Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kotohiro Nomura | -- | 1965 | 2024-02-05 21:30:20 | | | |
| 2 | Rita Xu | Meta information modification | 1965 | 2024-02-06 03:53:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nomura, K.; Wang, X. Aliphatic Polyesters by ADMET Polymerization and Hydrogenation. Encyclopedia. Available online: https://encyclopedia.pub/entry/54780 (accessed on 08 March 2026).
Nomura K, Wang X. Aliphatic Polyesters by ADMET Polymerization and Hydrogenation. Encyclopedia. Available at: https://encyclopedia.pub/entry/54780. Accessed March 08, 2026.
Nomura, Kotohiro, Xiuxiu Wang. "Aliphatic Polyesters by ADMET Polymerization and Hydrogenation" Encyclopedia, https://encyclopedia.pub/entry/54780 (accessed March 08, 2026).
Nomura, K., & Wang, X. (2024, February 05). Aliphatic Polyesters by ADMET Polymerization and Hydrogenation. In Encyclopedia. https://encyclopedia.pub/entry/54780
Nomura, Kotohiro and Xiuxiu Wang. "Aliphatic Polyesters by ADMET Polymerization and Hydrogenation." Encyclopedia. Web. 05 February, 2024.
Copy Citation
The recent developments of the synthesis of bio-based long-chain aliphatic polyesters by the acyclic diene metathesis (ADMET) polymerization of α,ω-dienes, derived from plant oils and bio-based chemicals, like bis(10-undecenoate) with isosorbide, using ruthenium-carbene catalysts. The development of subsequent (one-pot) tandem hydrogenation produced saturated polyesters under mild conditions.
bio-based
polyester
metathesis polymerization
plant oil
circular economy
chemical recycling
aliphatic polyester
bio-based polyester
1. Introduction
The development of sustainable polymers from renewable feedstocks attracts considerable attention from the viewpoints of the circular economy as well as green sustainable chemistry. Hydrocarbon-rich molecular biomasses, such as vegetable oils (castor, coconut, linseed, olive, palm, soybean, sunflower, etc.) presented as triglycerides with fatty acids, or fatty acid esters (FAEs) are naturally abundant and are recognized as low-cost molecular biomass products [1][2][3][4][5][6][7][8][9][10][11]. A study on bio-based advanced polyesters (exhibiting tunable mechanical properties and biodegradability), in particular, long-chain aliphatic polyesters (LCAPEs), are semicrystalline materials considered as a promising alternative of polyethylene [6][8]. The melting temperatures (Tm values) of polyesters are generally influenced by the methylene length (and the direction of dipoles called the odd–even effect) [6][12][13][14]; the placement of longer methylene units should be effective for the obtainment of polyesters without softening them at elevated temperatures. It has been considered that the precise polymerization technique provides a new strategy and methodology for the design of macromolecular architectures.
Two condensation polymerization approaches—(i) condensation polymerization by transesterification (dicarboxylic acid and diol, etc.) and (ii) acyclic diene metathesis (ADMET) polymerization (nonconjugated α,ω-dienes)—and subsequent hydrogenation (Scheme 1) are considered for the synthesis from FAEs [6][8]. The ring-opening polymerization (ROP) approach from cyclic monomers can also be considered, but the method has a limited monomer scope; the method also faces the difficulty of catalysts enabling the synthesis of high molar mass polymers [15][16]. Studies on alternative approaches to polymers are also under investigation [17][18][19][20]. Moreover, the recent progress in the development of olefin metathesis catalysts for the conversion of plant oils (FAEs) is well known [21][22][23][24].
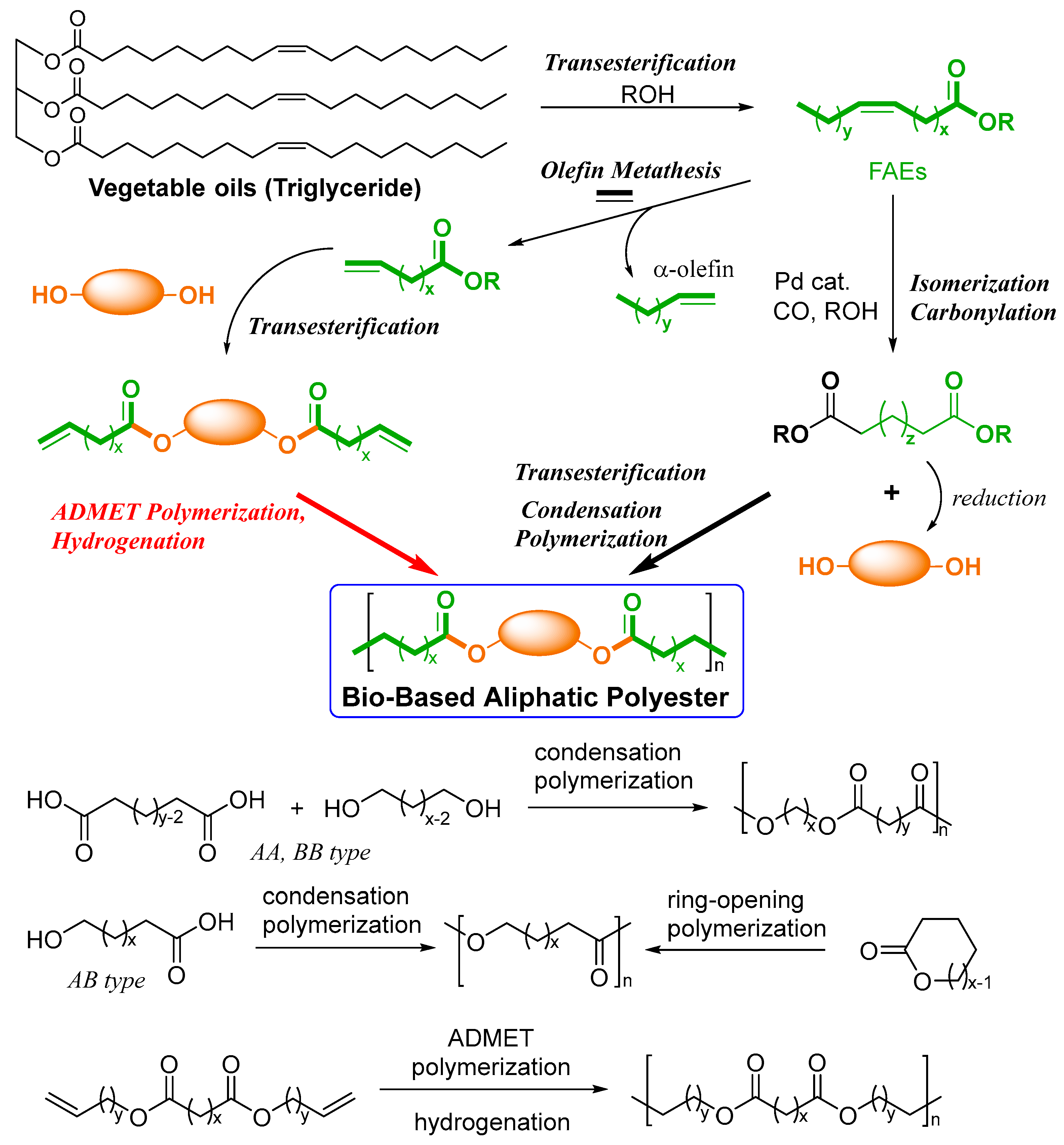
Scheme 1. Synthesis of bio-based polyesters from plant oil (triglycerides).
The conventional condensation polymerization approach through transesterification (ester bond exchange) requires high temperatures with the efficient removal of by-products (such as alcohols and water) to obtain high molar mass polymers with a high degree of polymerization (DPn). For example, the synthesis of poly(ethylene terephthalate) from terephthalic acid (which must be purified) with excess ethylene glycol requires high temperatures up to 290 °C under a reduced pressure [25]. This method, however, seems to be difficult to apply for the synthesis of LCAPEs due to the difficulty of removing diols with high boiling points (e.g., 1,12-dodecanediol, 189 °C/12 Torr; 1,16-hexadecane diol 197–199 °C/3 mmHg). Moreover, precise stoichiometric control (hydroxy and carboxylic groups) is needed for this purpose [6][26][27][28]; polymerization with the precise stoichiometric ratios of diols (algae oil) and diesters (C17 and C19) is required to create high molar mass polymers (Mn = 4.0 × 104) possessing a Tm value of 99 °C [28].
2. Synthesis of Aliphatic Polyesters by ADMET Polymerization and Hydrogenation
Reports on the synthesis of bio-based polyesters by ADMET polymerization, especially using commercially available (Grubbs-type) ruthenium carbene catalysts RuCl2(PCy3)2(CHPh) (G1; Cy = cyclohexyl), RuCl2(PCy3)(IMesH2)(CHPh) (G2; IMesH2 = 1,3-bis(2,4,6-trimethylphenyl)imidazolin-2-ylidene), and RuCl2(IMesH2)(CH-2-OiPr-C6H4) (HG2), shown in Scheme 2, are well known. These ruthenium catalysts have been employed [8] because these complexes can be readily available and do not require treatment with the strict Schlenk technique due to their insensitivities toward water and oxygen (better functional group tolerance) [29][30][31][32]. More recently, the example using a molybdenum-alkylidene catalyst (Mo cat.) [33][34][35], shown below, also demonstrates the synthesis of high molar mass polymers that exhibit good tensile properties [36].
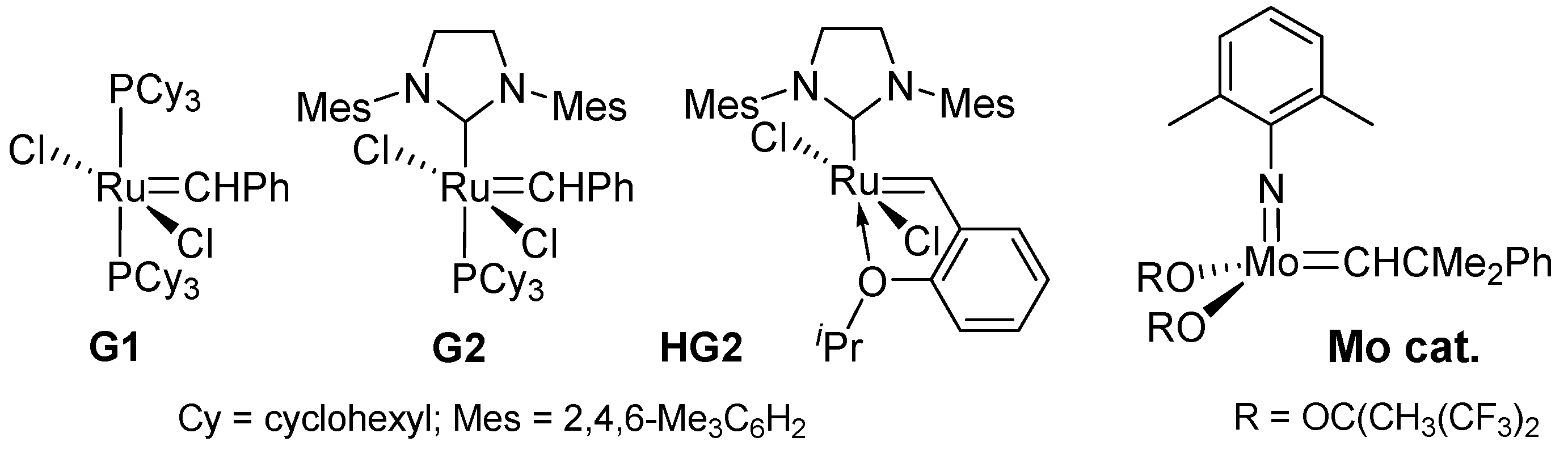
Scheme 2. Ruthenium-carbene and molybdenum-alkylidene catalysts for the synthesis of aliphatic polyesters by ADMET polymerization.
The synthesis of a bio-based polyester, expressed as PE1, by the ADMET polymerization of undec-10-en-1-yl undec-10-enoate (M1), prepared by the reaction of 10-undecenoic acid with 10-undecenol (derived from castor oil) was reported by the group of Meier in 2008 [37]. The resultant PE1 synthesized by G2 (0.5 or 1.0 mol%, 80 °C, 24 h, Scheme 3) possessed a rather high molecular weight (Mn = 22,000, 26,500), and the Mn values were controlled by the addition of terminal olefins, such as methyl 10-undecenoate and stearyl acrylate [37]. In contrast, the group reported that the polymerization of bis(undec-10-enoate) with isosorbide (M2, Scheme 3) conducted at 70–100 °C under bulk conditions yielded rather low-molecular-weight polymers (PE2) [38], whereas the Mn values seemed to improve when the polymerizations were conducted at high temperatures and/or under nitrogen-purge conditions (for the removal of by-produced ethylene). This was probably due to the catalyst decomposition caused by conducting the reaction at 70–100 °C [39][40][41][42][43][44], because these ruthenium catalysts are known to decompose under these conditions to produce ruthenium-hydride species [41] and/or nanoparticles [43], which induce olefin isomerization and/or certain side reactions by the formed radicals [39][40][41][42][43][44][45]. G2 showed a more significant degree of olefin isomerization compared to G1 and a higher percentage of isomerization (estimated by GC-MS, after treating the mixture with MeOH-H2SO4 under reflux conditions) [38]. Later, the degree of isomerization was extensively suppressed when the polymerizations were conducted in the presence of benzoquinone [45].
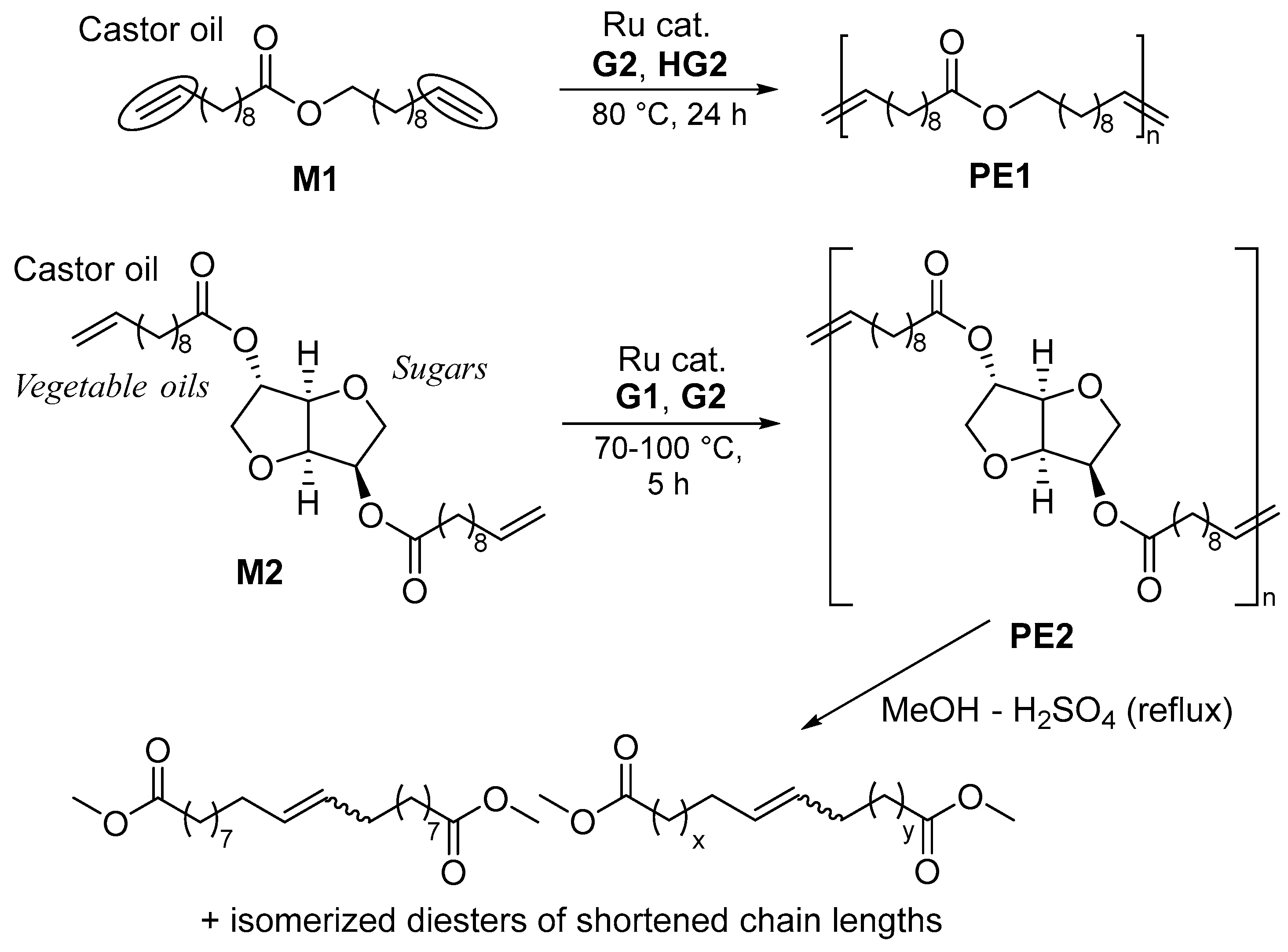
Scheme 3. ADMET polymerization of castor oil-derived monomers (M1, M2).
The ADMET polymerization of M1 by G1 under high-vacuum conditions for two days produced PE1 (Mn = 28,000, Mw/Mn = 1.9) and a subsequent hydrogenation step (Pd/C, 50 bar H2, 60 ºC) produced a saturated polyester (HPE1, PE-20.20, Scheme 4) [46]. The Tm value (103 °C) achieved was somewhat low compared to the HPE1 prepared by the condensation polymerization of 1,20-eicosanedioic acid with eicosane-1,20-diol (Tm = 108 °C) to form ‘regio-regular’ ester groups, C(O)-O, aligned with the polymer chain (Scheme 4). It was thus suggested that the microstructural control directly affected the thermal property, as described above [6][14]. ADMET polymerizations of α,ω-dienes with different methylene chain lengths, di(icos-19-en-1-yl)tricosanedioate (M3) and di(tricos-22-en-1-yl)tricosanedioate (M4), using G1 and the subsequent olefin hydrogenation conducted by Ru(CHOEt)Cl2(PCy)2 (40 bar H2, 70 °C, 2 d), prepared from G1, yielded the corresponding values of PE-38.23 (HPE3) and PE-44.23 (HPE4), respectively (Scheme 4) [47]. The polycondensation of 1,26-hexacosanedioate, prepared by the cross-metathesis of erucic acid, with the corresponding diol (produced by a reduction with LiAlH4) with Ti(OBu)4 also produced the corresponding polyester (HPE5, PE-26.26, Tm = 114 °C) [48]. The thermal properties (Tm values) of the resultant LCAPEs with different methylene lengths, prepared by ADMET [47] and polycondensation [48][49] approaches, revealed that the Tm values achieved a constant value (Figure 1a) [47]. A linear relationship between the Tm values and the number of ester groups in 1000 carbon atoms was observed (Figure 1b) [47]. Polyesters PE-26.26, PE-12.26 and PE-4.26 [48], and PE-18,18 [50] were also prepared by polycondensation.
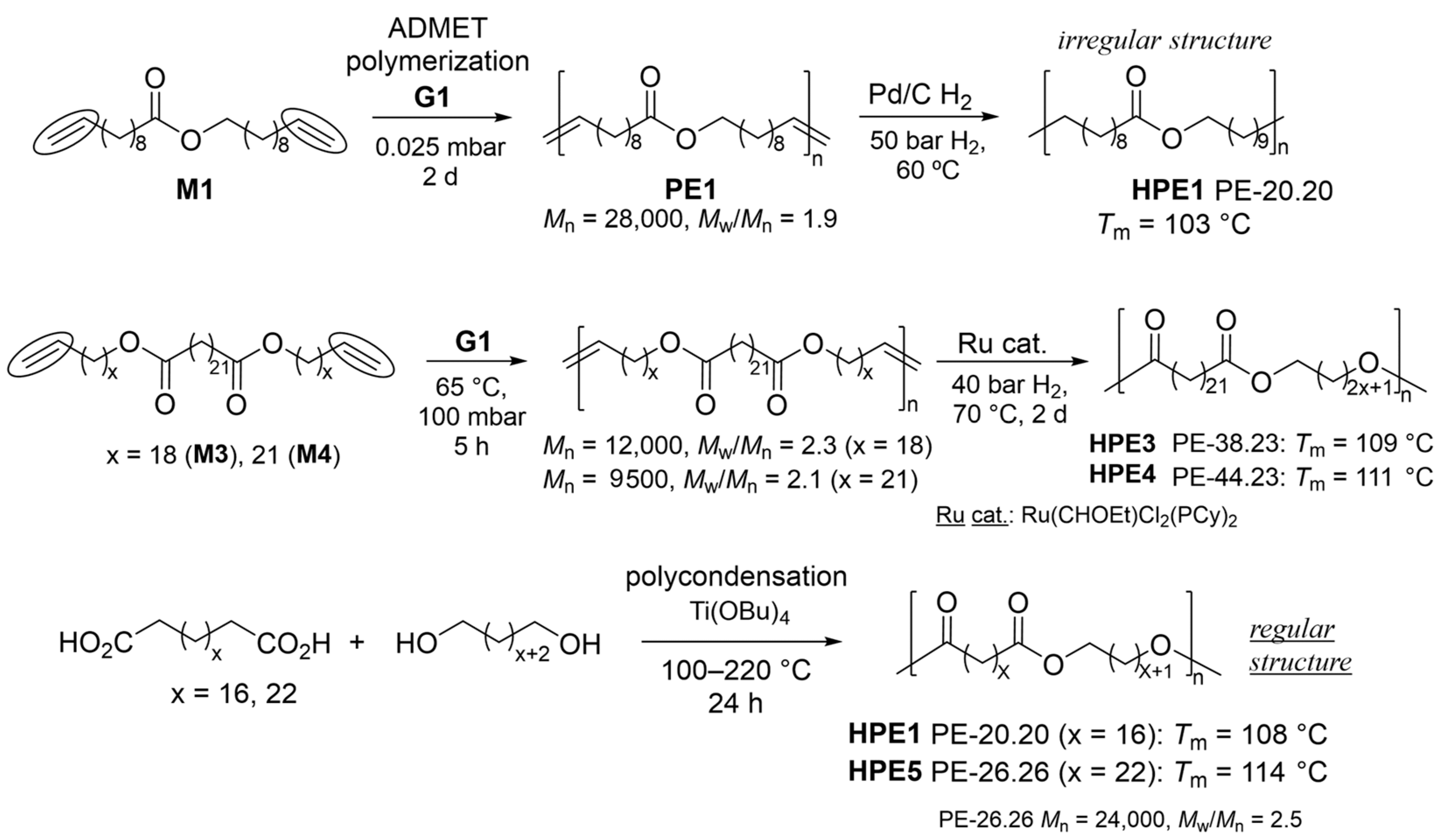
Scheme 4. Synthesis of linear polyesters (LCAPEs).
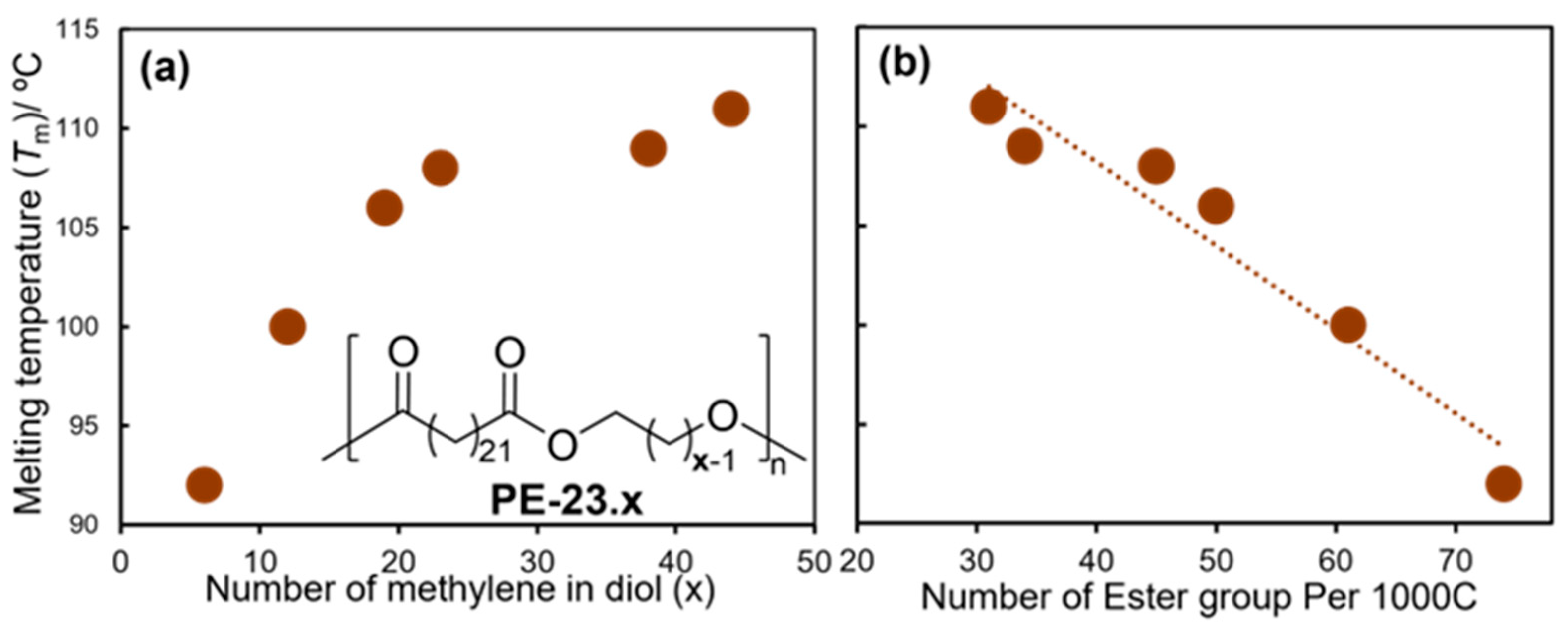
Figure 1. Plots of melting temperature (Tm) vs. number of (a) methylene units (x) in diol and (b) ester groups per 1000 C (methylene) in PE-23.x.
The one-pot synthetic method used for the bio-based aliphatic polyesters by ADMET polymerization and subsequent hydrogenation was demonstrated (Scheme 5) [51]. The polymerization of bis(undec-10-enoate)s with isosorbide (M2), isomannide (M6), 1,3-propanediol (M7), and 1,4-cyclohexanedimethanol (M8), derived from castor oil and glucose in chloroform by G2 or HG2 under a reduced pressure at 50 °C produced unsaturated polymers (expressed as PE2 and PE6–PE8, respectively) [51]. The Mn values in the produced polymers (Mn = 11,900–15,900) were somewhat higher than those reported previously (Mn = 4400–8400), conducted at 70–100 °C [38], and the Mn values did not change, even under rather scaled-up conditions [51]. One reason for the obtainment of high-molecular-weight product could be that the degree of the catalyst decomposition was significantly suppressed by conducting the polymerization at 50 °C (and the polymerization was conducted under a continuously reduced pressure) [51].
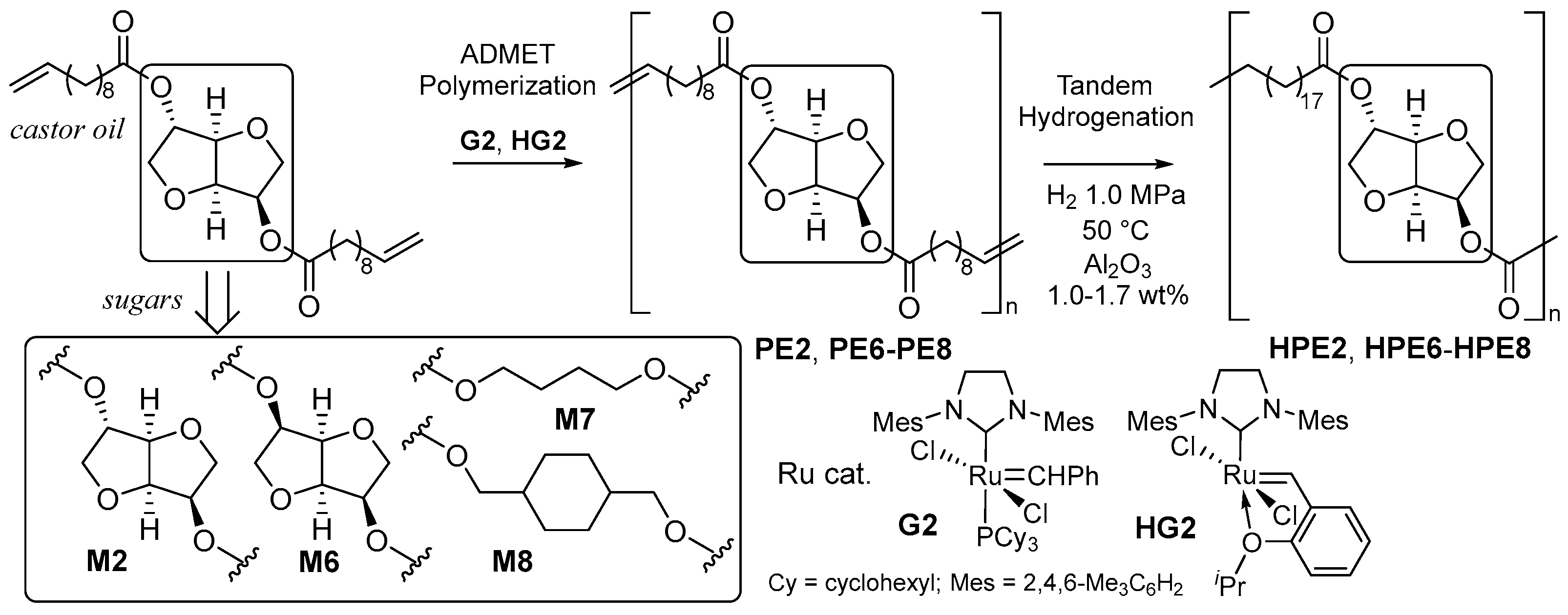
Scheme 5. One-pot synthesis of bio-based polyesters by Ru-catalyzed ADMET polymerization and hydrogenation.
As described above (Scheme 4) and below [52], conventional olefin hydrogenation requires a high hydrogen pressure and high temperature after the isolation of unsaturated polyesters after ADMET polymerization [46][47][52]. In contrast, one-pot hydrogenation under rather mild conditions (1.0 MPa, 50 °C, 3 h) was demonstrated following the addition of a small amount of Al2O3 (ca. 1 wt%) to the reaction mixture (Scheme 5). The completion of the olefin hydrogenation was confirmed by DSC thermograms (uniform compositions) due to the difficulty (accuracy of the integration of olefinic protons) of obtaining the 1H NMR spectra. No significant differences in the Mn and Mw/Mn values were observed before/after hydrogenation [51].
As shown in Figure 1b, the melting temperatures (Tm values) of the polyesters are influenced by the methylene unit number (n). As shown in Scheme 6, the copolymerization of M1 with undeca-1,10-diene (UDD) followed by olefin hydrogenation (H2 40 bar, 110 °C, 2 d) produced various LCAPEs with different chain lengths (ranging from 0.9 to 52.6 ester groups per 1000 carbon atoms), expressed as H2-poly(M1-co-UDD) [52]. A linear correlation of the melting temperatures (Tm values) with the average number of ester groups per methylene unit was thus demonstrated, whereas the ester group was incorporated in a random manner. A similar trend was observed in the copolymerization of M2 with 1,9-decadiene (DD) and the subsequent one-pot hydrogenation [53]. The saturated polymers possessed Tm values in the range of 71.7–107.6 °C, depending on the molar ratios of M2 and DD.
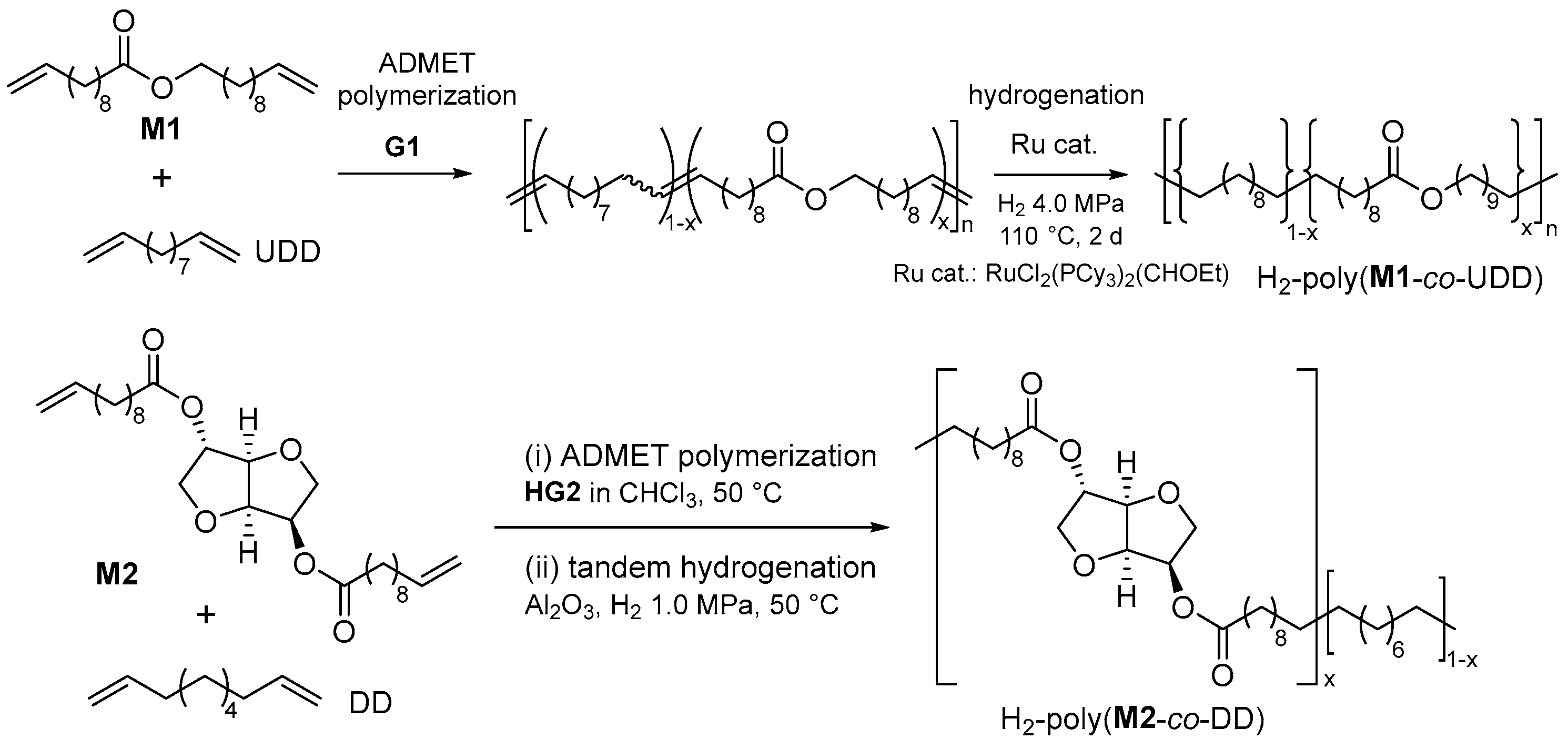
Scheme 6. ADMET copolymerization of undec-10-en-1-yl undec-10-enoate (M1) or bis(undec-10-enoate) with isosorbide (M2) with nonconjugated dienes, and subsequent hydrogenation.
The polymerization of bis(undec-10-enoate)s with D-xylose (1,2-O-isopropylidene-α-D-xylofuranose, M9c), and D-mannose (M10) by G2 was studied under a dynamic-vacuum (0.1 mbar) condition without solvent (bulk) conditions (60–90 °C, 20 h, Scheme 7) [54]. The molecular weights of the resultant polymers (PE9c, PE10) were affected by the polymerization temperature employed and the monomer/Ru molar ratios. Conducting the polymerization at 90 ºC under a low Ru concentration (0.1 mol%) seemed to be the optimized condition (PE9c: Ru, Mn = 7.14–7.16 × 104, Mw/Mn = 2.2–2.3, PE10: Mn = 3.24 × 104, Mw/Mn = 2.4) [46]. Due to the fact that the polymerization was conducted without a solvent, the PDI (Mw/Mn) values were rather high due to the difficulty pf controlling the stirring [54]. Later, the polymerizations of D-xylose diester analogs with different methylene lengths (M9, x = 0, 2, 8, Scheme 7) and the corresponding diether analogs (M11) were explored [55]. The Mn values of the resultant polymers decreased upon decreasing the methylene length, and the monomers did not possess a methylene spacer [55]. Some polymerization runs failed due to precipitation or the difficulty of performing isolations [55]. The resultant unsaturated polymers were amorphous, except PE11a, and both glass transition temperatures (Tg) increased after reducing the olefinic double bonds by treating them with p-toluenesulfonyl hydrazide as a reducing agent; most of the resultant saturated polymers (HPE9 and HPE11) were amorphous, except HPE9a and HPE11a derived from the castor oil (10-undecenoate), suggesting that the placement of the methylene spacer was important (as shown in Figure 1a and Figure 2) [55]. The resultant hydrogenated polymer films, especially the HPE11a-oriented film, exhibited a good tensile strength (43 MPa) with an elongation at a break of 155%; but, the hot-press film showed a much weaker tensile strength (7.8 MPa) with and improved elongation at the break (667%) [55].
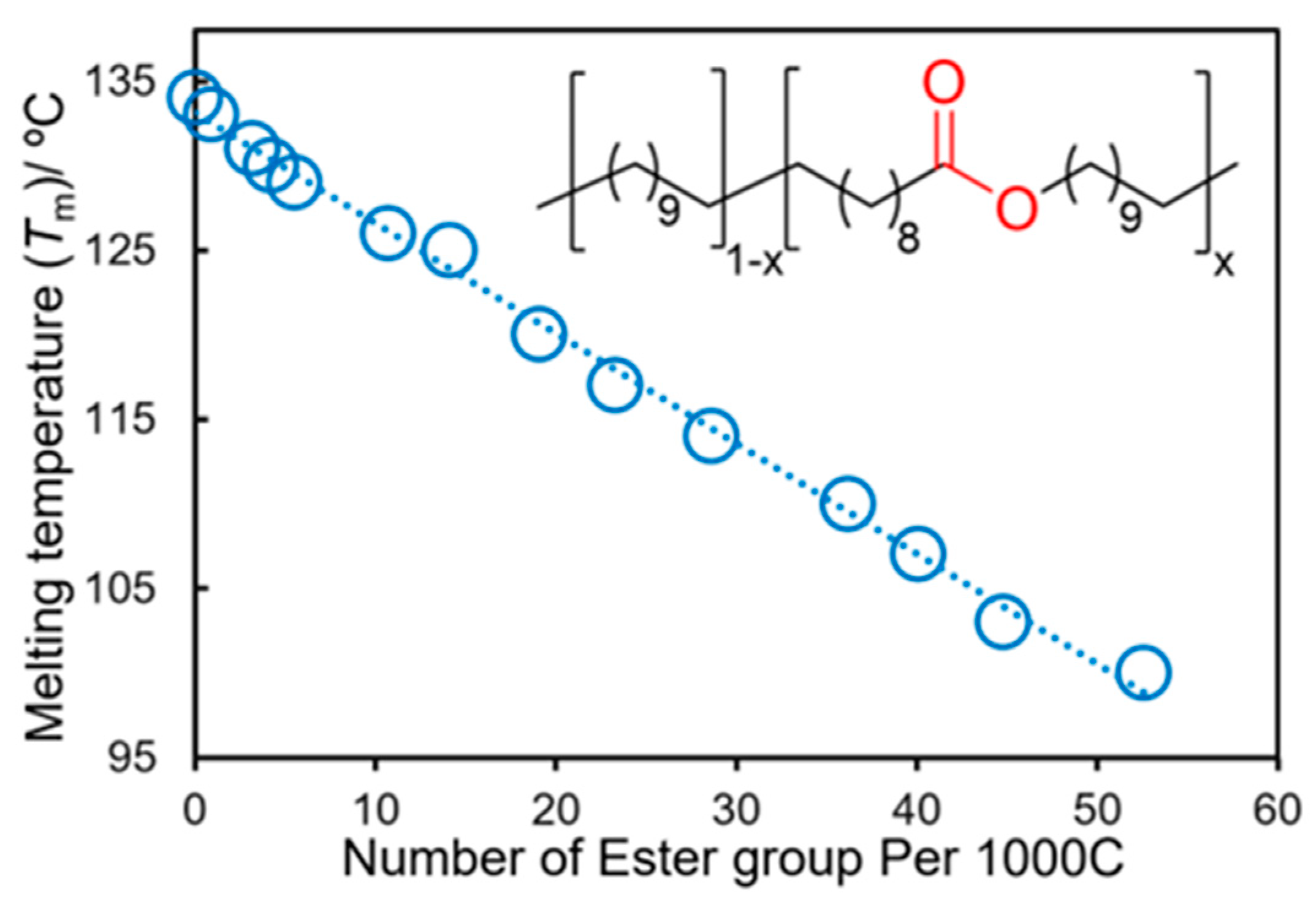
Figure 2. Plots of melting temperature (Tm) vs. number of ester groups per 1000 C (methylene units) in the hydrogenated copolymers, H2-poly(M1-co-UDD)s.
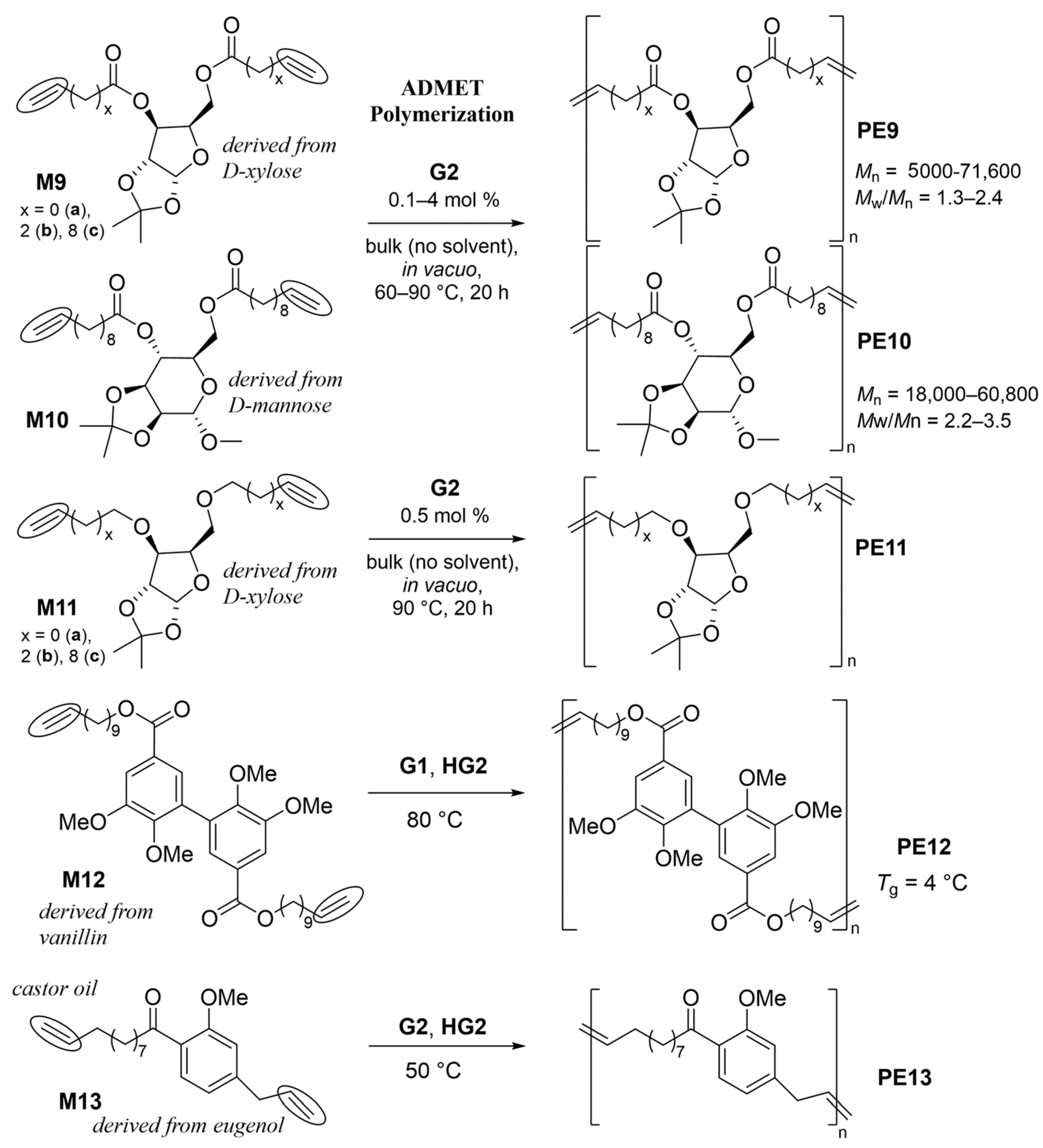
Scheme 7. ADMET polymerization of α,ω-dienes containing D-xylose, D-mannose, vanillin, and eugenol as the monomer units.
The syntheses of polyesters containing vanillin (PE12) [56] afforded high-molecular-weight PE12 (Mn = 10,000, Mw/Mn = 1.6) possessing a Tg value of 4 °C (Scheme 7), whereas the polymerization of 4-allyl-2-methoxyphenyl 10-undecenoate (M13) by G2 produced amorphous high molar mass polymers with low PDIs (Mw/Mn) with Tg at −9.6 °C [57]. The ADMET polymerization of M13 in the presence of 5-formylbenzene-1,2,3-triyl tris(undec-10-enoate) produced rather high molar mass network polymers [57].
The polymerization of trehalose bis(10-undecenoate) (M14) by HG2 (4.0 mol%) in THF at 45 ºC for 24 h (Scheme 8) produced semicrystalline polymers (PE14) possessing high molecular weights with unimodal molecular-weight distributions (Mn = 13,200, Mw/Mn = 2.1) with higher Tm values (156 °C) [58]. Both the molecular weights and melting temperatures (Tm values) of the resulting copolyesters with undec-10-en-1-yl undec-10-enoate (M1) decreased with the increase in the percentage of M1 [58].
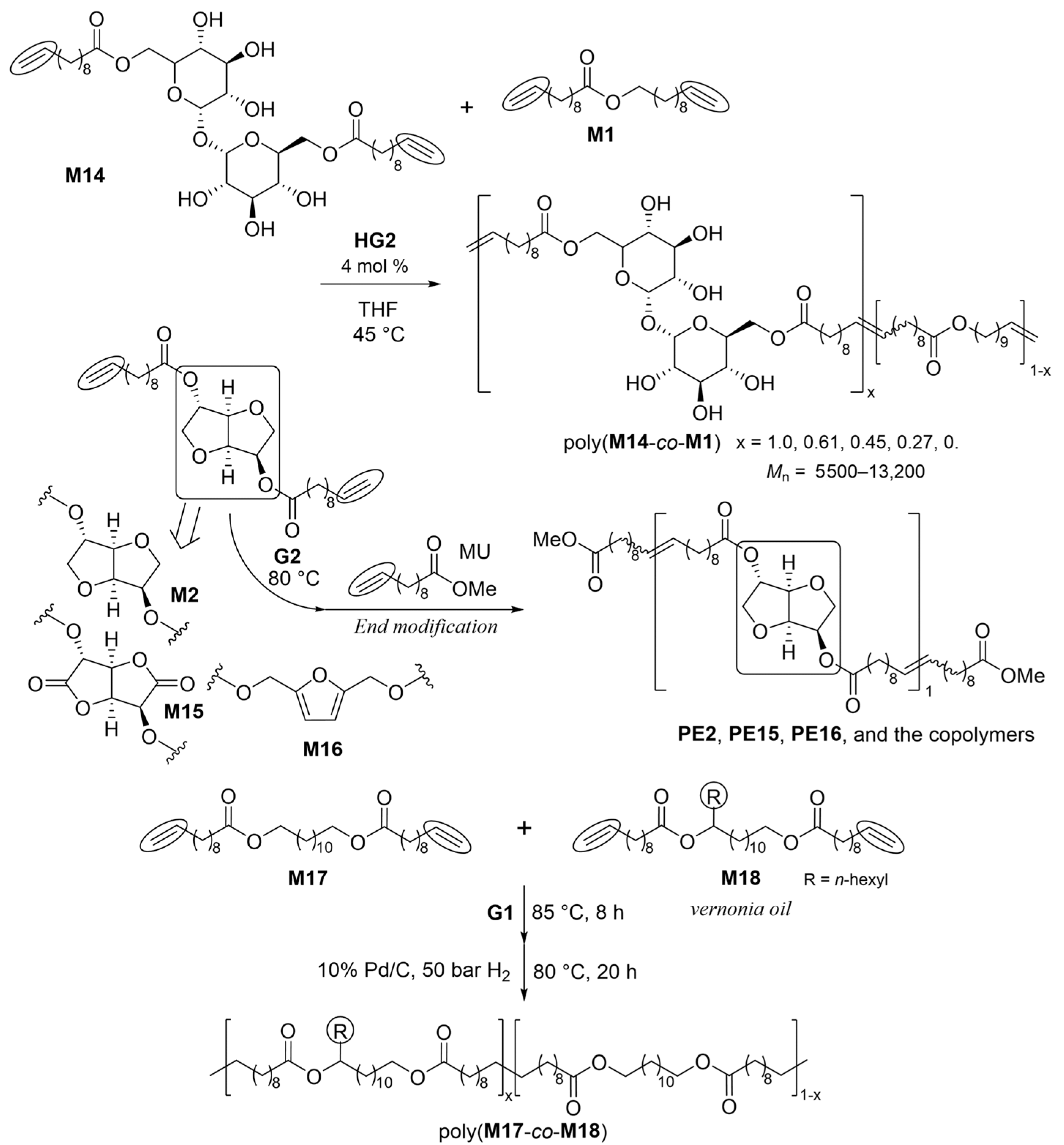
Scheme 8. Synthesis of bio-based copolyesters with different molar ratios.
References
- Gandini, A. Polymers from renewable resources: A Challenge for the future of macromolecular materials. Macromolecules 2008, 41, 9491–9504.
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802.
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909.
- Biermann, U.; Bornscheuer, U.; Meier, M.A.R.; Metzger, J.O.; Schäfer, H.J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871.
- Hillmyer, M.A.; Tolman, W.B. Aliphatic polyester block polymers: Renewable, degradable, and sustainable. Acc. Chem. Res. 2014, 47, 2390–2396.
- Stempfle, F.; Ortmann, P.; Mecking, S. Long-chain aliphatic polymers to bridge the gap between semicrystalline polyolefins and traditional polycondensates. Chem. Rev. 2016, 116, 4597–4641.
- Monomers and polymers from chemically modified plant oils and their fatty acids. In Polymers from Plant Oils, 2nd ed.; Gandini, A.; Lacerda, T.M. (Eds.) John Wiley & Sons, Inc.: Hoboken, NJ, USA; Scrivener Publishing LLC: Beverly, MA, USA, 2019; pp. 33–82.
- Nomura, K.; Awang, N.W.B. Synthesis of bio-based aliphatic polyesters from plant oils by efficient molecular catalysis: A selected survey from recent reports. ACS Sustain. Chem. Eng. 2021, 9, 5486–5505.
- Häußler, M.; Eck, M.; Rothauer, D.; Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 2021, 590, 423–427.
- Biermann, U.; Bornscheuer, U.T.; Feussner, I.; Meier, M.A.R.; Metzger, J.O. Fatty acids and their derivatives as renewable platform molecules for the chemical industry. Angew. Chem. Int. Ed. 2021, 60, 20144–20165.
- Worch, J.C.; Dove, A.P. 100th Anniversary of macromolecular science viewpoint: Toward catalytic chemical recycling of waste (and future) plastics. ACS Macro Lett. 2020, 9, 1494–1506.
- Ishioka, R.; Kitakuni, E.; Ichikawa, Y. Aliphatic polyesters: “Bionolle”. In Biopolymers; Steinbüchel, A., Doi, Y., Eds.; Wiley-VCH: Weinheim, Germany, 2002; Volume 4, pp. 275–297.
- Korshak, W.V.; Vinogradova, S.V. Polyesters of 1,20-eicosanediol. Bull. Acad. Sci. USSR Div. Chem. Sci. 1953, 2, 995–998.
- Bunn, C.W. The melting points of chain polymers. J. Polym. Sci. 1955, 16, 323–343.
- Gallagher, J.J.; Hillmyer, M.A.; Reineke, T.M. Isosorbide-based polymethacrylates. ACS Sustain. Chem. Eng. 2015, 3, 662–667.
- Wang, J.; Mahmud, S.; Zhang, X.; Zhu, J.; Shen, Z.; Liu, X. Bio-based amorphous polyesters with high Tg: Trade-off between rigid and flexible cyclic diols. ACS Sustain. Chem. Eng. 2019, 7, 6401–6411.
- Walther, G.; Deutsch, J.; Martin, A.; Baumann, F.; Fridag, D.; Franke, R.; Kçckritz, A. α,ω-Functionalized C19 Monomers. ChemSusChem 2011, 4, 1052–1054.
- Witt, T.; Häußler, M.; Kulpa, S.; Mecking, S. Chain multiplication of fatty acids to precise telechelic polyethylene. Angew. Chem. Int. Ed. 2017, 56, 7589–7594.
- Herrmann, N.; Köhnke, K.; Seidensticker, T. Selective product crystallization for concurrent product separation and catalyst recycling in the isomerizing methoxycarbonylation of methyl oleate. ACS Sustain. Chem. Eng. 2020, 8, 10633–10638.
- Pandey, S.; Rajput, B.S.; Chikkali, S.H. Refining plant oils and sugars to platform chemicals, monomers, and polymers. Green Chem. 2021, 23, 4255–4295.
- Wyrębek, P.; Małecki, P.; Sytniczuk, A.; Kośnik, W.; Gawin, A.; Kostrzewa, J.; Kajetanowicz, A.; Grela, K. Looking for the noncyclic(amino)(alkyl)carbene ruthenium catalyst for ethenolysis of cthyl oleate: Selectivity is on target. ACS Omega 2018, 3, 18481–18488.
- Sytniczuk, A.; Kajetanowicz, A.; Grela, K. “Inverted” cyclic(alkyl)(amino)carbene ligands allow olefin metathesis with ethylene at parts-per-billion catalyst loading. Chem. Catal. 2023, 3, 100713.
- Gawin, R.; Tracz, A.; Krajczy, P.; Kozakiewicz-Piekarz, A.; Martínez, J.P.; Trzaskowski, B. Inhibition of the decomposition pathways of ruthenium olefin metathesis catalysts: Development of highly efficient catalysts for ethenolysis. J. Am. Chem. Soc. 2023, 145, 25010–25021.
- Del Vecchio, A.; Talcik, J.; Colombel-Rouen, S.; Lorkowski, J.; Serrato, M.R.; Roisnel, T.; Vanthuyne, N.; Bertrand, G.; Jazzar, R.; Mauduit, M. Highly robust and efficient Blechert-type cyclic(alkyl)(amino)carbene ruthenium complexes for olefin metathesis. ACS Catal. 2023, 13, 6195–6206.
- Rogers, M.E.; Long, T.E. Synthetic Methods in Step-Growth Polymers; Wiley-Interscience: Hoboken, NJ, USA, 2003.
- Le Fevere de Ten Hove, C.; Penelle, J.; Ivanov, D.A.; Jonas, A.M. Encoding crystal microstructure and chain folding in the chemical structure of synthetic polymers. Nat. Mater. 2004, 3, 33–37.
- Menges, M.G.; Penelle, J.; Le Fevere de Ten Hove, C.; Jonas, A.M.; Schmidt-Rohr, K. Characterization of long-chain aliphatic polyesters: Crystalline and supramolecular structure of PE22,4 elucidated by X-ray scattering and nuclear magnetic resonance. Macromolecules 2007, 40, 8714–8725.
- Roesle, P.; Stempfle, F.; Hess, S.K.; Zimmerer, J.; Río Bartulos, C.; Lepetit, B.; Eckert, A.; Kroth, P.G.; Mecking, S. Synthetic polyester from Algae oil. Angew. Chem. Int. Ed. 2014, 53, 6800–6804.
- Trnka, T.M.; Grubbs, R.H. The development of L2X2Ru=CHR olefin metathesis catalysts: An organometallic success story. Acc. Chem. Res. 2001, 34, 18–29.
- Samojzowicz, C.; Bieniek, M.; Grela, K. Ruthenium-based olefin metathesis catalysts bearing N-heterocyclic carbene ligands. Chem. Rev. 2009, 109, 3708–3742.
- Grubbs, R.H.; Wenzel, A.G.; O’Leary, D.J.; Khosravi, E. (Eds.) Handbook of Metathesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2015.
- Vougioukalakis, G.; Grubbs, R.H. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 2010, 110, 1746–1787.
- Oskam, H.J.; Fox, H.H.; Yap, B.K.; McConville, H.D.; O’Dell, R.; Lichtenstein, J.B.; Schrock, R.R. Ligand variation in alkylidene complexes of the type Mo(CHR)(NR′)(OR″)2. J. Organomet. Chem. 1993, 459, 185–198.
- Schrock, R.R.; Hoveyda, A.H. Molybdenum and tungsten imido alkylidene complexes as efficient olefin-metathesis catalysts. Angew. Chem. Int. Ed. 2003, 42, 4592–4633.
- Miyashita, T.; Kunisawa, M.; Sueki, S.; Nomura, K. Synthesis of poly(arylene vinylene)s containing different end groups by combined acyclic diene metathesis polymerization with Wittig-type coupling. Angew. Chem. Int. Ed. 2017, 56, 5288–5293.
- Kojima, K.; Wang, X.; Go, L.; Makino, R.; Matsumoto, Y.; Shimoyama, D.; Abdellatif, M.M.; Kadota, J.; Hirano, H.; Nomura, K. Synthesis of high molecular weight bio-based aliphatic polyesters exhibiting tensile properties beyond polyethylene. ACS Macro Lett. 2023, 12, 1403–1408.
- Rybak, A.; Meier, M.A.R. Acyclic diene metathesis with a monomer from renewable resources: Control of molecular weight and one-step preparation of block copolymers. ChemSusChem 2008, 1, 542–547.
- Fokou, P.A.; Meier, M.A.R. Use of a renewable and degradable monomer to study the temperature-dependent olefin isomerization during ADMET polymerizations. J. Am. Chem. Soc. 2009, 131, 1664–1665.
- Ulman, M.; Grubbs, R.H. Ruthenium carbene-based olefin metathesis initiators: Catalyst decomposition and longevity. J. Org. Chem. 1999, 64, 7202–7207.
- Lehman, S.E.; Wagener, K.B. Comparison of the kinetics of acyclic diene metathesis promoted by Grubbs ruthenium olefin metathesis catalysts. Macromolecules 2001, 35, 48–53.
- Schmidt, B. Catalysis at the interface of ruthenium carbene and ruthenium hydride chemistry: Organometallic aspects and applications to organic synthesis. Eur. J. Org. Chem. 2004, 2004, 1865–1880.
- Hong, S.H.; Wenzel, A.G.; Salguero, T.T.; Day, M.W.; Grubbs, R.H. Decomposition of ruthenium olefin metathesis catalysts. J. Am. Chem. Soc. 2007, 129, 7961–7968.
- Higman, C.S.; Lanterna, A.E.; Marin, M.L.; Scaiano, J.C.; Fogg, D.E. Catalyst decomposition during olefin metathesis yields isomerization-active ruthenium nanoparticles. ChemCatChem 2016, 8, 2446–2449.
- Jawiczuk, M.; Marczyk, A.; Trzaskowski, B. Decomposition of ruthenium olefin metathesis catalyst. Catalysts 2020, 10, 887.
- Fokou, P.A.; Meier, M.A.R. Studying and suppressing olefin isomerization side reactions during ADMET polymerizations. Macromol. Rapid Commun. 2010, 31, 368–373.
- Trzaskowski, J.; Quinzler, D.; Bährle, C.; Mecking, S. Aliphatic long-chain C20 polyesters from olefin metathesis. Macromol. Rapid Commun. 2011, 32, 1352–1356.
- Stempfle, F.; Ortmann, P.; Mecking, S. Which polyesters can mimic polyethylene? Macromol. Rapid Commun. 2013, 34, 47–50.
- Vilela, C.; Silvestre, A.J.D.; Meier, M.A.R. Plant oil-based long-chain C26 monomers and their polymers. Macromol. Chem. Phys. 2012, 213, 2220–2227.
- Stempfle, F.; Quinzler, D.; Heckler, I.; Mecking, S. Long-chain linear C19 and C23 monomers and polycondensates from unsaturated fatty acid esters. Macromolecules 2011, 44, 4159–4166.
- Roumanet, P.-J.; Jarroux, N.; Goujard, L.; Le Petit, J.; Raoul, Y.; Bennevault, V.; Guégan, P. Synthesis of linear polyesters from monomers based on 1,18-(Z)-octadec-9-enedioic acid and their biodegradability. ACS Sustain. Chem. Eng. 2020, 8, 16853–16860.
- Nomura, K.; Chaijaroen, P.; Abdellatif, M.M. Synthesis of bio-based long-chain polyesters by acyclic diene metathesis polymerization and tandem hydrogenation and depolymerization with ethylene. ACS Omega 2020, 5, 18301–18312.
- Ortmann, P.; Mecking, S. Long-spaced aliphatic polyesters. Macromolecules 2013, 46, 7213–7218.
- Kojima, M.; Abdellatif, M.M.; Nomura, K. Synthesis of semi-crystalline long chain aliphatic polyesters by ADMET copolymerization of dianhydro-D-glucityl bis(undec-10-enoate) with 1,9-decadiene and tandem hydrogenation. Catalysts 2021, 11, 1098–1106.
- Piccini, M.; Leak, D.J.; Chuck, C.J.; Buchard, A. Polymers from sugars and unsaturated fatty acids: ADMET polymerisation of monomers derived from d-xylose, d-mannose and castor oil. Polym. Chem. 2020, 11, 2681–2691.
- Piccini, M.; Lightfoot, J.; Castro, D.B.; Buchard, A. Xylose-Based Polyethers and Polyesters Via ADMET Polymerizationtoward Polyethylene-Like Materials. ACS Appl. Polym. Mater. 2021, 3, 5870–5881.
- Llevot, A.; Grau, E.; Carlotti, S.; Greliera, S.; Cramail, H. ADMET polymerization of bio-based biphenyl compounds. Polym. Chem. 2015, 6, 7693–7700.
- Le, D.; Samart, C.; Kongparakul, S.; Nomura, K. Synthesis of new polyesters by acyclic diene metathesis polymerization of bio-based α,ω-dienes prepared from eugenol and castor oil (undecenoate). RSC Adv. 2019, 9, 10245–10252.
- Hibert, G.; Grau, E.; Pintori, D.; Lecommandoux, S.; Cramail, H. ADMET polymerization of α,ω-unsaturated glycolipids: Synthesis and physico-chemical properties of the resulting polymers. Polym. Chem. 2017, 8, 3731–3739.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
951
Revisions:
2 times
(View History)
Update Date:
06 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





