang | Meta information modification | 2035 | 2024-02-07 07:33:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ali, A.; Alhussaini, K.I. Pathogenesis of Helicobacter pylori. Encyclopedia. Available online: https://encyclopedia.pub/entry/54799 (accessed on 27 February 2026).
Ali A, Alhussaini KI. Pathogenesis of Helicobacter pylori. Encyclopedia. Available at: https://encyclopedia.pub/entry/54799. Accessed February 27, 2026.
Ali, Asghar, Khalid I. Alhussaini. "Pathogenesis of Helicobacter pylori" Encyclopedia, https://encyclopedia.pub/entry/54799 (accessed February 27, 2026).
Ali, A., & Alhussaini, K.I. (2024, February 06). Pathogenesis of Helicobacter pylori. In Encyclopedia. https://encyclopedia.pub/entry/54799
Ali, Asghar and Khalid I. Alhussaini. "Pathogenesis of Helicobacter pylori." Encyclopedia. Web. 06 February, 2024.
Copy Citation
Helicobacter pylori (H. pylori) is a Gram-negative bacterium that colonizes the gastric mucosa and is associated with various gastrointestinal disorders. H. pylori is a pervasive pathogen, infecting nearly 50% of the world’s population, and presents a substantial concern due to its link with gastric cancer, ranking as the third most common cause of global cancer-related mortality.
pathogenesis
virulence factors
1. Introduction
Helicobacter pylori, a ubiquitous, microaerophilic, spiral-shaped, Gram-negative bacterium residing in the human stomach, has profoundly impacted the landscape of gastroenterology and infectious diseases since its discovery in 1982 [1]. Before its identification, peptic ulcer disease was primarily attributed to stress and dietary factors, fundamentally altering researchers' understanding of its etiology. This revolutionary discovery earned Barry J. Marshall and Robin Warren the Nobel Prize in Physiology or Medicine in 2005 and laid the foundation for a comprehensive investigation into H. pylori’s role in various gastrointestinal disorders [2]. As the scientific community has continued to unravel the complexities of this pathogen, the field of H. pylori research has witnessed remarkable advancements. H. pylori colonizes the gastric mucosa of approximately half the world’s population, making it one of the most prevalent human infections [2]. Its ability to establish a persistent and often lifelong infection in the stomach lining has earned it a reputation as a formidable pathogen. The bacterium’s remarkable adaptability to the acidic and inhospitable gastric environment has led to a plethora of host responses and pathological outcomes [3][4]. From its initial association with peptic ulcers, researchers's understanding has expanded to include its role in gastritis, duodenal ulcers, gastric cancer, mucoid-associated lymphoid tissue (MALT) lymphoma, and a range of extra-gastric conditions, including neurological, ocular, hematologic, cardiovascular, and dermatological diseases, which afflict millions worldwide and have substantial economic and healthcare burdens [4][5][6]. The complex interplay between H. pylori and its human host have spurred intensive research efforts to elucidate its pathogenesis, develop accurate diagnostic methods, and refine the treatment strategies. The significance of H. pylori lies not only in its prevalence, but also in its wide-ranging impact on human health. Additionally, H. pylori is recognized as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC); it is the only bacterium on the list with a strong association with gastric adenocarcinoma and is one of the leading causes of cancer-related deaths globally [7]. Furthermore, the bacterium’s implications extend beyond the stomach, with the links to autoimmune disorders, cardiovascular diseases, and metabolic syndromes being explored. Understanding the pathogenesis, accurate diagnosis, and effective management of H. pylori infections are paramount in mitigating their impact on public health.
2. Pathogenesis of Helicobacter pylori
The pathogenesis of H. pylori may be studied at three distinct stages: the attachment to and colonization of the gastric mucosa, triggering and evading the immune responses of the host, and finally, the establishment of the disease [8].
2.1. Dispersal and Routes of Infection
H. pylori infections are often asymptomatic and usually acquired during childhood. However, around 30% of infected persons may show signs of gastrointestinal diseases, such as mild-to-severe cases of peptic ulcers, gastritis, and even gastric cancer and MALT lymphoma. H. pylori infections have a narrow host range, and therefore, the transmission routes usually include vertical and horizontal transmission. The former includes person-to-person transmission between genetically related individuals, while the latter includes persons exposed to infected people of a similar socioeconomic status [9]. While the exact route of transmission remains unclear, the four major routes of H. pylori infection include the fecal–oral route, the oral–oral route, the gastric–oral route, and gastro-gastric route which may occur through the ingestion of contaminated food, water or during endoscopic procedures [10].
2.2. Molecular Mechanisms of Infection
2.2.1. Attachment and Colonization
The molecular mechanisms orchestrating H. pylori infections are sophisticated processes crucial for the bacterium’s successful establishment in the gastric mucosa. At the forefront of infection initiation is H. pylori’s adhesion and colonization strategies. The colonization of the pathogen is initiated first by the chemotaxis of the bacterial cells to the target site, which is mediated by the presence of certain receptors present on the host cells, mainly belonging to the Tlp family [11]. These receptors are known to be triggered by the presence of chemical signals, including urea, lactic acid, ROS species, and gastric juice, facilitating chemotactic responses to the gastric epithelium [11]. Flagellar motility allows H. pylori to navigate through the mucous layer and reach the gastric epithelium, contributing to its ability to firmly adhere to and colonize the mucosa [12]. Additionally, H. pylori can form biofilms, structured bacterial communities embedded in an extracellular matrix, enhancing adherence and providing protection against the host defenses and treatment therapies [13]. Furthermore, the bacterial outer membrane proteins, aka the surface adhesins, including BabA, SabA, AlpA/B, and OipA, enable the bacteria to adhere to gastric epithelial cells, paving the way for its persistence within the stomach [14].
2.2.2. Production of Virulence Factors
Various virulence factors, including those involved in motility, adhesion, urease and cytotoxin production, are essential for the pathogenesis of this bacterium [15].
2.2.3. CagA and VacA
Upon attachment, H. pylori engages in intricate host interactions, deploying an arsenal of virulence factors that manipulate host cell signaling. Among these, the CagA (cytotoxin-associated gene A) pathogenicity island is the main orchestrator [16]. Injected into the host cells via a type IV secretion system (T4SS), CagA triggers a cascade of events, disrupting cellular functions and contributing to the development of gastric pathologies [17]. CagA undergoes tyrosine phosphorylation, leading to the activation of various cellular signaling pathways [18]. This includes the aberrant activation of the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways [19][20]. The dysregulation of these pathways contributes to cellular morphological changes, the disruption of cell polarity, and the initiation of oncogenic processes. This molecular hijacking of host cell signaling is a hallmark of H. pylori-induced pathogenesis [21]. The vacuolating toxin, VacA, is another key virulence factor, that exhibits multifaceted effects on the host cells [22]. As the name suggests, VacA disrupts the integrity of the gastric epithelial barrier by forming channels or pores in the cell membrane, leading to increased permeability [15]. Within the host cells, VacA modulates the apoptotic pathways, leading to both pro- and antiapoptotic effects depending on the cell type and environmental conditions. Furthermore, VacA toxin contributes to the formation of vacuoles within the host cells, impacting the cellular structure and function [23]. This toxin also interferes with and evades the immune responses by affecting the function of T cells and other immune cells. The consequences of these molecular interactions extend to the host’s immune response [24][25]. H. pylori induces a chronic inflammatory state marked by the release of multiple pro-inflammatory cytokines, such as IL-8, IL-1α. IL-1β, and TNF α, among others, and the recruitment of immune cells to the gastric mucosa [26]. This inflammatory milieu is closely associated with the development of peptic ulcers, as the delicate balance between the protective mucosal mechanisms and bacterial aggression is disrupted [27]. The CagA and VacA proteins work together to induce H. pylori-associated gastric cancer. A study conducted by Abdullah et al. (2019) showed the functional interplay between the two oncoproteins, whereby the absence of VacA aids the host’s system is able to degrade CagA, thereby preventing the accumulation of CagA in the gastric epithelial cells [28]. Importantly, the long-term consequences of H. pylori infection include an increased risk of developing gastric cancer. Chronic inflammation, coupled with the release of genotoxins and the induction of genetic instability, creates an environment conducive to oncogenesis and other gastric malignancies [29]. The interplay between H. pylori’s virulence factors, such as CagA, VacA, and OipA, host genetic susceptibility, and environmental factors contribute to the complexity of this association [30]. Understanding these intricate molecular mechanisms is paramount for developing targeted therapeutic approaches and preventive strategies against H. pylori infections. The advances in elucidating these processes provide critical insights into the pathogenesis of H. pylori-related diseases and inform the development of novel interventions to mitigate the associated health risks.
2.2.4. Urease Production and Survival at Low pH
Urease is one of the more abundantly produced proteins expressed by the pathogen, accounting for almost 15% of the total proteins of the bacterium [31]. The production of urease is a characteristic feature of H. pylori and is widely used in the diagnosis of the infection as well [32]. Several studies in this regard have proved the role of urease in enabling the survival of the bacterium in the extremely low-pH acidic environment of the stomach by breaking down the urea into ammonia and carbon dioxide, thereby forming a pH-neutral environment around the bacterial cells (Figure 1). The other enzymes, including carbonic anhydrase, arginase, glutamine synthase, glutamate dehydrogenase, and glutaminase, are also involved in the urease-dependent mechanism of survival under acidic conditions [33]. The hydrolysis of urea into ammonia also provides the pathogen with a steady source of nitrogen [34][35]. Survival at low pH is further supported by other urease-independent physiological factors, including the flagellar motility across the gastric mucus layer, which facilitates the movement of the bacterial cells in low-pH-level conditions [36]. Furthermore, DNA repair proteins, such as RecA, RecN, RecO, Hup, etc., are actively involved in repairing the DNA that may be damaged after being subjected to acid stress in the stomach [37][38]. H. pylori also exhibits chemotactic activity towards certain chemoattractants, such as carbonates and urea, which attract the bacterial cells towards the regions of higher pH in the gastric lining. Among the chemoreceptors of H. pylori, the Tlp family is crucial for the desirable chemotactic activity that promotes survival in an acid stress environment [39].
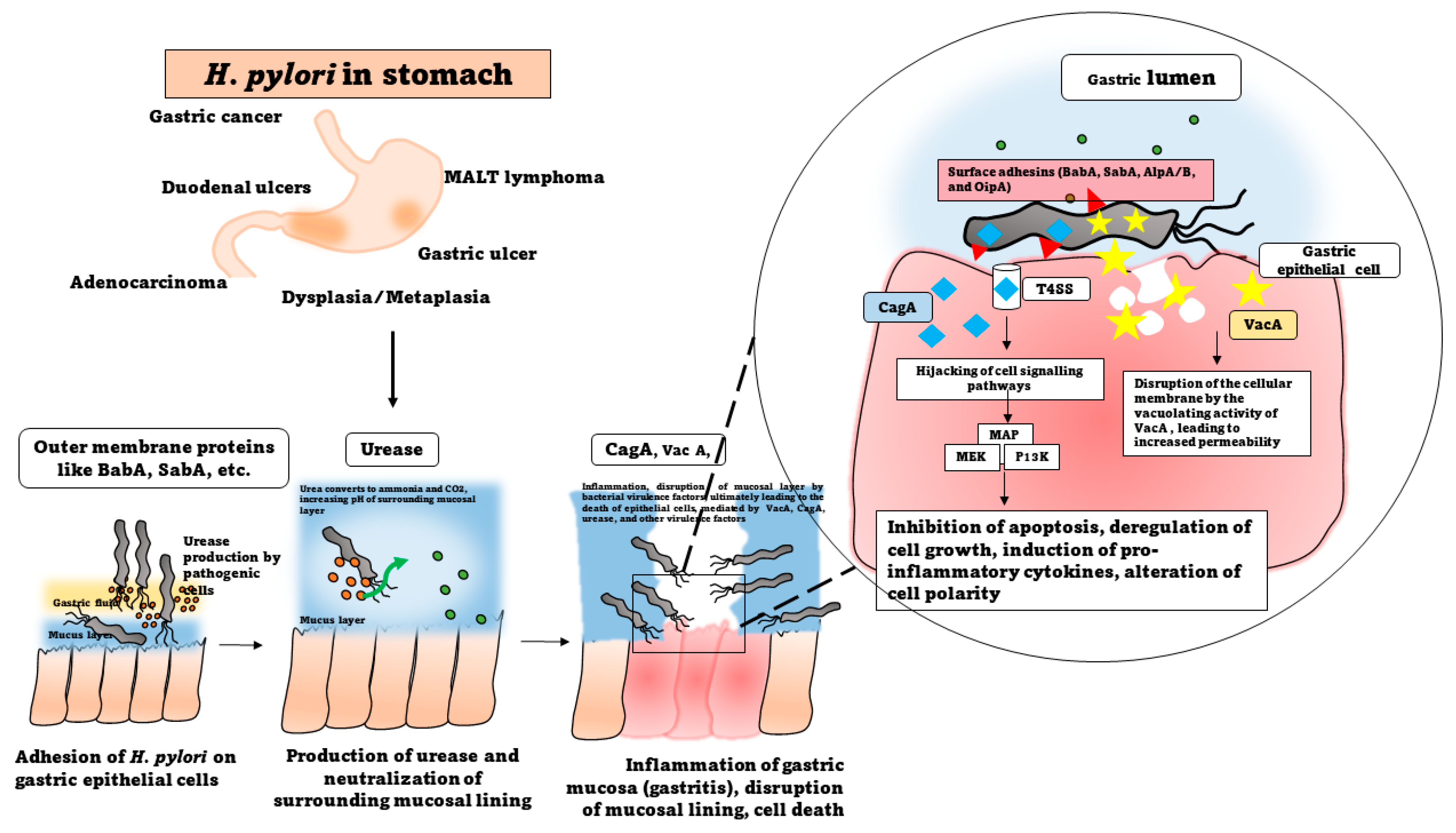
Figure 1. Pathogenesis of H. pylori.
2.3. Immune System Modulation and Induction of Inflammatory Responses
The immune response to H. pylori infection is a dynamic interplay between the bacterial factors and the host’s immune system [40]. The pathogenesis of H. pylori infections unfold through a complex and multifaceted localized gastric inflammatory response [41]. In the innate immune phase, H. pylori induces a chronic inflammatory response in the gastric mucosa, mediated by the lipopolysaccharides and the peptidoglycan of the cell wall, and characterized by the release of neutrophils, macrophages, lymphocytes, and pro-inflammatory cytokines (such as interleukin-1β, interleukin-6, and tumor necrosis factor-α) and the recruitment of immune cells [42][43]. Innate immune activation spurred by bacterial components, like lipopolysaccharide and peptidoglycan, further amplifies the inflammatory cascade through pattern recognition receptors such as Toll-like receptors [44]. This sets the stage for the adaptive immune response, where the CD4+ T-helper cells play a crucial role, particularly in promoting a Th1 response characterized by interferon-gamma secretion. Regulatory T cells are recruited, contributing to immune suppression, while the B cells produce antibodies against H. pylori [45][46]. However, the bacterium employs immune evasion strategies, such as urease production, antigenic variation, inhibition of recognition by PRRs, and molecular mimicry, to subvert recognition and signaling by the T and B cells [47]). The sustained presence of H. pylori leads to chronic inflammation, causing ongoing tissue damage and remodeling. The gastric epithelial cells undergo changes in turnover, with increased proliferation contributing to mucosal damage and ulcer formation [48]. Concurrently, disruptions in the tight junctions compromise the mucosal barrier integrity, facilitating H. pylori infiltration into the deeper mucosal layers [49]. The sustained release of inflammatory mediators, coupled with the induction of genetic instability, creates an environment conducive to oncogenesis [50]. This sustained inflammation is a central component of H. pylori-associated diseases and is implicated in the development of peptic ulcers and gastric cancer [2]. However, in the neighboring non-infected gastric cells, an adaptive immune response is triggered which prompts increased survival and proliferation, ultimately leading to the development of precancerous lesions in the gastric epithelium [51].
2.4. Modulation of Mucin Production
H. pylori influences mucin production in the gastric mucosa, impacting the protective mucous layer. The carbohydrate component of the mucins act as ligands that enable the binding of the bacterium to the gastric mucosal lining [52]. The bacterium can alter the expression of mucin genes and interact with mucins, like MUC5A and MUC1, directly, resulting in inhibition or the impairment of mucin turnover [53]. The changes in mucin production influence the composition of the mucosal glycocalyx, affecting the adherence and colonization of H. pylori. Moreover, this modulation of mucin production contributes to the bacterium’s ability to evade the host defenses and establish persistent infections [54]. Unraveling the nuances of immune system modulation in H. pylori infection is paramount for devising effective therapeutic interventions and preventive strategies in the ongoing pursuit of managing the associated diseases. The ongoing research continues to unveil the complexities of this host–pathogen interaction.
References
- Robin Warren, J.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 321, 1273–1275.
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713–739.
- Suzuki, H.; Moayyedi, P. Helicobacter pylori Infection in Functional Dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 168–174.
- Fong, I.W. Helicobacter pylori Infection: When Should It Be Treated? In Current Trends and Concerns in Infectious Diseases; Springer International Publishing: Cham, Switzerland, 2020; pp. 81–102. ISBN 978-3-030-36966-8.
- Gravina, A.G.; Zagari, R.M.; De Musis, C.; Romano, L.; Loguercio, C.; Romano, M. Helicobacter pylori and Extragastric Diseases: A Review. World J. Gastroenterol. 2018, 24, 3204–3221.
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori Infection—The Maastricht IV/ Florence Consensus Report. Gut 2012, 61, 646–664.
- International Agency for Research on Cancer; World Health Organization. Schistosomes, Liver Flukes and Helicobacter Pylori; IARC: Lyon, France, 1994; ISBN 9283212614.
- Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Abalkhail, A.; Anagreyyah, S.; Anajirih, N.; Almuzaini, A.M.; Rawway, M.; Alfadhel, A.; Draz, A.; et al. Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics 2023, 12, 191.
- Kayali, S.; Manfredi, M.; Gaiani, F.; Bianchi, L.; Bizzarri, B.; Leandro, G.; Di Mario, F.; De’angelis, G.L. Helicobacter pylori, Transmission Routes and Recurrence of Infection: State of the Art. Acta Biomed. 2018, 89, 72–76.
- Duan, M.; Li, Y.; Liu, J.; Zhang, W.; Dong, Y.; Han, Z.; Wan, M.; Lin, M.; Lin, B.; Kong, Q.; et al. Transmission Routes and Patterns of Helicobacter pylori. Helicobacter 2023, 28, e12945.
- Hikaru, H.; Engevik, K.A.; Matthis, A.L.; Ottemann, K.M.; Montrose, M.H.; Aihara, E. Helicobacter pylori Uses the TlpB Receptor To Sense Sites of Gastric Injury. Infect. Immun. 2019, 87, 10–1128.
- Gu, H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr. Microbiol. 2017, 74, 863–869.
- Yonezawa, H.; Osaki, T.; Kamiya, S. Biofilm Formation by Helicobacter pylori and Its Involvement for Antibiotic Resistance. Biomed. Res. Int. 2015, 2015, 914791.
- Salama, N.R.; Hartung, M.L.; Müller, A. Life in the Human Stomach: Persistence Strategies of the Bacterial Pathogen Helicobacter pylori. Nat. Rev. Microbiol. 2013, 11, 385–399.
- Reyes, V.E. Helicobacter pylori and Its Role in Gastric Cancer. Microorganisms 2023, 11, 1312.
- Backert, S.; Tegtmeyer, N.; Fischer, W. Composition, Structure and Function of the Helicobacter pylori Cag Pathogenicity Island Encoded Type IV Secretion System. Future Microbiol. 2015, 10, 955–965.
- HATAKEYAMA, M. Structure and Function of Helicobacter pylori CagA, the First-Identified Bacterial Protein Involved in Human Cancer. Proc. Jpn. Acad. Ser. B 2017, 93, 196–219.
- Yong, X.; Tang, B.; Li, B.-S.; Xie, R.; Hu, C.-J.; Luo, G.; Qin, Y.; Dong, H.; Yang, S.-M. Helicobacter pylori Virulence Factor CagA Promotes Tumorigenesis of Gastric Cancer via Multiple Signaling Pathways. Cell Commun. Signal. 2015, 13, 30.
- Kusters, J.G.; van Vliet, A.H.M.; Kuipers, E.J. Pathogenesis of Helicobacter pylori Infection. Clin. Microbiol. Rev. 2006, 19, 449–490.
- Maeda, S.; Mentis, A.F. Pathogenesis of Helicobacter pylori Infection. Helicobacter 2007, 12, 10–14.
- Messina, B.; Lo Sardo, F.; Scalera, S.; Memeo, L.; Colarossi, C.; Mare, M.; Blandino, G.; Ciliberto, G.; Maugeri-Saccà, M.; Bon, G. Hippo Pathway Dysregulation in Gastric Cancer: From Helicobacter pylori Infection to Tumor Promotion and Progression. Cell Death Dis. 2023, 14, 21.
- Cover, T.L.; Blanke, S.R. Helicobacter pylori VacA, a Paradigm for Toxin Multifunctionality. Nat. Rev. Microbiol. 2005, 3, 320–332.
- Foegeding, N.J.; Caston, R.R.; McClain, M.S.; Ohi, M.D.; Cover, T.L. An Overview of Helicobacter pylori VacA Toxin Biology. Toxins 2016, 8, 173.
- Chauhan, N.; Tay, A.C.Y.; Marshall, B.J.; Jain, U. Helicobacter pylori VacA, a Distinct Toxin Exerts Diverse Functionalities in Numerous Cells: An Overview. Helicobacter 2019, 24, e12544.
- Ahmed, S.; Belayneh, Y.M. Helicobacter pylori and Duodenal Ulcer: Systematic Review of Controversies in Causation. Clin. Exp. Gastroenterol. 2019, 12, 441–447.
- Dincă, A.L.; Meliț, L.E.; Mărginean, C.O. Old and New Aspects of H. pylori-Associated Inflammation and Gastric Cancer. Children 2022, 9, 1083.
- Yang, H.; Hu, B. Immunological Perspective: Helicobacter pylori Infection and Gastritis. Mediat. Inflamm. 2022, 2022, 2944156.
- Abdullah, M.; Greenfield, L.K.; Bronte-Tinkew, D.; Capurro, M.I.; Rizzuti, D.; Jones, N.L. VacA Promotes CagA Accumulation in Gastric Epithelial Cells during Helicobacter pylori Infection. Sci. Rep. 2019, 9, 38.
- Venerito, M.; Krieger, T.; Ecker, T.; Leandro, G.; Malfertheiner, P. Meta-Analysis of Bismuth Quadruple Therapy versus Clarithromycin Triple Therapy for Empiric Primary Treatment of Helicobacter pylori Infection. Digestion 2013, 88, 33–45.
- Yamaoka, Y. Mechanisms of Disease: Helicobacter pylori Virulence Factors. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 629–641.
- Ha, N.-C.; Oh, S.-T.; Sung, J.Y.; Cha, K.A.; Lee, M.H.; Oh, B.-H. Supramolecular Assembly and Acid Resistance of Helicobacter pylori Urease. Nat. Struct. Biol. 2001, 8, 505–509.
- Mobley, H.L.; Mendz, G.L.; Hazell, S.L. Helicobacter pylori: Physiology and Genetics; ASM Press: Washington, DC, USA, 2001; ISBN 1555812139.
- Ansari, S.; Yamaoka, Y. Survival of Helicobacter pylori in Gastric Acidic Territory. Helicobacter 2017, 22, e12386.
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.-M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori Infection. Nat. Rev. Dis. Primers 2023, 9, 19.
- Idowu, S.; Bertrand, P.P.; Walduck, A.K. Gastric Organoids: Advancing the Study of H. pylori Pathogenesis and Inflammation. Helicobacter 2022, 27, e12891.
- Fagoonee, S.; Pellicano, R. Helicobacter pylori: Molecular Basis for Colonization and Survival in Gastric Environment and Resistance to Antibiotics. A Short Review. Infect. Dis. 2019, 51, 399–408.
- Agarwal, N.; Jaiswal, N.; Gulati, K.; Gangele, K.; Nagar, N.; Kumar, D.; Poluri, K.M. Molecular Insights into Conformational Heterogeneity and Enhanced Structural Integrity of Helicobacter pylori DNA Binding Protein Hup at Low PH. Biochemistry 2021, 60, 3236–3252.
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori Infection and Antibiotic Resistance—From Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629.
- Huang, J.Y.; Goers Sweeney, E.; Guillemin, K.; Amieva, M.R. Multiple Acid Sensors Control Helicobacter pylori Colonization of the Stomach. PLoS Pathog. 2017, 13, e1006118.
- Kao, C.-Y.; Sheu, B.-S.; Wu, J.-J. Helicobacter pylori Infection: An Overview of Bacterial Virulence Factors and Pathogenesis. Biomed. J. 2016, 39, 14–23.
- White, J.R.; Winter, J.A.; Robinson, K. Differential Inflammatory Response to Helicobacter pylori Infection: Etiology and Clinical Outcomes. J. Inflamm. Res. 2015, 8, 137–147.
- Zeyaullah, M.d.; AlShahrani, A.M.; Ahmad, I. Association of Helicobacter pylori Infection and Host Cytokine Gene Polymorphism with Gastric Cancer. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8810620.
- D’Elios, M.M.; Amedei, A.; Cappon, A.; Del Prete, G.; de Bernard, M. The Neutrophil-Activating Protein of Helicobacter pylori (HP-NAP) as an Immune Modulating Agent. FEMS Immunol. Med. Microbiol. 2007, 50, 157–164.
- Su, B.; Ceponis, P.J.M.; Lebel, S.; Huynh, H.; Sherman, P.M. Helicobacter pylori Activates Toll-Like Receptor 4 Expression in Gastrointestinal Epithelial Cells. Infect. Immun. 2003, 71, 3496–3502.
- Larussa, T.; Leone, I.; Suraci, E.; Imeneo, M.; Luzza, F. Helicobacter pylori and T Helper Cells: Mechanisms of Immune Escape and Tolerance. J. Immunol. Res. 2015, 2015, 981328.
- Bergman, M.P.; D’Elios, M.M. Cytotoxic T Cells in H. pylori-Related Gastric Autoimmunity and Gastric Lymphoma. J. Biomed. Biotechnol. 2010, 2010, 104918.
- Reyes Victor, E.; Peniche, A.G. Helicobacter pylori Deregulates T and B Cell Signaling to Trigger Immune Evasion. In Molecular Mechanisms of Inflammation: Induction, Resolution and Escape by Helicobacter pylori; Backert, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 229–265. ISBN 978-3-030-15138-6.
- Kumar, S.; Patel, G.K.; Ghoshal, U.C. Helicobacter pylori-Induced Inflammation: Possible Factors Modulating the Risk of Gastric Cancer. Pathogens 2021, 10, 1099.
- Caron, T.J.; Scott, K.E.; Fox, J.G.; Hagen, S.J. Tight Junction Disruption: Helicobacter pylori and Dysregulation of the Gastric Mucosal Barrier. World J. Gastroenterol. 2015, 21, 11411–11427.
- Graham, D.Y.; Miftahussurur, M.; Yamaoka, Y. Helicobacter pylori as an Oncogenic Pathogen, Revisited. Expert Rev. Mol. Med. 2017, 19, e4.
- Díaz, P.; Valenzuela Valderrama, M.; Bravo, J.; Quest, A.F.G. Helicobacter pylori and Gastric Cancer: Adaptive Cellular Mechanisms Involved in Disease Progression. Front. Microbiol. 2018, 9, 5.
- Skoog, E. Helicobacter spp. Interactions with Mucins: Adhesion and Mucin Regulation of Pathogen Proliferation and Gene Expression; Department of Medical Biochemistry and Cell Biology, Institute of Biomedicine, Sahlgrenska Academy at University of Gothenburg: Göteborg, Sweden, 2014; ISBN 9789162888718.
- Nazanin, N.; Johansson, M.E.; Raghavan, S.; Lindén, S.K. Helicobacter pylori Infection Impairs the Mucin Production Rate and Turnover in the Murine Gastric Mucosa. Infect. Immun. 2013, 81, 829–837.
- Padra, M.; Benktander, J.; Robinson, K.; Lindén, S.K. Carbohydrate-Dependent and Antimicrobial Peptide Defence Mechanisms Against Helicobacter pylori Infections. In Molecular Mechanisms of Inflammation: Induction, Resolution and Escape by Helicobacter pylori; Backert, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 179–207. ISBN 978-3-030-15138-6.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Entry Collection:
Gastrointestinal Disease
Revisions:
2 times
(View History)
Update Date:
07 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





