Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shaopeng Wang | -- | 1953 | 2024-02-05 18:20:41 | | | |
| 2 | Sirius Huang | Meta information modification | 1953 | 2024-02-07 01:35:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chieng, A.; Wan, Z.; Wang, S. Real-Time Label-Free Detection of Small Molecules. Encyclopedia. Available online: https://encyclopedia.pub/entry/54778 (accessed on 07 February 2026).
Chieng A, Wan Z, Wang S. Real-Time Label-Free Detection of Small Molecules. Encyclopedia. Available at: https://encyclopedia.pub/entry/54778. Accessed February 07, 2026.
Chieng, Andy, Zijian Wan, Shaopeng Wang. "Real-Time Label-Free Detection of Small Molecules" Encyclopedia, https://encyclopedia.pub/entry/54778 (accessed February 07, 2026).
Chieng, A., Wan, Z., & Wang, S. (2024, February 05). Real-Time Label-Free Detection of Small Molecules. In Encyclopedia. https://encyclopedia.pub/entry/54778
Chieng, Andy, et al. "Real-Time Label-Free Detection of Small Molecules." Encyclopedia. Web. 05 February, 2024.
Copy Citation
The detection and analysis of small molecules, typically defined as molecules under 1000 Da, is of growing interest ranging from the development of small-molecule drugs and inhibitors to the sensing of toxins and biomarkers. However, due to challenges such as their small size and low mass, many biosensing technologies struggle to have the sensitivity and selectivity for the detection of small molecules.
small molecule
label-free
real-time detection
biosensor
optical transduction
electrochemical transduction
piezoelectric transduction
1. Introduction
Small molecules, typically defined as molecules under 1000 Da, have been of great interest due to their ubiquitous presence in biological processes and their intrinsic properties in human anatomy [1][2]. Notably, small molecules are capable of crossing the blood-brain barrier and have relative ease in cell membrane permeation compared to larger molecules; thus, small molecules can both enter the body readily and traverse to targets with high specificity [1][2]. These properties have great appeal in the pharmaceutical industry, with 15 of the 37 drugs approved by the FDA in 2022 being small molecules [3]. On the other hand, the properties that make small molecules appealing for drug development also make them effective toxins, carcinogens, mutagens, and endocrine disruptors both natural, such as several secondary metabolites of fungi, cyanobacteria, plants, and other organisms; and artificial, such as chemical warfare agents [1][2][4][5][6][7]. Furthermore, biological systems themselves utilize small molecules such as amino acids, steroids, sugars, or metabolites for many of the processes essential for life [1][2][7].
Considering the biological importance of small molecules, the development of biosensors capable of real-time detection has been in great demand with widespread applications such as: ensuring correct dosage of drugs, early diagnosis of disease, or continuous detection of toxins. Biosensors are generally composed of a receptor that captures an analyte and a transducer that changes in property upon the binding between the analyte and the receptor to produce a recordable signal [2]. Unfortunately, due to the low abundance and mass of small molecules, many techniques, especially those where the transducer’s signal strength scales with analyte mass, are challenging to achieve the sensitivity necessary for the effective detection of small molecules under biologically relevant conditions [7][8]. In addition, label-free techniques are greatly preferred for small molecules, even more so compared to larger analytes like proteins. The low mass and small size of small molecules only exacerbate the concerns of labeled detection techniques in that labels such as fluorescent proteins, fluorescent dyes, and quantum dots can alter the binding behavior and physical properties of an analyte [9][10]. In many cases, the label is much larger than the analyte itself; for example, green fluorescent protein, a common fluorescent label, has a molecular weight of 27 kDa to 69 kDa, and even small-molecule organic fluorescent probes such as fluorescein have a molecular weight of approximately 332 Da; a very significant addition to a small molecule with mass less than 1000 Da [9][10][11][12]. Currently, one of the common techniques for molecular detection is enzyme-linked immunosorbent assay (ELISA) and its variations, which offers high accuracy and sensitivity and can be used for small molecule detection; however, ELISA is an endpoint detection method that does not provide real-time kinetic information [13]. Small-molecule interaction kinetics and affinity with their target or receptor molecules are essential information for studying small molecule functions [14]. Thus, real-time measurement is preferred; correspondingly, transduction methods that can be measured continuously, such as changes in refractive index, surface conductance, and resonance frequency, have been developed into widespread label-free techniques, including surface plasmon resonance (SPR), field effect transistor (FET), and quartz crystal microbalance (QCM), for real-time small molecule detection using the corresponding receptor or target molecules as recognition elements [2][15][16][17][18][19][20]. The unique challenges to achieving the sensitivity and selectivity necessary to detect small molecules are leading to innovations to amplify signal and optimize surface chemistry as well as interesting methods to indirectly measure binding. These innovations can be broadly categorized as: physical amplification, cascading or chain reaction, and molecular switches (Figure 1). These strategies attempt to circumvent the limiting attributes of small molecules by measuring the change of alternative properties upon binding, correlating the concentration of analyte to a more easily measured product, or using a conformational change induced by analyte binding to produce a signal, respectively.
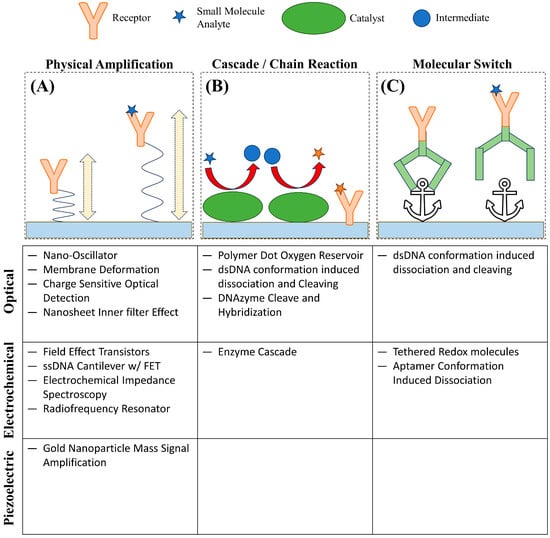
Figure 1. Representative novel strategies for small molecule detection. (A) Small-molecule binding induces a change in amplitude of a charge-sensitive oscillation. (B) A small molecule analyte undergoes a series of reactions to produce a product that can be detected. (C) Small-molecule binding results in a conformational change releasing a structure from an anchor point.
2. Optical Transduction
Optical transducers have great potential in the real-time detection of small molecules, but also face intrinsic challenges to fully utilizing their advantages. Optical transductions can measure a variety of signals related to the presence of small molecules, such as absorption, scattering, luminescence, or refractive index [2]. Optical biosensors typically offer fast results with high temporal resolution for real-time monitoring of binding, making them ideal for diagnostics and point-of-care devices [7]. However, the signal of optical transducers generally scales with the size and/or mass of the analyte of interest, especially when avoiding the use of fluorescent labels [2][7][21]. For example, one of the commonly used label-free optical detection techniques, surface plasmon resonance, has been lauded as the gold standard for molecular binding kinetic measurement, being used for analytes such as proteins, DNA, RNA, peptides, and other biological macromolecules, but attempts to utilize SPR for the detection of small molecules required advanced instrumentation or significant enhancement of receptor surface [15][22]. Thus, the development of optical techniques for the measurement of small molecules has primarily focused on either enhancing the weak signal of small-molecule binding or by measuring binding indirectly via measuring phenomena induced by analyte-binding events.
Surface plasmon resonance utilizes surface plasmons, electron oscillations at a metal-dielectric interface, typically gold-coated glass, which respond via oscillation at resonance with a light wave [23]. The evanescent waves of this oscillation are sensitive to changes close to the metal surface, notably as a change in refractive index due to the binding of an analyte, which shifts the SPR signal and produces a signal proportional to the analyte’s mass [24]. Unfortunately, this signal dependency on the mass of an analyte makes it challenging for SPR to detect small molecules, often requiring enhancement of either binding site density or signal strength such as through the usage of dextran chips or by utilizing localized surface plasmon resonance with nanostructures [15]. Thus, there has been great interest in developing sensing platforms with high sensitivity to small molecules, requiring simpler sample preparation and instrument operation, and for diagnostic use, having high portability and low cost.
Since the emergence of SPR in the 1970s, surface plasmon-based techniques have been developed with great interest, and different strategies have emerged to expand techniques into the range of small molecules and overcome their inherent mass dependency [25]. The use of gold-coated optical fibers instead of traditional gold chips for SPR has been in development since the 1990s, offering reduced cost and size compared to traditional SPR; which facilitates its application in point-of-care or diagnostic fields and the ability to optimize sensor attributes to suit the sensor’s purpose by changing the fabrication of the fiber probe [26][27].
3. Electrochemical Transduction
Electrochemical transducers share many of the advantages of optical transducers in that they also offer fast, real-time measurements of binding and have the added benefit of generally requiring low-cost instrumentations that can be designed for high portability and accessibility for use with little to no training. Furthermore, the signal of electrochemical transducers is generally not significantly dependent on the size or mass of analytes, but rather on their electronic properties, such as electrochemical reactivity or charge [16][28][29]. Traditionally, electrochemical biosensors utilized potentiometry or amperometry to detect redox reactions at an electrode surface, offering a mass-independent signal, but this greatly limits the range of molecules that can be detected via electrochemical biosensors to electroactive molecules only. Thus, although real-time electrochemical detection of oxygen and glucose has already reached the commercialization stage for diagnostics and point-of-care monitoring, the detection of other small molecules through electrochemical detection is still developing [30]. For this reason, innovations in the field have explored other means for analytes to modify the electrical properties of transducers. In particular, impedance-based transducers are promising alternatives that are primarily dependent on the charge of small molecules. Thus, impedance biosensors such as FET sensors have grown in popularity for the detection of small molecules due to their detection mechanism, in which the signal is produced by a change in conductance upon binding of charged molecules, allowing high-sensitivity mass independent measurement [17][18]. Unfortunately, charge-sensitive techniques face challenges from nonspecific binding and Debye screening from the biologically relevant high ionic strength that hinder their sensitivity in serum, plasma, or even phosphate-buffered saline (PBS) [6][31]. Sensing platforms for small molecules have developed methods to use electrochemical transduction that can detect a larger range of different analytes by enhancing the signal strength of impedance-based biosensors to compensate for charge screening in biologically relevant ionic concentrations. Furthermore, enhancement of signal strength increases the sensitivity of electrochemical instrumentation, and helps to detect the relatively low abundance small molecules typically have in nature [32][33].
One of the weaknesses of electrochemical detection, particularly in amperometry or potentiometry, is that the analyte of interest must be electrochemically active in order to produce a signal. One workaround to this weakness adapted commercially personal glucose meter for the detection of ATP by using a cascade enzymatic reaction promoted by hexokinase and pyruvate kinase [34]. The amount of ATP is inversely proportional to glucose through the catalyzation of glucose to glucose 6-phosphate in which ATP is converted to ADP [34]. Pyruvate kinase catalyzes the regeneration of ATP from ADP to further react glucose and amplify the signal. Concentrations of ATP as low as 2.5 × 10−8 g/mL can be detected [34]. This technique offers a potential means to indirectly measure small molecules that are not electroactive by instead measuring the proportional signal of an electroactive product from an enzymatic reaction. Kurbanoglu et al. developed a methimazole (MT) enzyme cascade blocking biosensor using a nanocomposite of magnetic nanoparticles and iridium oxide nanoparticles on screen-printed electrodes and obtained an LOD of 6.85 × 10−10 g/mL [35]. By utilizing the inhibition of tyrosinase via chelating copper and forming thioquinone with MT, the concentration of MT can be measured via amperometry resulting in a miniaturized lab on a chip biosensor that can be adapted to other small molecules that can inhibit enzymes [35].
4. Piezoelectric Transduction
In comparison to optical and electrochemical transducers, piezoelectric transducers are a relatively recent addition to the repertoire of techniques for detecting small molecules, most popularly utilizing quartz crystal microbalances (QCM) [19]. Piezoelectricity, the phenomenon in which a material produces voltage under mechanical stress or vice versa, allows for the fabrication of sensors that utilize anisotropic crystals that oscillate upon the application of voltage [19][36]. Piezoelectric biosensors typically measure change in oscillation due to analyte binding for the measurement of analyte properties and kinetic information [36]. For example, in a conventional quartz crystal microbalance, the added mass upon binding increases the damping of the oscillation and a change in dissipation rate upon ceasing of voltage application that is related to the mass of the bound analyte following the Sauerbrey Equation [20][37]. Unfortunately, Piezoelectric transducers find difficulty in the measurement of small-molecule binding due to the mass dependency of the oscillation’s frequency change; additionally, though piezoelectric transducers are resistant to interference from non-transparent mediums compared to optical transducers, they are responsive to changes in viscosity [20]. Nevertheless, piezoelectric biosensors can be versatile and robust methods for small molecule detection and much progress has been made in enhancing the sensitivity of piezoelectric-based techniques. Furthermore, most relevant biosensing conditions require the sensor to be in liquid, which produces an additive damping to the measured frequency. Thus, the development of piezoelectric biosensors for small-molecule detection has been in amplifying the change of frequency upon binding or utilizing alternative means to collect data from the piezoelectric transducer.
References
- Fechner, P.; Bleher, O.; Ewald, M.; Freudenberger, K.; Furin, D.; Hilbig, U.; Kolarov, F.; Krieg, K.; Leidner, L.; Markovic, G.; et al. Size Does Matter! Label-Free Detection of Small Molecule-Protein Interaction. Anal. Bioanal. Chem. 2014, 406, 4033–4051.
- Peltomaa, R.; Glahn-Martínez, B.; Benito-Peña, E.; Moreno-Bondi, M.C. Optical Biosensors for Label-Free Detection of Small Molecules. Sensors 2018, 18, 4126.
- Tiz, D.B.; Bagnoli, L.; Rosati, O.; Marini, F.; Santi, C.; Sancineto, L. FDA-Approved Small Molecules in 2022: Clinical Uses and Their Synthesis. Pharmaceutics 2022, 14, 2538.
- Wu, X.; Zhang, Q.; Guo, Y.; Zhang, H.; Guo, X.; You, Q.; Wang, L. Methods for the Discovery and Identification of Small Molecules Targeting Oxidative Stress-Related Protein–Protein Interactions: An Update. Antioxidants 2022, 11, 619.
- Singh, S.; Srivastava, A.; Oh, H.M.; Ahn, C.Y.; Choi, G.G.; Asthana, R.K. Recent Trends in Development of Biosensors for Detection of Microcystin. Toxicon 2012, 60, 878–894.
- Vogiazi, V.; De La Cruz, A.; Mishra, S.; Shanov, V.; Heineman, W.R.; Dionysiou, D.D. A Comprehensive Review: Development of Electrochemical Biosensors for Detection of Cyanotoxins in Freshwater. ACS Sens. 2019, 4, 1151–1173.
- Moulahoum, H.; Ghorbanizamani, F.; Guler Celik, E.; Zihnioglu, F.; Timur, S. Nanoconjugated Materials as Sensors in Point-of-Care Diagnostic Tools Detection of Small Molecules and Viruses; Elsevier B.V.: Amsterdam, The Netherlands, 2023; Volume 102, ISBN 978-0-443-13199-8.
- Sayago, I.; Matatagui, D.; Fernández, M.J.; Fontecha, J.L.; Jurewicz, I.; Garriga, R.; Muñoz, E. Graphene Oxide as Sensitive Layer in Love-Wave Surface Acoustic Wave Sensors for the Detection of Chemical Warfare Agent Simulants. Talanta 2016, 148, 393–400.
- Crivat, G.; Taraska, J.W. Imaging Proteins inside Cells with Fluorescent Tags. Trends Biotechnol. 2012, 30, 8–16.
- Jensen, E.C. Use of Fluorescent Probes: Their Effect on Cell Biology and Limitations. Anat. Rec. 2012, 295, 2031–2036.
- Fu, Y.; Finney, N.S. Small-Molecule Fluorescent Probes and Their Design. RSC Adv. 2018, 8, 29051–29061.
- Wysocki, L.M.; Lavis, L.D. Advances in the Chemistry of Small Molecule Fluorescent Probes. Curr. Opin. Chem. Biol. 2011, 15, 752–759.
- Powers, J.L.; Rippe, K.D.; Imarhia, K.; Swift, A.; Scholten, M.; Islam, N. A Direct, Competitive Enzyme-Linked Immunosorbent Assay (ELISA) as a Quantitative Technique for Small Molecules. J. Chem. Educ. 2012, 89, 1587–1590.
- Hämäläinen, M.D.; Markgren, P.-O.; Schaal, W.; Karlén, A.; Classon, B.; Vrang, L.; Samuelsson, B.; Hallberg, A.; Danielson, U.H. Characterization of a Set of HIV-1 Protease Inhibitors Using Binding Kinetics Data from a Biosensor-Based Screen. J. Biomol. Screen. 2000, 5, 353–359.
- Myszka, D.G. Analysis of Small-Molecule Interactions Using Biacore S51 Technology. Anal. Biochem. 2004, 329, 316–323.
- Wadhera, T.; Kakkar, D.; Wadhwa, G.; Raj, B. Recent Advances and Progress in Development of the Field Effect Transistor Biosensor: A Review. J. Electron. Mater. 2019, 48, 7635–7646.
- Vu, C.A.; Chen, W.Y. Predicting Future Prospects of Aptamers in Field-Effect Transistor Biosensors. Molecules 2020, 25, 680.
- Shoorideh, K.; Chui, C.O. Optimization of the Sensitivity of FET-Based Biosensors via Biasing and Surface Charge Engineering. IEEE Trans. Electron. Devices 2012, 59, 3104–3110.
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448.
- García-Martinez, G.; Bustabad, E.A.; Perrot, H.; Gabrielli, C.; Bucur, B.; Lazerges, M.; Rose, D.; Rodriguez-Pardo, L.; Fariña, J.; Compère, C.; et al. Development of a Mass Sensitive Quartz Crystal Microbalance (QCM)-Based DNA Biosensor Using a 50 MHz Electronic Oscillator Circuit. Sensors 2011, 11, 7656–7664.
- Guo, X. Surface Plasmon Resonance Based Biosensor Technique: A Review. J. Biophotonics 2012, 5, 483–501.
- Singh, P. SPR Biosensors: Historical Perspectives and Current Challenges. Sens. Actuators B Chem. 2016, 229, 110–130.
- Wang, Q.; Ren, Z.H.; Zhao, W.M.; Wang, L.; Yan, X.; Zhu, A.S.; Qiu, F.M.; Zhang, K.K. Research Advances on Surface Plasmon Resonance Biosensors. Nanoscale 2022, 14, 564–591.
- Gosu, R.; Zaheer, S.M. Principle of Surface Plasmon Resonance (OneStep) BT—Methods for Fragments Screening Using Surface Plasmon Resonance; Zaheer, S.M., Gosu, R., Eds.; Springer: Singapore, 2021; pp. 5–7. ISBN 978-981-16-1536-8.
- Pockrand, I.; Swalen, J.D.; Gordon, J.G.; Philpott, M.R. Surface Plasmon Spectroscopy of Organic Monolayer Assemblies. Surf. Sci. 1978, 74, 237–244.
- Zhao, Y.; Tong, R.; Xia, F.; Peng, Y. Current Status of Optical Fiber Biosensor Based on Surface Plasmon Resonance. Biosens. Bioelectron. 2019, 142, 111505.
- Jorgenson, R.C.; Yee, S.S. A Fiber-Optic Chemical Sensor Based on Surface Plasmon Resonance. Sens. Actuators B Chem. 1993, 12, 213–220.
- Xiao, F.; Wang, L.; Duan, H. Nanomaterial Based Electrochemical Sensors for in Vitro Detection of Small Molecule Metabolites. Biotechnol. Adv. 2016, 34, 234–249.
- Chen, R.J.; Choi, H.C.; Bangsaruntip, S.; Yenilmez, E.; Tang, X.; Wang, Q.; Chang, Y.L.; Dai, H. An Investigation of the Mechanisms of Electronic Sensing of Protein Adsorption on Carbon Nanotube Devices. J. Am. Chem. Soc. 2004, 126, 1563–1568.
- Wang, J. Electrochemical Glucose Biosensors. Electrochem. Sens. Biosens. Biomed. Appl. 2008, 108, 814–825.
- Bogomolova, A.; Komarova, E.; Reber, K.; Gerasimov, T.; Yavuz, O.; Bhatt, S.; Aldissi, M. Challenges of Electrochemical Impedance Spectroscopy in Protein Biosensing. Anal. Chem. 2009, 81, 3944–3949.
- Wiegand, C.; Pflugmacher, S. Ecotoxicological Effects of Selected Cyanobacterial Secondary Metabolites a Short Review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218.
- Regenthal, R.; Krueger, M.; Koeppel, C.; Preiss, R. Drug Levels: Therapeutic and Toxic Serum/Plasma Concentrations of Common Drugs. J. Clin. Monit. Comput. 1999, 15, 529–544.
- Ahn, J.K.; Kim, H.Y.; Park, K.S.; Park, H.G. A Personal Glucose Meter for Label-Free and Washing-Free Biomolecular Detection. Anal. Chem. 2018, 90, 11340–11343.
- Kurbanoglu, S.; Mayorga-Martinez, C.C.; Medina-Sánchez, M.; Rivas, L.; Ozkan, S.A.; Merkoçi, A. Antithyroid Drug Detection Using an Enzyme Cascade Blocking in a Nanoparticle-Based Lab-on-a-Chip System. Biosens. Bioelectron. 2015, 67, 670–676.
- Skládal, P. Piezoelectric Biosensors. TrAC—Trends Anal. Chem. 2016, 79, 127–133.
- Jandas, P.J.; Prabakaran, K.; Luo, J.; Derry Holaday, M.G. Effective Utilization of Quartz Crystal Microbalance as a Tool for Biosensing Applications. Sens. Actuators A Phys. 2021, 331, 113020.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
530
Revisions:
2 times
(View History)
Update Date:
07 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




