Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Shaopeng Wang and Version 2 by Sirius Huang.
The detection and analysis of small molecules, typically defined as molecules under 1000 Da, is of growing interest ranging from the development of small-molecule drugs and inhibitors to the sensing of toxins and biomarkers. However, due to challenges such as their small size and low mass, many biosensing technologies struggle to have the sensitivity and selectivity for the detection of small molecules.
- small molecule
- label-free
- real-time detection
- biosensor
- optical transduction
- electrochemical transduction
- piezoelectric transduction
1. Introduction
Small molecules, typically defined as molecules under 1000 Da, have been of great interest due to their ubiquitous presence in biological processes and their intrinsic properties in human anatomy [1][2][1,2]. Notably, small molecules are capable of crossing the blood-brain barrier and have relative ease in cell membrane permeation compared to larger molecules; thus, small molecules can both enter the body readily and traverse to targets with high specificity [1][2][1,2]. These properties have great appeal in the pharmaceutical industry, with 15 of the 37 drugs approved by the FDA in 2022 being small molecules [3]. On the other hand, the properties that make small molecules appealing for drug development also make them effective toxins, carcinogens, mutagens, and endocrine disruptors both natural, such as several secondary metabolites of fungi, cyanobacteria, plants, and other organisms; and artificial, such as chemical warfare agents [1][2][4][5][6][7][1,2,4,5,6,7]. Furthermore, biological systems themselves utilize small molecules such as amino acids, steroids, sugars, or metabolites for many of the processes essential for life [1][2][7][1,2,7].
Considering the biological importance of small molecules, the development of biosensors capable of real-time detection has been in great demand with widespread applications such as: ensuring correct dosage of drugs, early diagnosis of disease, or continuous detection of toxins. Biosensors are generally composed of a receptor that captures an analyte and a transducer that changes in property upon the binding between the analyte and the receptor to produce a recordable signal [2]. Unfortunately, due to the low abundance and mass of small molecules, many techniques, especially those where the transducer’s signal strength scales with analyte mass, are challenging to achieve the sensitivity necessary for the effective detection of small molecules under biologically relevant conditions [7][8][7,8]. In addition, label-free techniques are greatly preferred for small molecules, even more so compared to larger analytes like proteins. The low mass and small size of small molecules only exacerbate the concerns of labeled detection techniques in that labels such as fluorescent proteins, fluorescent dyes, and quantum dots can alter the binding behavior and physical properties of an analyte [9][10][9,10]. In many cases, the label is much larger than the analyte itself; for example, green fluorescent protein, a common fluorescent label, has a molecular weight of 27 kDa to 69 kDa, and even small-molecule organic fluorescent probes such as fluorescein have a molecular weight of approximately 332 Da; a very significant addition to a small molecule with mass less than 1000 Da [9][10][11][12][9,10,11,12]. Currently, one of the common techniques for molecular detection is enzyme-linked immunosorbent assay (ELISA) and its variations, which offers high accuracy and sensitivity and can be used for small molecule detection; however, ELISA is an endpoint detection method that does not provide real-time kinetic information [13]. Small-molecule interaction kinetics and affinity with their target or receptor molecules are essential information for studying small molecule functions [14]. Thus, real-time measurement is preferred; correspondingly, transduction methods that can be measured continuously, such as changes in refractive index, surface conductance, and resonance frequency, have been developed into widespread label-free techniques, including surface plasmon resonance (SPR), field effect transistor (FET), and quartz crystal microbalance (QCM), for real-time small molecule detection using the corresponding receptor or target molecules as recognition elements [2][15][16][17][18][19][20][2,15,16,17,18,19,20]. The unique challenges to achieving the sensitivity and selectivity necessary to detect small molecules are leading to innovations to amplify signal and optimize surface chemistry as well as interesting methods to indirectly measure binding. These innovations can be broadly categorized as: physical amplification, cascading or chain reaction, and molecular switches (Figure 1). These strategies attempt to circumvent the limiting attributes of small molecules by measuring the change of alternative properties upon binding, correlating the concentration of analyte to a more easily measured product, or using a conformational change induced by analyte binding to produce a signal, respectively.
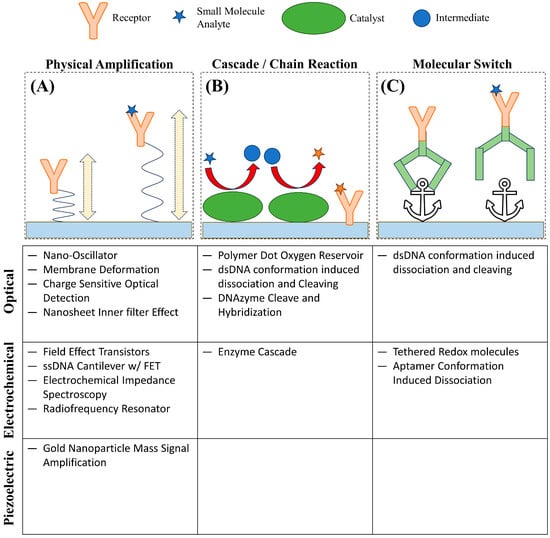
Figure 1. Representative novel strategies for small molecule detection. (A) Small-molecule binding induces a change in amplitude of a charge-sensitive oscillation. (B) A small molecule analyte undergoes a series of reactions to produce a product that can be detected. (C) Small-molecule binding results in a conformational change releasing a structure from an anchor point.
2. Optical Transduction
Optical transducers have great potential in the real-time detection of small molecules, but also face intrinsic challenges to fully utilizing their advantages. Optical transductions can measure a variety of signals related to the presence of small molecules, such as absorption, scattering, luminescence, or refractive index [2]. Optical biosensors typically offer fast results with high temporal resolution for real-time monitoring of binding, making them ideal for diagnostics and point-of-care devices [7]. However, the signal of optical transducers generally scales with the size and/or mass of the analyte of interest, especially when avoiding the use of fluorescent labels [2][7][21][2,7,24]. For example, one of the commonly used label-free optical detection techniques, surface plasmon resonance, has been lauded as the gold standard for molecular binding kinetic measurement, being used for analytes such as proteins, DNA, RNA, peptides, and other biological macromolecules, but attempts to utilize SPR for the detection of small molecules required advanced instrumentation or significant enhancement of receptor surface [15][22][15,25]. Thus, the development of optical techniques for the measurement of small molecules has primarily focused on either enhancing the weak signal of small-molecule binding or by measuring binding indirectly via measuring phenomena induced by analyte-binding events.
Surface plasmon resonance utilizes surface plasmons, electron oscillations at a metal-dielectric interface, typically gold-coated glass, which respond via oscillation at resonance with a light wave [23][26]. The evanescent waves of this oscillation are sensitive to changes close to the metal surface, notably as a change in refractive index due to the binding of an analyte, which shifts the SPR signal and produces a signal proportional to the analyte’s mass [24][27]. Unfortunately, this signal dependency on the mass of an analyte makes it challenging for SPR to detect small molecules, often requiring enhancement of either binding site density or signal strength such as through the usage of dextran chips or by utilizing localized surface plasmon resonance with nanostructures [15]. Thus, there has been great interest in developing sensing platforms with high sensitivity to small molecules, requiring simpler sample preparation and instrument operation, and for diagnostic use, having high portability and low cost.
Since the emergence of SPR in the 1970s, surface plasmon-based techniques have been developed with great interest, and different strategies have emerged to expand techniques into the range of small molecules and overcome their inherent mass dependency [25][28]. The use of gold-coated optical fibers instead of traditional gold chips for SPR has been in development since the 1990s, offering reduced cost and size compared to traditional SPR; which facilitates its application in point-of-care or diagnostic fields and the ability to optimize sensor attributes to suit the sensor’s purpose by changing the fabrication of the fiber probe [26][27][29,30].
