Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Douglas A. Steinmacher | -- | 8421 | 2024-02-04 00:13:31 | | | |
| 2 | Jason Zhu | -74 word(s) | 8347 | 2024-02-04 02:45:24 | | | | |
| 3 | Taras P. Pasternak | Meta information modification | 8347 | 2024-02-05 17:58:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pasternak, T.P.; Steinmacher, D.A.; Pasternak, T. Plant Growth Regulation in Cell and Tissue Culture. Encyclopedia. Available online: https://encyclopedia.pub/entry/54735 (accessed on 07 February 2026).
Pasternak TP, Steinmacher DA, Pasternak T. Plant Growth Regulation in Cell and Tissue Culture. Encyclopedia. Available at: https://encyclopedia.pub/entry/54735. Accessed February 07, 2026.
Pasternak, Taras P., Douglas A. Steinmacher, Taras Pasternak. "Plant Growth Regulation in Cell and Tissue Culture" Encyclopedia, https://encyclopedia.pub/entry/54735 (accessed February 07, 2026).
Pasternak, T.P., Steinmacher, D.A., & Pasternak, T. (2024, February 04). Plant Growth Regulation in Cell and Tissue Culture. In Encyclopedia. https://encyclopedia.pub/entry/54735
Pasternak, Taras P., et al. "Plant Growth Regulation in Cell and Tissue Culture." Encyclopedia. Web. 04 February, 2024.
Copy Citation
Precise knowledge of all aspects controlling plant tissue culture and in vitro plant regeneration is crucial for plant biotechnologists and their correlated industry, as there is increasing demand for such scientific knowledge, which results in more productive and resilient in vitro plant propagation and acclimatization in the field. Surprisingly, but so far, researchers/industry-based protocols on the 60-year-old concepts ignore new plant physiology achievements. Namely, high nitrogen and halogens used in the culture medium and exogenous auxin/cytokinin ratio regulate plant morphogenesis.
auxin
biotechnology
culture media
somatic embryogenesis
1. Regenerative Pathways
1.1. Organizer Cell Type Controlling Plant Regeneration
By being sessile, plants have evolved with a complex network of structures and signals to regulate and coordinate their development. One of these strategies is the occurrence of stem cell niches which might develop into a new organ or even a new plant, upon certain conditions. Nowadays, plant shoot (less) and root meristems are by far the most studied stem cell niches, but plant tissue culture has basically evolved from this observation and the occurrence of this phenomenon [1][2]. This is compatible with the concept presented by Mayer et al., (1998) [3], which suggests that although there is ample evidence that shoot and root meristems employ different sets of regulatory genes, both stem cells may be specified by a central organizer cell. In roots, the organizer cell corresponds to the quiescent center, and WUSCHEL and its HOMEOBOX (WUS–WOX) expression is a marker for this cell type [4][5]. Also, in the root model and using different strategies, Sabatini et al. [6] have shown that cells from different types of tissue are competent to form organizer cell types in response to high levels of auxin. Evidence has proven the interconnection between auxin and WOX5 pathways, and recent studies have also shown that WOX5 induces TAA1-mediated auxin biosynthesis [7]. Therefore, auxin biosynthesis and accumulation are crucial facets for plant regeneration. In the root, the stem cell niche is formed as a result of auxin accumulation in the xylem pole pericycle cells, through xylem-mediated (as the initial stage) and active auxin transport responsible for stem cell formation [8]. In the shoot stem cell, the opposite situation occurs. A group of small cells formed either from callus or from an organ’s surface (direct regeneration), this group can be named as the organizer cell niche and should produce its own auxin from an auxin gradient. These cells (WUS active) are relatively dormant and have a special nuclei feature (compact nuclei with small nucleoli, histone hyperacetylation) [9]. Organizer cells can also be formed from the competent cells in plant organs, which do not connect with the xylem/vasculature and cannot canalize auxin in this way. In the case of xylem formation, only the root can form. Showing that this set of genes is essential for plant regeneration, ectopic WUS expression might, therefore, be a relevant strategy for enhanced plant regeneration. For further details see [10].
1.2. Organogenesis
Organogenesis is the most commonly applied micropropagation technique for a massive (large-scale) plant propagation method, using relatively small plant tissue explants from the mother plant [11]. These plants grow in vitro in sterile conditions under applications of growth regulators. Micropropagation can be scientifically divided into three steps, each of which requires significantly different conditions: 1. de novo induction of “organizer cell”, which is required for de novo shoot formation; 2. growth of new shoots by creating auxin gradients followed by cell specification, induction of multiple cell fate-specific auxin biosynthesis pathways with specific chromatin organization in each cell type; 3. induction of root from formed shoot by creation of temporary auxin maximum in xylem-adjusted cell, cell cycle activation and directional auxin transport. Growing mother plants on optimized culture medium led to an increase in tissue response and a rise in the propagation ratio.
1.3. Somatic Embryogenesis
Somatic embryogenesis (SE) is the induction de novo of embryos from somatic plant cells. This morphogenetic pathway can be induced by a number of auxinic herbicides, 2,4-Dichlorophenoxyacetic acid (2,4-D), Picloram, and Dicamba, at a relatively high concentration [12]. These synthetic auxins, in addition to their auxinic effect, bind to F-box proteins as transport inhibitor response 1 (TIR1) [13] and can induce endogenous auxin biosynthesis, but can slightly inhibit its efflux. The competent cells, presenting regular ploidy and regular nuclei structure, and are able to start auxin biosynthesis (via TAA1-IPA pathway) performing initial auxin accumulation, results in the formation of “organizer cell”, which, in the future, divide periphally to form globular structures with surrounding epidermis [14]. However, such a system is not very effective since non-embryo competent cells also enter in cell division and form callus because of exogenous auxin. In this case, the resources available from the culture medium can dissipate as the division rate of callus cells is usually higher. One simple way to improve this step might be to slightly reduce auxinic herbicides’ concentration and increase auxin endogenous accumulation in the organizer cells with the inhibition of auxin efflux (pH, salicylic acid (SA), Iron (Fe), Copper (Cu) at a certain concentration). In this case, the effect of 2,4-D and other synthetic auxins will be more targeted, avoiding callus formation and accelerating organizer cell formation, thus increasing their number and, therefore, the plant regeneration capacity.
1.4. Haploid Induction In Vitro
Using the doubled haploid system is the simplest and fastest method of homozygotic hybrid production, a very important strategy for breeding programs. The most used method is microspore embryogenesis. This method has been established for many important crops (barley, pepper, rapeseed, rice, sugar beet, and wheat). The physiological mechanism of microspore embryogenesis is similar to that of classical somatic embryogenesis and requires stress factors and auxin interactions with further epigenetic modifications to switch from gametophytic to sporophyte pathways [15].
1.5. Protoplast Culture
Plant protoplast culture has two main applications: somatic hybridization, including asymmetric somatic hybridization [16], and the study of cell totipotence [17]. Somatic hybridization is especially important for hybridization between species or genera that cannot be forced to cross-breed using traditional sexual hybridization techniques. In addition, protoplast can serve as a powerful tool for testing different gene constructions in transient assays, including modern techniques of target gene editing like CRISPR. In this case, plant regeneration is not required [18][19].
However, so far, for plant regeneration, protoplast cultures are rarely used since, contrary to plant regeneration from explants, they require much more effort and are strongly genotype dependent [17].
2. Nutrient Balance In Vitro
Although researchers are facing the advent of new approaches and knowledge of molecular aspects of plant regeneration, mineral nutrients form a significant component of culture media but are often overlooked as possible morphogenic elicitors [20]. It is well known that each ion is a possible morphogenic factor/elicitor [21]. Recent investigations indicate that the signaling pathways for the majority of nutrients are subject to epigenetic regulation [22][23][24]. Therefore, optimal nutrition is key to the success of plant tissue culture, and when choosing the culture medium, one needs to consider the role of each element/ion in morphogenesis based on classical plant physiology [25].
Interestingly, some popular culture media based on another nutritional concepts have been adjusted for soft callus and, after that, mistakenly extrapolated to whole plants. The most famous culture medium used in plant micropropagation is Murashige and Skoog medium (1962) (MS) [26], and it presents the following formula: N-K-Cl-Ca-S-Mg-P-Fe-Mn-B-Zn-Mo-I-Cu-Co with an N:P ratio of 40:1. It is important to mention that the original purpose of the this formula was to develop a revised medium for the rapid growth of tobacco cells from pith tissues and was not focused on plant regeneration and plant quantity. This formula made halides (chloride) a major anion after nitrate, which in turn, based on the ability of chloride-induced rapid post-mitotic cell expansion, resulted in rapid soft callus growth. Nowadays, several authors, who also work on the optimization of the composition of culture media, have also suggested that the MS formula is far from optimal for micropropagation (plant regeneration) and rooting [27][28].
Additionally, all nutrients/ions in each culture medium can be divided into four main groups, namely major macros (N, K, P), minor macros (Ca, Mg, S), mediate macros (Fe) and micros (Mn, B, Mo, Zn, I, Cu, Co). Macro nutrients serve as structural components or are involved in several metabolic reactions (N, P, Ca), while microelements serve as cofactors of numerous hormone- and stress-related reactions/responses, with significant interactions occurring among those nutrients. The basic nutritional formula for plants/plant cells is as follows: N-K-P-Ca-Mg-S-Fe-Mn-B-Zn-Cl-Mo-Cu-Co-I with an N:P ratio of (5–12):1. This formula is based on results from more than 100 years of research on plant physiology and considers the role of each element. In addition, is necessary to consider a very complicated interaction between micro and macro nutrient in terms of cell uptake and cell signaling [29].
In summary, it is important to note that in the case of plants growing in the soil, ions present mainly in the form of complexes but not in pure form. Therefore, most of the plant nutrition investigation was carried out in water/hydroponic cultures. Since plant cells respond in completely similar ways in vitro and in nature, the results from the hydroponic culture were easily extrapolated to the in vitro system. Similarly, the molecular and physiological mechanisms of the stress response were very similar in vitro and in soil, which further pointed out the similarities between in vitro and in-soil systems.
2.1. Macro Elements
Carbon
Carbon (C) is a main component of almost all macromolecules in the plant body (cell wall, protein, DNA, RNA). Carbon starvation is lethal for plants from the onset of seedling growth [30]. Some different types of carbohydrates, including sucrose, glucose, and maltose, can be used for in vitro plant regeneration. Sucrose is the most useable compound since it is inexpensive and resistant to autoclaving.
Glucose is the most used carbon source by plants in nature; however, there are significant disadvantages to using it directly for in vitro plant regeneration. Firstly, glucose is unstable in solution at pH 5.6 and can spontaneously open the ring to convert to lactic acid. This process is significantly accelerated during autoclaving [31]. Conversely, sucrose is relatively resistant to autoclaving but can be caramelized after prolonged heating. Sucrose concentration depends on the task: 1% can be for photosynthetic plants and up to 6% can be sued for embryogenic callus. Usually, 2% to 6% is used for initial plant growth. This concentration cannot be considered osmotic and has a metabolic function as a carbon source for in vitro plant growth [32].
Nitrogen
Nitrogen (N) is key to plant nutrition and its concentration in media must be the second highest after carbon. Nitrogen is a major component of amino acids (proteins) as well as other important biological macromolecules. The form of nitrogen is also extremely important for plants. On the whole plant level, the most beneficial form of nitrogen is strongly dependent on the pH. Ammonium is directly involved in metabolic pathways, while nitrate requires conversion to ammonium through nitrate and nitrite reductase activity. Thus, on one hand, ammonium is beneficial for plant nutrition. On the other hand, the application of ammonium only to plant cells leads to extremely quickly uptake and nitrogen overloading [33][34][35][36]. Several recent investigations have suggested that nitrogen signaling is directly linked with auxin signaling [37][38] and epigenetic modifications [35]. The optimal nitrogen concentration for the majority of plant species is ~15 mM N, with a nitrogen to phosphate (N:P) ratio of 5–6:1 for shoot induction and 10–12:1 for plant growth.
Potassium
Potassium (K) is the third most important ion that plants require. Contrary to nitrogen, potassium is not a structural element but plays an essential role in plant development. Potassium is a symporter with hydrogen and can increase auxin transport. The first link between auxin distribution and potassium was reported in 1962 [39]. Later, a detailed investigation of potassium nutrition led to the conclusion that potassium deficiency leads to a significant alteration in auxin distribution [40] through several potassium transporters. A recent review by Johnson et al. [41] further confirmed the role of potassium in the regulation of cell-to-cell communications and stress responses in plants. Additionally, potassium is used for agar-solidification and an extra source of K can reduce hyperhydricity and promote cell division, especially when it is caused by auxin deficiency (barley microspore, wheat ovary, etc.). researchers suggest using at least two times less potassium than nitrogen and even less for induction [42]. Potassium is an ion with extremely high mobility in plants and plays a key role in regulating cell-to-cell communication and transport (N, C), including auxin transport. In many cases, high potassium nutrition alleviates stress effects [43]. Based on these properties, potassium positively affects plant growth in vitro during de novo shoot development, rooting, and by avoiding hyperhydricity. However, high potassium levels may have a negative effect on the induction stage, while potassium deficiency or low levels can be useful for creating organizer cells, which should be semi-autonomous (personal observation).
Phosphate
Phosphate (P) is the fourth element in the classical plant nutrition formula and plays a role as a structural element (phospholipid, DNA) and as a metabolic molecule involved in phosphorylation/dephosphorylation, ATP/ADP transition, etc. [44][45]. In contrast to nitrogen, P is much more labile (it is only non-utilizable in membranes). There are many reports on how phosphate nutrition regulates processes in PTC [46].
Calcium
Calcium (Ca) is a known signal transducer involved in the regulation of many kinases, the cell wall structure, and responses to stimuli. Calcium plays an important role in plant tissue culture [47][48] through its involvement in different signaling processes. The normal concentration of Ca in culture media is the same as that of P and should be around 2–3 mM. In this respect, the calcium source is key to plants’ in vitro regeneration. In the majority of cases, based on MS culture medium, researchers use calcium chloride (CaCl2) as a Ca source and even studied the effect of removing Ca or adding extra CaCl2. This, in turn, made chloride (Cl) a major anion after nitrate with its numerous negative effects on PTC (for details, see chloride section).
Magnesium and Sulphur
Deficiency of both magnesium (Mg) and sulphur (S) components has a very negative impact on plant development in soil since both are involved in many vital reactions as cofactors (Mg) and as structural components (S) as parts of amino acid and the central hub of thiol [49]. The optimal concentration of both components is around 1.5 mM, so magnesium sulfur (MgSO4) is an ideal salt for PTC. It is also important to note that both Mg deficiency and Mg excess have significant negative effects on plants and plant cells, for example, on Prunus micropropagation [50].
Iron
Iron (Fe) is an essential macro element for plants [51][52]. Iron plays an important role in accepting and donating electrons as well as an essential role in the electron transport chains of photosynthesis and respiration. The optimal concentration of iron in the medium is 70–100 µM, in the form of iron(II) sulfate heptahydrate (FeSO4·7H2O), a commonly used salt. However, Fe is not soluble and is rarely accessible to plants; therefore, it is used more effectively in a Fe chelate form, as ferric ethylenediaminetetraacetic acid (FeEDTA) chelated from FeSO4, or in a directly chelated form, as ethylenediamine di-2-hydroxyphenyl acetate ferric (FeEDDHA), among others [53]. The chelated form FeEDDHA is more stable at different pH levels and has shown relevant results for different species evaluated, including date palm [54], walnut [55], and hazelnut [56]. It is also important to note that excess iron may cause iron stress, which can effect embryogenesis [57]. As expected, the iron effect directly relates to epigenetic regulation [58].
2.2. Microelements
Boron
The primary functions of boron (B) relate to cell-to-cell communication and its structural role in the cell wall [59]. Its roles include involvement in membrane integrity, seed production, root elongation, and sugar metabolism [60]. Pioneering work on the boron deficiency effect was performed in 1920 [61][62]. A recent investigation pointed out the role of boron at the molecular level also during fruit development [63] and its link with auxin transport/metabolism. Boron is not a re-utilizable element, and it accumulates in the rigid cell wall of mature cells. In PTC, low boron levels may be useful for the de novo shoot induction stage, as a critical minimum content in the meristematic cells is necessary to elicit mitosis [64]. However, given its essential role in different aspects of cell maintenance and plant growth, including interaction with hormonal pathways [65], reaching this minimum threshold is not an easy task, and considering that the earliest defects following B starvation are growth arrest and aberrant meristem formation [64], it is better to keep the boron concentration constant during all stages of PTC.
Manganese
Manganese (Mn) is a metal and an essential cofactor for the oxygen-evolving complex (OEC), playing a role in photosynthesis and many other functions [66][67]. While Mn deficiency/excess affects plant morphogenesis, the difference between its thresholds of deficiency and toxicity is very small, as shown in Nicotiana [68]. Therefore, if the objective is not to test the manganese effect, researchers do not recommend interfering with it because the effect can be very complicated. A higher Mn concentration can help in the induction stage, but generally, excess Mn is highly toxic [69]. The recommended concentration of manganese in the medium is from 20 to 100 µM, and researchers should consider using manganese chloride (MnCl2) as a salt source, keeping both ions at a micronutrient concentration.
Molibdenium
Molibdenium (Mo) is a trace element required for plants in micro doses [70]. Mo-containing enzymes play a role in the redox cycles of nitrogen, carbon, and sulfur. Mo remains biologically inactive until it becomes complex enough to form a Mo-cofactor [71]. Based on the classical culture medium composition, Mo concentration is around 0.2 to 1 µM. So far, there are no published studies on the effect of excess or deficient molybdenum.
Copper
In plants, copper (Cu) is a cofactor of many reactions and was first described in 1939 [66][72]. Also, Cu serves as an epigenetic regulator [73]. The optimal concentration of Cu is around 1–2 µM (not 0.1 µM as in the medium for callus). Increasing the copper concentration to 20 mg/L (80 µM) had a positive result for plant regeneration in several plant species [74]. Cu stress (20–30 µM) may serve as a trigger of cell division and plant regeneration [75].
Zinc
Zinc (Zn) is an essential ion that serves as a cofactor of Cu/Zn Superoxidedismutase (SOD) in chloroplasts and cytoplasm. In addition, investigations found the important role of Zn as a catalysator of auxin biosynthesis [76][77][78]. The optimal concentration of Zn is around 5–10 µM as ZnSO4 salt.
2.3. Halogens
The halogens group is, in nature, represented by three major members: chloride (Cl), bromide (Br), and iodine (I). So far, two (Cl and I) have been proven as micronutrients essential for plant development.
Iodine
Iodine (I) was recognized as a micronutrient as early as 1961 [79]. Recent investigation showed that iodine modulated gene expression, interacted with protein by iodination, activated multiple metabolic pathways, and was mostly involved in defense responses [80]. For a review, see [81]. The optimal concentration of iodine in plant culture medium is in the range of 0.2–5 µM. While variation in iodine concentration may have some effects on plant tissue culture for specific species, such as Cocos nucifera [82], the effects may be very complicated.
Chloride
Chloride (Cl) is the most contradictory ion in plant tissue culture in vitro. Chloride is a halogen element recognized as a micro nutrient and plays a specific role in proton transfer reactions in photosynthesis [83]. However, chloride can accumulate in other acidic compartments besides chloroplasts which further increases its acidity. This function is explored in plants in two cases: in micro doses (10 µM), it can be useful for acidification of the stroma to enhance the rate of photosynthesis. This function is not very relevant for in vitro culture regarding carbohydrate supply. In macro doses (6 mM in MS culture medium [26], up to 34 mM in Y3 culture medium [83]), chloride is rapidly taken up in the middle acid vacuole (gamma-type),and helps to convert it to a lytic vacuole with increased turgor pressure. This application is relevant if soft calluses are desired, as in [26][84] or it might have a role in plant cell elongation [85]. However, although an effect might be seen on the plant growth rate, this might be linked to higher water uptake by plantlets [86], and due to in vitro conditions, it might also result in hyperhydric plantlets with a lower ex vitro survival rate. Therefore, for in vitro plant regeneration, chloride should be omitted or used only as a micronutrient with 20–100 µM concentrations during the plant/root elongation phases.
3. Organic Components
3.1. Bacto-Tryptone
Bacto-tryptone (casein hydrolysate) is an extremely important culture medium component since it prevents ion precipitation [87]. In case, researchers have used this compound not as a nitrogen source and not as an amino acid mixture, but only as a compound to prevent precipitation. researchers suggest using 100 mg/L (which is usually equal to a max 13 mg of nitrogen) and a very low concentration of some amino acids, which can be ignored as a nutrient source.
3.2. Ascorbic Acid
Ascorbate or ascorbic acid (ASC) is a low-molecular-weight antioxidant and can be considered a “paradoxical” compound [88]. In plants, the main ascorbic acid pool is related to mature organs, linked with plastids (APX), and has a low level in the meristematic zone [89][90]. Ascorbate biosynthesis-deficient mutants do not demonstrate visible phenotypes during morphogenesis in plants. Changes in the endogenous ASC pool, as well as the effect of exogenous ASC on plant cells in vitro, clearly demonstrated that ASC is a negative regulator of cell proliferation but can protect mature cells from damage and from excess phenolic compound accumulation during callus growth [91]. Adding ASC directly to culture medium has some direct implications. At pH 5.6, ASC’s half-life is just a few hours, after which it converts to dehydroascorbate (DHA) and further to oxalic acid [92]. Moreover, ASC is a highly charged molecule that plant cells cannot directly absorb. So, first, ascorbate is oxidized to DHA, it is absorbed by plants in this form, and as a neutral molecule, it is rapidly absorbed and immediately converted back to ASC by DHA-reductase within plant cells. Once ASC reaches the cytoplasm, it becomes stable since cytoplasmic pH is around 7.
3.3. Glutathione
Glutathione (GSH) represents a class of thiol and is considered an antioxidant. However, despite this function, GSH also regulates auxin response [90][93][94][95]. In plants, GSH exists in several isoforms. Such divergence is related to possible different GSH functions. In the Fabaceae species, GSH significantly promotes cell cycle progressions and plant regeneration [93][94]. GSH application in PTC requires special attention since at millimolar concentrations, it serves as a strong buffer in a pH range of 4–4.5 [89] and requires adjustment in the pH level after addition.
3.4. Amino Acids
Several authors suggest adding all 20 amino acids at low concentrations to the medium. However, there are better decisions than this. Among amino acids, researchers suggest using glutamine, which, in turn, serves as an epigenetic regulator (see below) at a concentration of 20 mg/L or similar. This concentration is widely used for the improvement of embryogenesis in several plant species [95][96]. The auxin precursor tryptophan may also positively affect some plant species [97].
3.5. Sodium (Potassium) Humate
Humic acid has a very positive effect on plant productivity in soil by alleviating many kinds of stresses; see [98] for a detailed description. In tissue culture, neutralized sodium (potassium) salt of humic acid has been used at a concentration 5–15 mg/L mainly for the improvement of explant rooting. This protocol is very effective for root formation and growth in some recalcitrant species and is highly recommended [99][100][101].
3.6. Vitamins
Vitamins are necessary compounds synthesized and utilized in plants. The effects of vitamins on plants were first described more than 80 years ago [102][103]. In tissue culture media, the effects of different vitamins were first studied by Linsmaer and Skoog (1965) [104]. The addition of exogenous vitamins has a significant positive effect on plants in vitro (for review, see [105]). Among vitamins, B1 and B6 are two of the most important ones and are widely used.
3.7. B1 as Auxin Cofactor
B1 is the most useful vitamin since it serves as a cofactor in endogenous auxin biosynthesis [103][104][105]. Linsmaer and Skoog (1965) [104] demonstrated the exclusive role of thiamine (B1) in tobacco callus growth. Digby, J. and Skoog, F. (1966) demonstrated the induction of B1 synthesis in plants by cytokinin as an auxin biosynthesis inducer [106]. Gamborg (1968) proposed an increase in B1 content to 10 mg/L, and currently, this concentration is widely used in PTC [107]. An even higher concentration of B1 (however, not in PTC) showed superior results in Jatropha clonal propagation [108].
3.8. B6 as a Promoter of Rooting
B6 plays a key role in auxin homeostasis in roots [109] and can be helpful for de novo rooting. An amount of 1–2 mg/L of B6 is a standard concentration in the medium. Other vitamins may play a specific role, and in some cases, “vitamin stress” may positively affect PTC. Nevertheless, playing with these compounds may be quite complicated, and researchers suggest using the basic vitamin formula B1:B6:PP (nicotinic acid) in a 10:1:1 ratio (in mg/L) [110].
4. Phytohormones
Exogenous phytohormones serve as a tool for the regulation of different processes in PTC. That is why most researchers mention exogenous phytohormones as regulators of morphogenesis. However, plant morphogenesis is regulated exclusively by endogenous hormones, and exogenous ones can serve as modulators of their action only.
4.1. Auxin(s)
Auxin is an essential hormone responsible for all processes in PTC. It is the only exclusive hormone that can transport polarly, create gradients, and regulate cell fate. So, auxin is responsible for both shoot and root morphogenesis. In plants, local auxin biosynthesis was responsible for one or more morphogenic processes [111][112][113]. For a review, see Verma S. et al., 2021 [114]. The next question is about endogenous or exogenous auxin. Recently, it was shown that the main role in PTC belongs to endogenous auxin [115][116]. Since plants are represented as having continuous auxin gradients with different origins, applying exogenous auxin in PTC leads to disturbance in plant morphogenesis and should only be carried out in the induction stage. Among exogenous auxins (auxin analogs), 1-Naphthaleneacetic acid (NAA) is the closest to natural Indole-3-acetic acid (IAA) in terms of its function but is more stable; other analogs like 2,4-D, 2,3,5-T, dicamba, and picloram can be considered rather as “auxinic herbicides”, can induce endogenous auxin synthesis [117], and have some stress effects. That is why “auxinic herbicides” are widely used for somatic embryo induction.
Auxin is the most common plant growth regulator used to induce somatic embryogenesis [118]. It has been shown that auxins act like molecular glue, binding to their TIR1 receptor and promoting ubiquitin-dependent degradation of Aux/IAA repressor proteins [113][119]. Naturally occurring auxin (IAA) and synthesized auxin analogs (NAA and 2,4-D) showed the same activity [13][119]. In plants, auxin biosynthesis is represented by several pathways, the main one of which is tryptophan dependent. In the frame of this pathway, first, Indole-3-pyruvic acid (IPA) was formed by the action of the TAA1 gene, and thereafter, numerous YUCCA genes were converted from IPA to IAA. Recently, the key role of the TAA1 gene and several auxin transporters in the process of the induction of embryos in plants was shown [119][120]. However, auxin itself, auxin “concentration” or level, cannot be used as a marker for any morphogenic processes. In plants, several “homological” genes (YUCCA, TAA1) are responsible for auxin production, and each plays its own role. In tomatoes, for example, there are at least seven YUCCA genes; each of them shows activity in different stages of cotyledon development and they cannot replace each other [121].
In tissue culture, exogenous 2,4-D induced endogenous auxin biosynthesis, which may relate to further auxin independence and somatic embryo induction [57]. Later, it was shown that the LEC2 gene promoted the embryogenic pathway through auxin biosynthesis and the role of TAA1 in this process was pointed out [122]. There is some evidence about the role of TAA1 in plant regeneration. First, this includes the exact colocalization of TAA1 with the shoot organizer cells [123] with potentially competent cells in the leaf. Second, TAA1 is colocalized with the wox5/wus gene [7], responsible for shoot regeneration [124]. Third, WUS (stem cell organizer) is induced by auxin [125].
In summary, de novo shoot formation requires the presence of the auxin source in a specific cell type (organizer cell, which divides very slowly in the center and has a specific chromatin status). These cells synthesized IPA through the TAA1 pathway, which further converted to IAA and influenced the action of YUCCA 2. Cells on the periphery of the cluster divide faster and possess auxin canalization through PINs action to form an auxin gradient, which, in turn, leads to epigenetic modification with the formation of different cell types. Endogenous auxin also plays a primary role in the rooting step as part of the plant micropropagation procedure. Namely, through the root is considered the simplest means of auxin canalization, and the root can form only after the formation of a sieve element vessel. In PTC, pulse application of exogenous auxin can induce de novo root primordia in the already formed xylem pole “pericycle” cell, which can be realized after removing exogenous auxin. There are two key factors for successful rooting: a high rate of auxin biosynthesis in new shoots and a high rate of auxin flux from these shoots through the vessel. Nutrient medium composition is critical in this case. A possible model of plant regeneration is summarized in Figure 1.
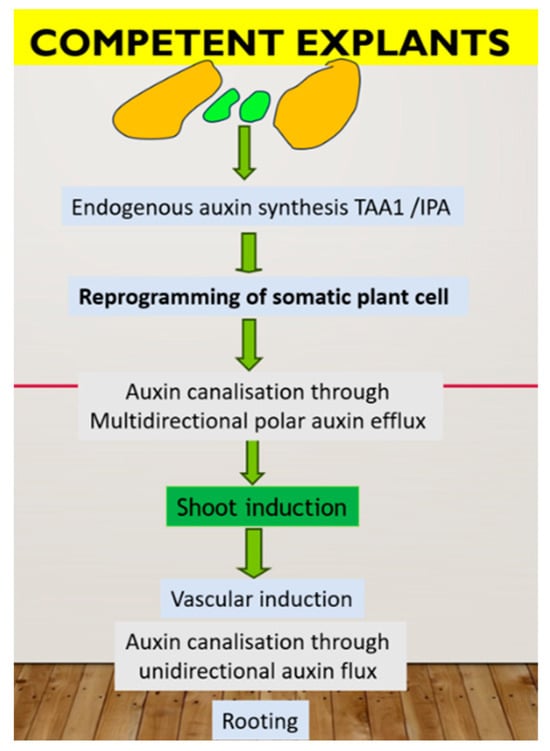
Figure 1. Hypothetic mechanism of plant regeneration in tissue culture in vitro. In green are representative of organizer cell, controlling in vitro response through auxin biosynthesis.
4.2. Cytokinin(s)
The main effect of cytokinin in tissue culture in vitro is shoot induction [126], which occurs through auxin biosynthesis induction and maintenance [127][128]. There are several natural cytokinins [Zeatin (Zea) and its riboside (Zea-R)] and artificial cytokinin-like compounds [Benzilaminopurine (BAP), Kinetin (Kin), 2-isopentenyladenine (2-IP)]. In addition, Thidiazuron (TDZ; N-phenyl-N′-1,2,3-thiadiazol-5-ylurea) was recognized as a potent regulator in vitro as early as 1990, with cytokinin-likes responses [129]. In addition to its cytokinin-like effects, TDZ can also be considered as a stress factor [130]. Currently, TDZ is used successfully in many plant species (for a review, see [131][132]). Cytokinin action/effect is dependent on the ability of the cell to produce endogenous auxin by one pathway or another. Only the competence of the cell to activate the TAA1 pathway (linked with the “organizer” cell) can induce shoots. A direct link between TAA1 and auxin has been confirmed by the presence in the TAA1 promoter of two cis elements of ARR1 response. Therefore, transcriptional regulation of TAA1 serves as a prerequisite for further regulation of auxin (IPA) biosynthesis. In the majority of dicotyledon shoot explants (leaf/cotyledon), exogenous cytokinin induces either callus or shoots or roots. These phenomena represent three faces of cytokinin, which are dependent on the origin and type of auxin biosynthesis pathway. Namely, in tomato cotyledon, the “soft callus” forms mainly from mesophyll cells (direct organogenesis), and somatic embryogenesis forms from cell layers near the epidermis with the active TAA1 gene, while auxin canalization to the xylem leads to the formation of large sieve elements with further possibility of root induction after the exogenous cytokinin level is lowered [133]. However, in monocotyledon, leaf auxin transport is directed from the meristem to the leaf tips (opposite to dicotyledons), so the leaf is not able to produce endogenous auxin and, therefore, cannot react to exogenous cytokinin application.
4.3. Other Hormones
Salicylic Acid
Salicylic acid at least partially performs specific action as an inhibitor of cell-to-cell communications. This function, indeed, is very useful in PTC since it promotes auxin accumulation in putative stem cells (during the induction phase) and should be used in low–middle concentration (25–100 µM) in combination with endogenous auxin synthesis (induced by cytokinins) or exogenous cytokinins [134][135][136][137]. However, prolonged incubation with high SA inhibited embryo development [138]. A recent investigation demonstrated a positive effect of 40 µM SA on olive somatic embryogenesis if applied with a cytokinin-like compound (TDZ + BAP) [139]. SA’s effect on inhibiting cell-to-cell communication and reducing water content in plant cells is an effective way to increase cell viability and dehydration tolerance required for successful cryopreservation [140][141].
Abscisic Acid
Abscisic acid (ABA) is linked to the “stress hormone”. Endogenous ABA plays a role in seed maturation (desiccation). Exogenous ABA application is useful for de novo embryo/shoot induction [142][143][144] by the initial induction of auxin accumulation and further cell re-programming (auxin biosynthesis and epigenetic regulation). Exogenous ABA modulates the auxin/cytokinin effect in PTC and leads to an increase in the SE rate in barley [142].
Gibberellic Acid
The gibberellic acid (GA) effect on PTC was first described in 1958 [145]. This hormone promotes cell expansion and can be used at low concentrations during shoot proliferation in combination with cytokinin [146]. However, if conditions are optimal, endogenous hormone synthesis should be enough for shoot development. GA biosynthesis inhibitor paclobutrazol (PBZ) showed a positive effect during de novo organ induction and can be used for somatic embryo or shoot induction by pulse application [147].
Ethylene
Ethylene is an important regulator of plant development, and its actions were described a relatively long time ago [148][149][150]. Ethylene can be considered a typical stress hormone [151]; therefore, it has a dual role during plant morphogenesis in vitro. Ethylene should get special attention in PTC since plant growth is relatively small in isolated volumes with lower air exchange rates than in natural conditions, a system with a high risk of ethylene accumulation. During the induction step, high ethylene concentration has a positive effect on somatic embryo induction [130] by promoting auxin biosynthesis and the inhibition of transport [152][153]. Moreover, ethylene also influences mineral nutrition through auxin signaling [154][155]. However, in the shoot proliferation stage, ethylene has a number of inhibitory effects: inhibition of shoot growth, hyperhydricity, etc. That is why ethylene accumulation should be reduced by increasing the air exchange rate and using ethylene inhibitors like silver nitrate (AgNO3) or cobalt.
5. Culture Conditions
5.1. pH as Growth Regulator
pH is one of the most potent regulators of plant development since it directly regulates transport, including auxin transport. Most of the reactions in plant cells are pH dependent; therefore, pH must be precisely balanced. There are three different types of pH in PTC: cytoplasmic pH (less or more constant, around 7, very buffering); extracellular (medium) pH, and vacuolar pH. According to the vacuolar pH value, one can distinguish three types of vacuoles: alfa type (PSV—protein storage vacuole), associated with stem cells in both shoot and root; beta type, associated with dividing cells; and gamma type (lytic vacuole), associated with fully expanding/apoptotic cells. The transition from beta to alfa type can be associated with the formation of shoot stem cells. Local auxin accumulation may serve as a factor that induces PSV. On the other hand, chloride can promote the induction of a lytic vacuole by rapidly transiting to the vacuole, inducing acidification and subsequent water uptake.
The most manipulable pH is medium pH. Slow artificial medium acidification promoted plant morphogenesis [57]. In addition, all plant species can be divided into acidic, neutral, and alkaline, depending on optimal soil growth conditions. More details on this classification are needed. Barley and sugar beet are typical alkaline plants, and rice is acidic. Alkaline plants induce extremely rapid external medium acidification by high proton efflux from the plants to the medium. This efflux, in turn, may induce cytoplasm alkalinization. Alkalinization can be a main problem related to plants growing in soil with a high pH in nature and is generally recalcitrant for plant morphogenesis in vitro.
In natural conditions, plants must create an acid (local) environment to facilitate nutrient uptake. A recent investigation pointed out the key role of pH homeostasis in the plant morphogenetic process [156]. In plant tissue culture, for the maintenance of a pH balance, in most cases, researchers use a chemical buffer to keep the pH of media in the physiological range. As an example, researchers cite the addition to the medium of 5–10 mM MES [2-(N-Morpholino)ethanesulfonic acid] buffer but never controlled the pH after cultivation. Another essential factor that drastically affects the pH of culture media is the addition of nitrogen, ammonium (NH4+), and nitrate (NO3−). Ammonium absorption by plant cells might occur in a higher pH environment and is more metabolically efficient than NO3−, requiring relatively less energy for its assimilation. However, this, in turn, is coupled with the H+ efflux, which will rapidly acidify the culture medium, and NH4+ might easily reach toxic levels within plant cells. NO3−, in turn, might be more absorbed at acidic pH levels in a more controlled way by plant cells, and this nitrate uptake releases OH–, again increasing culture medium pH. The carbonic acid/bicarbonate buffer is considered the most critical system for cell pH homeostasis.
5.2. Light
Light is a key environmental factor of plant-regulated growth. In PTC, the actual light intensity provided by a fluorescent lamp in the growth chamber is up to 100–120 µmol/m2/s. However, natural sunlight intensity may be high at 2000 µmol/m2/s. In the shade illuminated by a clear blue sky, midday − 20,000 Lux = 400 µmol/m2/s. Moreover, if very little shade occurs in nature (field), significant shade can occur in a standard growth chamber. Light can also induce high reactive oxygen species (ROS) production in the callus, and this ROS can serve as the most simple and powerful tool for shoot induction. The simplest method is to insert plates/tubes during induction for strong light (700–800 µmol or direct sunlight) for 1–2 h and return to regular light (100 µmol) after that.
In many cases, such tricks can increase shoot formation efficiency as a temporary stress factor, which does not require transfer to the new medium. Also, in line with this, light might have the same effects as a morphogen (i.e., direct influence on plant morphogenesis) or as a stress factor for plants growing in vitro. Usually, up to 100 µmol/m2/s would have an effect as a morphogen, as photosynthesis in micropropagation conditions is irrelevant. In addition to the amount of light, different light spectrums significantly affect plant tissue culture. Many light-emitting diodes (LEDs) sources with different ranges are currently available and need to be considered as energy spectra [157]. One of the possible mechanisms of different light source effects is an alteration in the endogenous hormone levels [158]. These factors also need to be considered.
6. Stress Factor and Epigenetic
6.1. Stress Factors
Stress factors (but not stress by itself) have a dual role in PTC. In plants, stress-induced agents inhibited cell-to-cell communication [159] and induced branching both in roots and shoots through re-distribution of auxin signaling. This, in turn, lead to an increase in plant regeneration ability [160]. ROS also play a role in stem cell induction and fate regulation (for a review, see [161]). Moreover, the positive effect of hydrogen peroxide on regeneration capacity has been reported as well [162]. On the whole plant level, the application of stress factors leads to hormone re-distribution and, through it, the induction of several epigenetic changes locally, differently in different cell types. In PTC, stress-inducing factors are key for morphogenesis induction through creating the organizer cell niche by the initial accumulation of high auxin contents, the induction of histone hyperacetylation, and auxin biosynthesis induction in specific cell types [163]. In this case, with a few exceptions (i.e., heat shock on microspore embryogenesis), this effect of the stress-induced agents can be achieved only in combination with exogenous 2,4,-D [164] or cytokinin (TDZ) [130]. Stress factors in general play a key role in embryo induction during microspore embryogenesis through changes in epigenetics and, therefore, auxin homeostasis [165]. Paraquat, alloxan, and menadione promote compact cell division and the formation of colonies independent from exogenous auxin [75][164]. Conversely, the inhibition of ROS production with diphenylene iodonium (DPI) or scavenging ROS with N,N′-dimethylthiourea (DMTU) or ascorbic acid prevents cell cycle and shoot induction [164].
However, it is important to note that stress factors should be used very carefully since they inhibit shoot growth. This means that stress factors should be applied only briefly during de novo stem cell induction. Once stem cells and primary shoots are induced, stress factors need to be removed for proper shoot outgrowth. Continued application of stress factors leads to numerous small shoots that do not expand and tend to form calluses because of auxin overaccumulation in young leaves.
6.2. Epigenetic Regulators
Epigenetic regulation plays a central role in all plant morphogenic processes, including plant morphogenesis in vitro [166][167][168][169]. Recently, it was shown that plant morphogenesis in vitro has an epigenetic mechanism similar to that of plants [170]. Epigenetic regulation includes dynamic changes in DNA methylation, histone modification, chromatin accessibility, and other processes that determine the transition of somatic cells to embryogenesis [165][171][172]. However, “direct epigenetic regulators” were rarely used in PTC since during morphogenesis, the process of DNA methylation/histone modification is very dynamic and, in many cases, it occurs differently in different types of plant cells during embryo/shoot induction [171]. As an example, researchers can cite [172], which claimed that mutation in the histone deacetylases (HDAC) gene inhibited plant regeneration. This is very logical since histone hyperacetylation is required only for the formation of an organizer stem cell niche, and thereafter, histone performs deacetylation to form leaf primordia. So, constitutive histone hyperacetylation inhibited shoot growth. In this case, applying certain compounds for all cells in forming morphogenesis will negatively affect the de novo shoot/embryo. Among direct epi regulators, only Trichostatin (TCA) is widely used mainly in pollen embryogenesis since histone hyperacetylation is a key step in organizer cell induction, and there are no other effects since pollen is a single-cell system, [173] and references therein. Some authors have applied 5-Azacytidine, a demethylation agent in the cell cycle, and increased morphogenic responses were observed on a regeneration level [174]; however, the most powerful and suitable epigenetic regulator is phytohormone auxin: application of exogenous auxin/cytokinin on a single cell level significantly changes chromatin organization in plant cells and, therefore, leads to cellular re-programming [175][176]. In plants, auxin gradient formation during de novo organ induction is key for cell fate specification and chromatin remodeling.
7. Plant Medium Preparation—Focus on Ions Chemistry
Typical plant media contains 12–14 ions, which have their own chemistry and potentially can interact with each other to form precipitates [87]. One of the most problematic ions is iron because it has relatively poor solubility. To improve iron efficiency, the majority of the medium iron is prepared as chelate. However, one should consider that complexes readily dissociate in solutions with a pH level less than 6, and EDTA can easily chelate other ions. Besides Fe, Ca, Mg, Cu, Zn, and Mn can precipitate during cultivation [177]. Altogether, such chemical behavior creates a serious problem with nutrient availability and may lead to poor plant growth and morphogenesis. It is fascinating that in freshly prepared media, the concentrations of free divalent cations like Cu, Ca, etc., were much less than those added during medium preparation because they exist in the form of chelates. However, during plant cultivation, most ions become free and can be absorbed by plant cells. In the natural soil, this problem has been solved by the presence of organic matter from complexes with divalent cations. In a culture medium, adding a trace amount of organic matter can significantly help and ultimately prevent precipitation even after autoclaving. This is especially important for the liquid medium for bioreactors and high-volume suspension culture.
8. Competent Explants
8.1. Choosing the Right Explants
Corresponding explants are the key to success in PTC. There are dramatic differences between monocotyledon in dicotyledon plants since in the case of monocotyledon cells, they were very rapidly terminally differentiated after exiting from the meristematic zone (except intercalary meristem, which formed after spike induction). That is why the best initial explant for cereal is scutellular tissue from immature embryos or leaf base from young seedlings. Interestingly, cereal leaves (contrary to dicotyledon leaf) showed an auxin transport direction from the base to the tips, suggesting an absence of auxin synthesis in cereal leaf which is the main inducer of cell division and further morphogenesis.
For the dicotyledon plants, leaf/cotyledon serve as an auxin source, and auxin transport is directed from leaf tips to the stem/hypocotyl and roots. That is why dicotyledon leaf/cotyledon were able to induce cell division and, in some cases, de novo shoots also in natural conditions. Leaf and cotyledon may serve as good/optimal explants for de novo shoot induction. Cotyledon has some advantages compared with leaf since it is a more standard system, while the ability of leaf regeneration is dependent on many factors that need to be considered. Cotyledon can serve as the most suitable explant for many dicotyledon species [133][178][179] because of the high capacity of auxin synthesis through the IPA pathway [180]. However, cotyledon is competent for plant regeneration only in a relatively short “window”: before the induction of shoot apical meristem. At this stage, cotyledon serves as a main auxin source for root growth. However, once shoot induced, the cotyledon is subjected to very rapid differentiation with endoreduplication, chromatin condensation, and limited auxin synthesis capacity. From this moment, cotyledon switches to photosynthesis as its main task and, therefore, is not competent for plant regeneration anymore.
The hypocotyl is part of the stem of young seedlings below the cotyledons and roots. In seedlings, the hypocotyl serves as a potential source of adventitious roots and shoots. The system of plant regeneration from the hypocotyl explants was established a long time ago [178][179]. The main features of the hypocotyl are local auxin metabolism [180] and a “sink “for the cotyledon-derived auxin. Currently, this regeneration system is widely used in plant tissue culture [181][182]. Recent investigations of gene expressions in cotton hypocotyl regeneration systems suggest that auxin synthesis and polar transport are essential for de novo embryo/shoot induction [183]. There are two possible regeneration protocols: new shoot induction by the application of exogenous cytokinin (BAP/kin) or on hormone-free medium by endogenous hormones [184]. In the latter case, hypocotyl-derived auxin first induces adventitious root, and root-derived cytokinin is able to induce de novo auxin synthesis, accumulation, and cell re-programming in the apical part of the hypocotyl. In the case of direct cytokinin application, the shoot originates directly from the competent cell in the epidermis-attached tissue.
In contrary to dicotyledonous plants, monocotyledons have more “compact “sites of auxin synthesis and cell cycles correspondingly. In most cereals, this site is restricted to just a few millimeters from the shoot–root junction with an auxin transport direction from the shoot–root junction towards the leaf tips. This also explains why cereals have a very restricted morphogenic capacity, and ideal explants for plant regeneration are immature organs at a very specific stage (expanding but not differentiated), as, for instance, scutella from 12- to 14-day-old wheat seedlings are the most suitable. Recent investigation shows that scutella have a very high auxin metabolism [185][186].
Plant regeneration from callus requires special knowledge and some tricks. Callus is an unorganized cell mass in which the majority of cells are equal and require exogenous hormone (auxin/cytokinin) application or endogenous hormone production [114][115]. In many cases, callus originates from certain cell types (mesophyll cells in some dicotyledonous species, for example) that have high ploidy levels and cannot regenerate diploid plants. In addition, during callus culture, a lot of somaclonal variations and other abnormalities can be induced, which, in turn, lead to plants with characteristics diverging from their mother plant [187][188].
Altogether, this makes a callus a difficult object for true-to-type plant regeneration in the majority of species (except model objects like tobacco and Solanum nigrum, for example). The main pre-request of de novo shoot formation is the induction of organizer cells with specific features (auxin source plus relative dormancy because of histone hyperacetylation). Only this type of cell is responsible for creating a de novo auxin gradient and developmental gradients. In this case, applying pulse stress factors in combination with exogenous auxin/cytokinin may trigger the creation of an “organizer cell” from the callus. However, this step must be performed very carefully, and treatments must be designed for each type of callus/explant individually.
In plant tissue culture, callus culture has another practical approach: the production of secondary metabolites for the pharmacological industry and the production of biologically active compounds [189][190][191]. The most important feature of this method is the possibility of regulating the level of bioactive compound synthesis relatively easily by changing hormonal signaling [192].
8.2. Rooting of New Regenerated Plants
Rooting of de novo forming shoots is an essential step in plant tissue culture for completing the micropropagation step and performing successful plant adaptation in soil conditions [193]. In some species, this step is limiting of the whole procedure. In order to avoid these difficulties and perform successful rooting, one needs to understand the physiological mechanism of this morphological response. From a physiological point of view, through the root is a natural means of canalization of the shoot-derived auxin.
There are three main pre-requests of successful rooting: high auxin production in certain xylem-adjusted cells in the stem. The presence of vessels (sieve elements) is key for plant rooting since only this system allows the creation of enough powerful channels for auxin transport and the open ability to canalize through roots. Several protocols for de novo root induction have been used [194], which include pulse treatments with relatively high auxin contents (IBA) and darkness. In this case, two possible problems in natural rooting have been solved: tissue (stem) with vessel elements gets higher auxin content and was able to induce temporary auxin maximum to activate cell division in a xylem-pole cell with de novo root induction.
8.3. Hyprehydricity
Hyperhydricity is a common physiological disorder in de novo-formed shoots [195][196]. The main physiological reason for such a disorder is an imbalance between new cell formation and growth. The molecular basis of this phenomenon is multiplying auxin functions. Auxin is responsible for cell division and cell expansion. In plants, this role is separated by different auxin synthesis pathways located in different cell types. Once the shoot grew, the multiply auxin biosynthesis pathway was activated. In this case, high auxin production in the mesophyll cell may lead to very rapid cell expansion through vacuolar growth. This leads to excess water uptake and cell deformation. In turn, excess auxin in the mesophyll cell cannot canalize through vascular tissue and inhibit rooting. There are several basic tricks to prevent excess water uptake: reduce auxin production or increase the rate of auxin efflux. One of the possible methods to reduce hyperhydricity is to reduce the ethylene level with silver nitrate [197] or by increasing the air exchange rate. The next trick is nutritional balance: lower nitrogen and chloride. At this stage, chloride is rapidly absorbed by the cell, increasing vacuolar acidity and water uptake. The final method is a reduction in exogenous cytokinin which, in turn, will reduce the auxin biosynthesis rate.
References
- Fambrini, M.; Usai, G.; Pugliesi, C. Induction of Somatic Embryogenesis in Plants: Different Players and Focus on WUSCHEL and WUS-RELATED HOMEOBOX (WOX) Transcription Factors. Int. J. Mol. Sci. 2023, 23, 15950.
- Wang, J.; Su, Y.; Kong, X.; Ding, Z.; Zhang, X.S. Initiation and maintenance of plant stem cells in root and shoot apical meristems. Abiotech 2020, 1, 194–204.
- Mayer, K.F.; Schoof, H.; Haecker, A.; Lenhard, M.; Jürgens, G.; Laux, T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998, 95, 805–815.
- Jha, P.; Ochatt, S.J.; Kumar, V. WUSCHEL: A master regulator in plant growth signaling. Plant Cell Rep. 2020, 39, 431–444.
- Wan, Q.; Zhai, N.; Xie, D.; Liu, W.; Xu, L. WOX11: The founder of plant organ regeneration. Cell Regen. 2023, 12, 1.
- Sabatini, S.; Beis, D.; Wolkenfelt, H.; Murfett, J.; Guilfoyle, T.; Malamy, J.; Benfey, P.; Leyser, O.; Bechtold, N.; Weisbeek, P.; et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 1999, 99, 463–472.
- Savina, M.S.; Pasternak, T.; Omelyanchuk, N.A.; Novikova, D.D.; Palme, K.; Mironova, V.V.; Lavrekha, V.V. Cell dynamics in WOX5-overexpressing root tips: The impact of local auxin biosynthesis. Front. Plant Sci. 2020, 11, 560169.
- Du, Y.; Scheres, B. Lateral root formation and the multiple roles of auxin. J. Exp. Bot. 2018, 69, 155–167.
- Verdeil, J.L.; Alemanno, L.; Niemenak, N.; Tranbarger, T.J. Pluripotent versus totipotent plant stem cells: Dependence versus autonomy? Trends Plant Sci. 2007, 12, 245–252.
- Lee, K.; Kim, J.H.; Park, O.S.; Jung, Y.J.; Seo, P.J. Ectopic expression of WOX5 promotes cytokinin signaling and de novo shoot regeneration. Plant Cell Rep. 2022, 41, 2415–2422.
- Sluis, A.; Hake, S. Organogenesis in plants: Initiation and elaboration of leaves. Trends Genet. 2015, 31, 300–306.
- Ramírez-Mosqueda, M.A. Overview of Somatic Embryogenesis. In Somatic Embryogenesis: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–8.
- Tan, X.; Calderon-Villalobos, L.I.A.; Sharon, M.; Zheng, C.; Robinson, C.V.; Estelle, M.; Zheng, N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007, 446, 640–645.
- Smetana, O.; Mäkilä, R.; Lyu, M.; Amiryousefi, A.; Sánchez Rodríguez, F.; Wu, M.F.; Solé-Gil, A.; Gavarrón, M.L.; Siligato, R.; Miyashima, S.; et al. High levels of auxin signalling define the stem-cell organizer of the vascular cambium. Nature 2020, 565, 485–489.
- Niazian, M.; Shariatpanahi, M.E. In vitro-based doubled haploid production: Recent improvements. Euphytica 2020, 216, 69.
- Imandi, S.; Bahadur, V. A review on protoplast culture, fusion and its construction, identification and characterization of somatic hybrids and cybrids. Pharma Innov. J. 2023, 12, 2052–2055.
- Pasternak, T.; Lystvan, K.; Betekhtin, A.; Hasterok, R. From single cell to plants: Mesophyll protoplasts as a versatile system for investigating plant cell reprogramming. Int. J. Mol. Sci. 2020, 21, 4195.
- Yue, J.J.; Yuan, J.L.; Wu, F.H.; Yuan, Y.H.; Cheng, Q.W.; Hsu, C.T.; Lin, C.S. Protoplasts: From isolation to CRISPR/Cas genome editing application. Front. Genome Ed. 2021, 3, 717017.
- Reyna-Llorens, I.; Ferro-Costa, M.; Burgess, S.J. Plant protoplasts in the age of synthetic biology. J. Exp. Bot. 2023, 74, 3821–3832.
- Singhal, R.K.; Fahad, S.; Kumar, P.; Choyal, P.; Javed, T.; Jinger, D.; Singh, P.; Saha, D.; Prathibha; Bose, B.; et al. Beneficial elements: New Players in improving nutrient use efficiency and abiotic stress tolerance. Plant Growth Regul. 2023, 100, 237–265.
- Ramage, C.M.; Williams, R.R. Mineral nutrition and plant morphogenesis. In Vitro Cell. Dev. Biol.-Plant 2002, 38, 116–124.
- Zhang, H.; Zhang, X.; Xiao, J. Epigenetic Regulation of Nitrogen Signaling and Adaptation in Plants. Plants 2023, 12, 2725.
- Li, A.; Hu, B.; Chu, C. Epigenetic regulation of nitrogen and phosphorus responses in plants. J. Plant Physiol. 2021, 258, 153363.
- Séré, D.; Martin, A. Epigenetic regulation: Another layer in plant nutrition. Plant Signal. Behav. 2020, 15, 1–6.
- Bevan, L.; Jones, M.; Zheng, Y. Optimisation of nitrogen, phosphorus, and potassium for soilless production of Cannabis sativa in the flowering stage using response surface analysis. Front. Plant Sci. 2021, 12, 2587.
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497.
- Page, S.R.; Monthony, A.S.; Jones, A.M.P. DKW basal salts improve micropropagation and callogenesis compared with MS basal salts in multiple commercial cultivars of Cannabis sativa. Botany 2021, 99, 269–279.
- Zarei, A.; Davis, B.; Feyissa, B.A.; Dinani, E.T.; Simons, B. Improvement of mineral nutrition and rooting efficiency of Cannabis sativa L. for in vitro large-scale propagation. In Vitro Cell. Dev. Biol.-Plant 2023, 59, 95–105.
- Fan, X.; Zhou, X.; Chen, H.; Tang, M.; Xie, X. Cross-talks between macro-and micronutrient uptake and signaling in plants. Front. Plant Sci. 2021, 12, 663477.
- Pasternak, T.; Kircher, S.; Palme, K.; Pérez-Pérez, J.M. Regulation of early seedling establishment and root development in Arabidopsis thaliana by light and carbohydrates. Planta 2023, 258, 76.
- Leitzen, S.; Vogel, M.; Steffens, M.; Zapf, T.; Müller, C.E.; Brandl, M. Quantification of degradation products formed during heat sterilization of glucose solutions by LC-MS/MS: Impact of autoclaving temperature and duration on degradation. Pharmaceuticals 2021, 14, 1121.
- Gago, J.; Martínez-Núñez, L.; Landin, M.; Flexas, J.; Gallego, P.P. Modeling the effects of light and sucrose on in vitro propagated plants: A multiscale system analysis using artificial intelligence technology. PLoS ONE 2014, 9, e85989.
- Liu, Y.; von Wirén, N. Ammonium as a signal for physiological and morphological responses in plants. J. Exp. Bot. 2017, 68, 2581–2592.
- Gezahegn, G.; Feyissa, T.; Rezene, Y. Replacement of ammonium nitrate by alternative nitrogen sources in MS medium to enhance ginger (Zingiber officinale Rosc.) in vitro regeneration. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 154, 89–95.
- Zhang, H.; Jin, Z.; Cui, F.; Zhao, L.; Zhang, X.; Chen, J.; Zhang, J.; Li, Y.; Li, Y.; Niu, Y.; et al. Epigenetic modifications regulate cultivar-specific root development and metabolic adaptation to nitrogen availability in wheat. Nat. Commun. 2023, 14, 8238.
- Zhang, H.; Wang, C.; Luo, H.; Chen, J.; Kuang, M.; Yang, J. Iron Nanoparticles Protected by Chainmail-structured Graphene for Durable Electrocatalytic Nitrate Reduction to Nitrogen. Angew. Chem. 2023, 135, e202217071.
- Li, T.; Feng, Z.; Zhang, T.; You, C.; Zhou, C.; Wang, X. The nitrate-responsive transcription factor MdNLP7 regulates callus formation by modulating auxin response. J. Integr. Agric. 2023, 22, 3022–3033.
- Abualia, R.; Riegler, S.; Benkova, E. Nitrate, Auxin and Cytokinin—A Trio to Tango. Cells 2023, 12, 1613.
- Ilan, I. A specific stimulatory action of indolyl-3-acetic acid on potassium uptake by plant cells, with concomitant inhibition of ammonium uptake. Nature 1962, 194, 203–204.
- Ashley, M.K.; Grant, M.; Grabov, A. Plant responses to potassium deficiencies: A role for potassium transport proteins. J. Exp. Bot. 2006, 57, 425–436.
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69.
- Utsumi, Y.; Utsumi, C.; Tanaka, M.; Ha, V.T.; Matsui, A.; Takahashi, S.; Seki, M. Formation of friable embryogenic callus in cassava is enhanced under conditions of reduced nitrate, potassium and phosphate. PLoS ONE 2017, 12, e0180736.
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 2020, 11, 904.
- Abel, S.; Ticconi, C.A.; Delatorre, C.A. Phosphate sensing in higher plants. Physiol. Plant. 2002, 115, 1–8.
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42.
- Puri, S.; Heriansyah, P.; Nopsagiarti, T. Potassium Dihydrogen Phosphate (KH2PO4) and Kinetin Enhance The Growth of Dendrobium Sonia Somatic Embryos. J. Biol. Indones. 2022, 18, 41–50.
- Loneragan, J.F.; Snowball, K.; Simmons, W.J. Response of plants to calcium concentration in solution culture. Aust. J. Agric. Res. 1968, 19, 845–857.
- Askari, N.; Ghahremani, R. The role of phosphorus, potassium and calcium on in vitro culture of lily bulblet. Agric. Biotechnol. J. 2023, 15, 27–42.
- Sun, O.J.; Payn, T.W. Magnesium nutrition and photosynthesis in Pinus radiata: Clonal variation and influence of potassium. Tree Physiol. 1999, 19, 535–540.
- Troyanos, Y.; Hipps, N.; Moorby, J.; Ridout, M.S. The effects of external magnesium concentration on the growth and magnesium inflow rates of micropropagated cherry rootstocks ‘F.12/1’ (Prunus avium L.) and ‘Colt’ (Prunus avium L. × Prunus pseudocerasus L.). Plant Soil 1997, 197, 25–33.
- Sági-Kazár, M.; Solymosi, K.; Solti, Á. Iron in leaves: Chemical forms, signaling, and in-cell distribution. J. Exp. Bot. 2022, 73, 1717–1734.
- Liang, G. Iron uptake, signaling, and sensing in plants. Plant Communications. Plant Commun. 2022, 3, 100349.
- Glinushkin, A.; Akimova, S.; Nikulina, E.; Tsirulnikova, N.; Kirkach, V.; Kalinitchenko, V.; Radzhabov, A.; Radkevich, E.; Marchenko, L.; Solovyov, A.; et al. Preliminary Study: Micropropagation Using Five Types of Chelated Iron and the Subsequent Acclimation of Blue Honeysuckle (Lonicera caerulea var. kamtschatica Sevast.). Forests 2023, 14, 821.
- Al-Mayahi, A.M.W. In vitro plant regeneration system for date palm (Phoenix dactylifera L.): Effect of chelated iron sources. J. Genet. Eng. Biotechnol. 2021, 19, 83.
- Licea-Moreno, R.J.; Contreras, A.; Morales, A.V.; Urban, I.; Daquinta, M.; Gomez, L. Improved walnut mass micropropagation through the combined use of phloroglucinol and FeEDDHA. Plant Cell Tiss Organ Cult. 2015, 123, 143–154.
- Garrison, W.; Dale, A.; Saxena, P.K. Improved shoot multiplication and development in hybrid hazelnut nodal cultures by ethylenediamine di-2-hydroxy-phenylacetic acid (Fe-EDDHA). Can. J. Plant Sci. 2013, 93, 511–521.
- Pasternak, T.P.; Prinsen, E.; Ayaydin, F.; Miskolczi, P.; Potters, G.; Asard, H.; Van Onckelen, H.A.; Dudits, D.; Fehér, A. The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiol. 2002, 129, 1807–1819.
- Su, J.; Yao, Z.; Wu, Y.; Lee, J.; Jeong, J. Minireview: Chromatin-based regulation of iron homeostasis in plants. Front. Plant Sci. 2022, 13, 959840.
- González-Fontes, A.; Rexach, J.; Navarro-Gochicoa, M.T.; Herrera-Rodríguez, M.B.; Beato, V.M.; Maldonado, J.M.; Camacho-Cristóbal, J.J. Is boron involved solely in structural roles in vascular plants? Plant Signal. Behav. 2008, 24, 24–26.
- Bolaños, L.; Abreu, I.; Bonilla, I.; Camacho-Cristóbal, J.J.; Reguera, M. What Can Boron Deficiency Symptoms Tell Us about Its Function and Regulation? Plants 2023, 12, 777.
- Warington, K. The effect of boric acid and borax on the broad bean and certain other plants. Ann. Bot. 1923, 37, 629–672.
- Sommer, A.L.; Sorokin, H. Effects of the absence of boron and of some other essential elements on the cell and tissue structure of the root tips of Pisum sativum. Plant Physiol. 1928, 3, 237.
- Thakur, S.; Sinha, A.; Ghosh Bag, A. Boron-A Critical Element for Fruit Nutrition. Commun. Soil Sci. Plant Anal. 2023, 54, 2899–2914.
- Lovatt, C.J. Evolution of Xylem Resulted in a Requirment for Boron in the Apical Meristems of Vascular Plants. New Phytol. 1985, 99, 509–522.
- Chen, X.; Smith, S.M.; Shabala, S.; Yu, M. Phytohormones in plant responses to boron deficiency and toxicity. J. Exp. Bot. 2023, 74, 743–754.
- Stout, P.R.; Arnon, D.I. Experimental methods for the study of the role of copper, manganese, and zinc in the nutrition of higher plants. Am. J. Bot. 1939, 26, 144–149.
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300.
- Santandrea, G.; Schiff, S.; Bennici, A. Effects of manganese on Nicotiana species cultivated in vitro and characterization of regenerated Mn-tolerant tobacco plants. Plant Sci. 1998, 132, 71–82.
- Williams, D.E.; Vlamis, J. Manganese toxicity in standard culture solutions. Plant Soil 1957, 8, 183–193.
- Arnon, D.I.; Stout, P.R. Molybdenum as an essential element for higher plants. Plant Physiol. 1939, 14, 599.
- Mendel, R.R.; Kruse, T. Cell biology of molybdenum in plants and humans. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2012, 1823, 1568–1579.
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883.
- Attar, N.; Campos, O.A.; Vogelauer, M.; Cheng, C.; Xue, Y.; Schmollinger, S.; Salwinski, L.; Mallipeddi, N.V.; Boone, B.A.; Yen, L.; et al. The histone H3-H4 tetramer is a copper reductase enzyme. Science 2020, 369, 59–64.
- Phogat, S.; Poudel, A.; Bhurta, R.; Kalwan, G.; Madhavan, J.; Padaria, J.C.; Singh, P.K.; Vinutha, T.; Mandal, P.K. An improved in-vitro regeneration protocol using scutellum of mature and immature embryos of wheat. Indian J. Genet. Plant Breed. 2023, 83, 195–204.
- Pasternak, T. Oxidative stress inducing agents’ copper and alloxan accelerate cell cycle re-entering of somatic plant cells in the presence of suboptimal exogenous auxin. bioRxiv 2020, preprint.
- Skoog, F. Relationships between zinc and auxin in the growth of higher plants. Am. J. Bot. 1940, 27, 939–951.
- Tsui, C. The role of zinc in auxin synthesis in the tomato plants. Am. J. Bot. 1948, 35, 172–179.
- Wang, J.; Yang, S. Dose-dependent responses of Arabidopsis thaliana to zinc are mediated by auxin homeostasis and transport. Environ. Exp. Bot. 2021, 189, 104554.
- Borst Pauwels, G.W.F.H. Iodine as a micronutrient for plants. Plant Soil 1961, 14, 377–392.
- Ramachandran, L.K. Protein-iodine interaction. Chem. Rev. 1956, 56, 199–218.
- Kiferle, C.; Martinelli, M.; Salzano, A.M.; Gonzali, S.; Beltrami, S.; Salvadori, P.A.; Hora, K.; Holwerda, H.T.; Scaloni, A.; Perata, P. Evidences for a nutritional role of iodine in plants. Front. Plant Sci. 2021, 12, 616868.
- Riyazuddin, R.; Singh, K.; Iqbal, N.; Nisha, N.; Rani, A.; Kumar, M.; Khatri, N.; Siddiqui, M.H.; Yasheshwar; Kim, S.T.; et al. Iodine: An emerging biostimulant of growth and stress responses in plants. Plant Soil 2023, 486, 119–133.
- Brahmachari, U.; Guo, Z.; Konecny, S.E.; Obi, E.N.C.; Barry, B.A. Engineering proton transfer in photosynthetic oxygen evolution: Chloride, Nitrate, and Trehalose reorganize a hydrogen-bonding network. J. Phys. Chem. B 2018, 122, 6702–6711.
- Eeuwens, C.J. Mineral Requirements for Growth and Callus Initiation of Tissue Explants Excised from Mature Coconut Palms (Cocos nucifera) and Cultured in vitro. Physiol. Plant. 1976, 36, 23–28.
- Chen, Z.C.; Yamaji, N.; Fujii-Kashino, M.; Ma, J.F. A cation–chloride cotransporter gene is required for cell elongation and osmoregulation in rice. Plant Physiol. 2016, 171, 494–507.
- Franco-Navarro, J.D.; Brumós, J.; Rosales, M.A.; Cubero-Font, P.; Talón, M.; Colmenero-Flores, J.M. Chloride regulates leaf cell size and water relations in tobacco plants. J. Exp. Bot. 2016, 67, 873–891.
- Dalton, C.C.; Iqbal, K.; Turner, D.A. Iron phosphate precipitation in Murashige and Skoog media. Physiol. Plant. 1983, 57, 472–476.
- Osiecki, M.; Ghanavi, P.; Atkinson, K.; Nielsen, L.K.; Doran, M.R. The ascorbic acid paradox. Biochem. Biophys. Res. Commun. 2010, 400, 466–470.
- Potters, G.; Jansen, M.A.; Horemans, N.; Guisez, Y.; Pasternak, T. Dehydroascorbate and glutathione regulate the cellular development of Nicotiana tabacum L. SR-1 protoplasts. In Vitro Cell. Dev. Biol.-Plant 2010, 46, 289–297.
- Pasternak, T.; Asard, H.; Potters, G.; Jansen, M.A. The thiol compounds glutathione and homoglutathione differentially affect cell development in alfalfa (Medicago sativa L.). Plant Physiol. Biochem. 2014, 74, 16–23.
- Golubitskii, G.B.; Budko, E.V.; Basova, E.M.; Kostarnoi, A.V.; Ivanov, V.M. Stability of ascorbic acid in aqueous and aqueous-organic solutions for quantitative determination. J. Anal. Chem. 2007, 62, 742–747.
- Ndakidemi, C.F.; Mneney, E.; Ndakidemi, P.A. Effects of ascorbic acid in controlling lethal browning in in vitro culture of Brahylaena huillensis using nodal segments. Am. J. Plant Sci. 2014, 5, 42300.
- Kudełko, K.; Gaj, M.D. Glutathione (GSH) induces embryogenic response in in vitro cultured explants of Arabidopsis thaliana via auxin-related mechanism. Plant Growth Regul. 2019, 89, 25–36.
- Pasternak, T.; Palme, K.; Paponov, I.A. Glutathione enhances auxin sensitivity in Arabidopsis roots. Biomolecules 2020, 10, 1550.
- Pawar, B.; Prashant, K.A.L.E.; Bahurupe, J.; Jadhav, A.; Anil, K.A.L.E.; Pawar, S. Proline and glutamine improve in vitro callus induction and subsequent shooting in rice. Rice Sci. 2015, 22, 283–289.
- El-Dawayati, M.M.; Ghazzawy, H.S.; Munir, M. Somatic embryogenesis enhancement of date palm cultivar Sewi using different types of polyamines and glutamine amino acid concentration under in-vitro solid and liquid media conditions. Int. J. Biosci. 2018, 12, 149–159.
- Shahsavari, E. Impact of tryptophan and glutamine on the tissue culture of upland rice. Plant Soil Environ. 2011, 57, 7–10.
- Benito, P.; Bellón, J.; Porcel, R.; Yenush, L.; Mulet, J.M. The biostimulant, potassium humate ameliorates abiotic stress in Arabidopsis thaliana by increasing starch availability. Int. J. Mol. Sci. 2023, 24, 12140.
- Bekseitov, T.; Anikina, I. Improving the adaptation properties of plants in vitro using growth regulator natrium humate. Arch. Zootech. 2015, 18, 61.
- Canellas, L.P.; Canellas, N.O.; da, S. Irineu, L.E.S.; Olivares, F.L.; Piccolo, A. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 2020, 7, 12.
- Mitrofanova, I.; Brailko, V.; Lesnikova-Sedoshenko, N.; Mitrofanova, O. Clonal micropropagation and some physiology aspects of essential oil roses valuable cultivars regeneration in vitro. Poljopr. I Sumar. 2016, 62, 73.
- Schopfeb, W.H. Auxin action of vitamin B1 on a micro-organism. C. R. L’acad. Sci. 1935, 200, 1965–1967.
- Arnon, D.I. Vitamin B1 in relation to the growth of green plants. Science 1940, 92, 2386.
- Linsmaier, E.M.; Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 1965, 18, 100–127.
- Abrahamian, P.; Kantharajah, A. Effect of vitamins on in vitro organogenesis of plant. Am. J. Plant Sci. 2011, 2, 669.
- Digby, J.; Skoog, F. Cytokinin activation of thiamine biosynthesis in tobacco callus cultures. Plant Physiol. 1966, 41, 647–652.
- Gamborg, O.L.; Miller, R.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158.
- Dhillon, R.S.; Hooda, M.S.; Pundeer, J.S.; Ahlawat, K.S.; Chopra, I. Effects of auxins and thiamine on the efficacy of techniques of clonal propagation in Jatropha curcas L. Biomass Bioenergy 2011, 35, 1502–1510.
- Boycheva, S.; Dominguez, A.; Rolcik, J.; Boller, T.; Fitzpatrick, T.B. Consequences of a deficit in vitamin B6 biosynthesis de novo for hormone homeostasis and root development in Arabidopsis. Plant Physiol. 2015, 167, 102–117.
- Havaux, M.; Ksas, B.; Szewczyk, A.; Rumeau, D.; Franck, F.; Caffarri, S.; Triantaphylidès, C. Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biol. 2009, 9, 130.
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell 2018, 47, 306–318.
- Zhao, Y. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu. Rev. Plant Biol. 2018, 69, 417–435.
- Lv, B.; Yan, Z.; Tian, H.; Zhang, X.; Ding, Z. Local auxin biosynthesis mediates plant growth and development. Trends Plant Sci. 2019, 24, 6–9.
- Verma, S.; Attuluri, V.P.S.; Robert, H.S. An essential function for auxin in embryo development. Cold Spring Harb. Perspect. Biol. 2021, 13, a039966.
- Pasternak, T.; Paponov, I.A.; Kondratenko, S. Optimizing protocols for Arabidopsis shoot and root protoplast cultivation. Plants 2021, 10, 375.
- Sakamoto, Y.; Kawamura, A.; Suzuki, T.; Segami, S.; Maeshima, M.; Polyn, S.; De Veylder, L.; Sugimoto, K. Transcriptional activation of auxin biosynthesis drives developmental reprogramming of differentiated cells. Plant Cell 2022, 34, 4348–4365.
- Dudits, D.; Györgyey, J.; Bögre, L.; Bakó, L. Molecular biology of somatic embryogenesis. In In Vitro Embryogenesis in Plants; Springer: Dordrecht, The Netherlands, 1995; pp. 267–308.
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460.
- Tan, C.; Liang, M.; Luo, Q.; Zhang, T.; Wang, W.; Li, S.; Men, S. AUX1, PIN3, and TAA1 collectively maintain fertility in Arabidopsis. Planta 2023, 258, 68.
- Luo, P.; Di, D.W. Precise Regulation of the TAA1/TAR-YUCCA Auxin Biosynthesis Pathway in Plants. Int. J. Mol. Sci. 2023, 24, 8514.
- Expósito-Rodríguez, M.; Borges, A.A.; Borges-Pérez, A.; Hernández, M.; Pérez, J.A. Cloning and biochemical characterization of ToFZY, a tomato gene encoding a flavin monooxygenase involved in a tryptophan-dependent auxin biosynthesis pathway. J. Plant Growth Regul. 2007, 26, 329–340.
- Wójcikowska, B.; Jaskóła, K.; Gąsiorek, P.; Meus, M.; Nowak, K.; Gaj, M.D. LEAFY COTYLEDON2 (LEC2) promotes embryogenic induction in somatic tissues of Arabidopsis, via YUCCA-mediated auxin biosynthesis. Planta 2013, 238, 425–440.
- Kneuper, I.; Teale, W.; Dawson, J.E.; Tsugeki, R.; Katifori, E.; Palme, K.; Ditengou, F.A. Auxin biosynthesis and cellular efflux act together to regulate leaf vein patterning. J. Exp. Bot. 2021, 72, 1151–1165.
- Bueno, N.; Cuesta, C.; Centeno, M.L.; Ordás, R.J.; Alvarez, J.M. In vitro plant regeneration in conifers: The role of WOX and KNOX gene families. Genes 2021, 12, 438.
- Su, Y.H.; Zhao, X.Y.; Liu, Y.B.; Zhang, C.L.; O’Neill, S.D.; Zhang, X.S. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009, 59, 448–460.
- Šmeringai, J.; Schrumpfová, P.P.; Pernisová, M. Cytokinins–regulators of de novo shoot organogenesis. Front. Plant Sci. 2023, 14, 1239133.
- Di, D.W.; Wu, L.; Zhang, L.; An, C.W.; Zhang, T.Z.; Luo, P.; Gao, H.H.; Kriechbaumer, V.; Guo, G.Q. Functional roles of Arabidopsis CKRC2/YUCCA8 gene and the involvement of PIF4 in the regulation of auxin biosynthesis by cytokinin. Sci. Rep. 2016, 6, 36866.
- Yan, Z.; Liu, X.; Ljung, K.; Li, S.; Zhao, W.; Yang, F.; Wang, M.; Tao, Y. Type B response regulators act as central integrators in transcriptional control of the auxin biosynthesis enzyme TAA1. Plant Physiol. 2017, 175, 1438–1454.
- Murthy, B.N.S.; Murch, S.J.; Saxena, P.K. Thidiazuron: A potent regulator of in vitro plant morphogenesis. In Vitro Cell. Dev. Biol.-Plant 1998, 34, 267–275.
- Nautiyal, A.; Ramlal, A.; Agnihotri, A.; Rashid, A. Stress-induced somatic embryogenesis on seedlings of Azadirachta indica A. Juss. by thidiazuron and its inhibition by ethylene modulators. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 153, 357–366.
- Ali, H.M.; Khan, T.; Khan, M.A.; Ullah, N. The multipotent thidiazuron: A mechanistic overview of its roles in callogenesis and other plant cultures in vitro. Biotechnol. Appl. Biochem. 2022, 69, 2624–2640.
- Catalano, A.; Ceramella, J.; Iacopetta, D.; Mariconda, A.; Scali, E.; Bonomo, M.G.; Saturnino, C.; Longo, P.; Aquaro, S.; Sinicropi, M.S. Thidiazuron: New trends and future perspectives to fight Xylella fastidiosa in olive trees. Antibiotics 2022, 11, 947.
- Yaroshko, O.; Pasternak, T.; Larriba, E.; Pérez-Pérez, J.M. Optimization of Callus Induction and Shoot Regeneration from Tomato Cotyledon Explants. Plants 2023, 12, 2942.
- Roustan, J.P.; Latche, A.; Fallot, J. Inhibition of ethylene production and stimulation of carrot somatic embryogenesis by salicylic acid. Biol. Plant. 1990, 32, 273–276.
- Hutchinson, M.J.; Saxena, P.K. Acetylsalicylic acid enhances and synchronizes thidiazuron-induced somatic embryogenesis in geranium (Pelargonium × hortorum Bailey) tissue cultures. Plant Cell Rep. 1996, 15, 512–515.
- Luo, J.P.; Jiang, S.T.; Pan, L.J. Enhanced somatic embryogenesis by salicylic acid of Astragalus adsurgens Pall.: Relationship with H2O2 production and H2O2-metabolizing enzyme activities. Plant Sci. 2021, 161, 125–132.
- Kumar, V.; Ramakrishna, A.; Ravishankar, G.A. Influence of different ethylene inhibitors on somatic embryogenesis and secondary embryogenesis from Coffea canephora P ex Fr. In Vitro Cell. Dev. Biol.-Plant 2007, 43, 602–607.
- Grzyb, M.; Kalandyk, A.; Mikuła, A. Effect of TIBA, fluridone and salicylic acid on somatic embryogenesis and endogenous hormone and sugar contents in the tree fern Cyathea delgadii Sternb. Acta Physiol. Plant. 2018, 40, 1.
- Bashir, M.A.; Silvestri, C.; Salimonti, A.; Rugini, E.; Cristofori, V.; Zelasco, S. Can ethylene inhibitors enhance the success of olive somatic embryogenesis? Plants 2022, 11, 168.
- Bettoni, J.C.; Kretzschmar, A.A.; Bonnart, R.; Shepherd, A.; Volk, G.M. Cryopreservation of 12 Vitis species using apical shoot tips derived from plants grown in vitro. HortScience 2019, 54, 976–981.
- Ayala-Hernández, D.D.; Ruiz-Saénz, D.R.; Cruz-Gutiérrez, E.J.; López-Delgado, H.A. Ácido salicílico induce tolerancia al estrés por criogenia en Solanum tuberosum. Rev. Mex. Cienc. Agríc. 2019, 10, 1505–1515.
- Pasternak, T.P.; Rudas, V.A.; Lörz, H.; Kumlehn, J. Embryogenic callus formation and plant regeneration from leaf base segments of barley (Hordeum vulgare L.). J. Plant Physiol. 1999, 155, 371–375.
- Nishiwaki, M.; Fujino, K.; Koda, Y.; Masuda, K.; Kikuta, Y. Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 2020, 211, 756–759.
- Senger, S.; Mock, H.P.; Conrad, U.; Manteuffel, R. Immunomodulation of ABA function affects early events in somatic embryo development. Plant Cell Rep. 2001, 20, 112–120.
- Nickell, L.G. Gibberellin and the growth of plant tissue cultures. Nature 1958, 181, 499–500.
- Ahmad, A.; Ahmad, N.; Anis, M.; Alatar, A.A.; Abdel-Salam, E.M.; Qahtan, A.A.; Faisal, M. Gibberellic acid and thidiazuron promote micropropagation of an endangered woody tree (Pterocarpus marsupium Roxb.) using in vitro seedlings. Plant Cell Tissue and Organ Cult. (PCTOC) 2021, 144, 449–462.
- Abdalla, N.; Taha, N.; Bayoumi, Y.; El-Ramady, H.R.; Shalaby, T. Paclobutrazol applications in agriculture, plant tissue cultures and its potential as stress ameliorant: A mini review. Environ. Biodivers. Soil Secur. 2021, 5, 245–257.
- Lieberman, M. Biosynthesis and action of ethylene. Annu. Rev. Plant Physiol. 1979, 30, 533–591.
- Sisler, E.C.; Yang, S.F. Ethylene, the gaseous plant hormone. Bioscience 1984, 34, 234–238.
- Schaller, G.E. Ethylene and the regulation of plant development. BMC Biol. 2012, 10, 9.
- Van de Poel, B.; de Vries, J. Evolution of ethylene as an abiotic stress hormone in streptophytes. Environ. Exp. Bot. 2023, 214, 105456.
- Růžička, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212.
- Yu, Q.; Cheng, C.; Zhou, X.; Li, Y.; Hu, Y.; Yang, C.; Zhou, Y.; Soliman, T.M.A.; Zhang, H.; Wang, Q.; et al. Ethylene controls cambium stem cell activity via promoting local auxin biosynthesis. New Phytol. 2023, 239, 964–978.
- Hu, C.-H.; Yuan, S.-D.; Tong, C.-L.; Zhang, D.-J.; Huang, R.H. Ethylene modulates root growth and mineral nutrients levels in trifoliate orange through the auxin-signaling pathway. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13269.
- Ma, B.; Ma, T.; Xian, W.; Hu, B.; Chu, C. Interplay between ethylene and nitrogen nutrition: How ethylene orchestrates nitrogen responses in plants. J. Integr. Plant Biol. 2023, 65, 399–407.
- Zhang, H.; Zhou, J.; Kou, X.; Liu, Y.; Zhao, X.; Qin, G.; Wang, M.; Qian, G.; Li, W.; Huang, Y.; et al. Syntaxin of plants71 plays essential roles in plant development and stress response via regulating pH homeostasis. Front. Plant Sci. 2023, 14, 1198353.
- Batista, D.S.; Felipe, S.H.S.; Silva, T.D.; de Castro, K.M.; Mamedes-Rodrigues, T.C.; Miranda, N.A.; Ríos-Ríos, A.M.; Faria, D.V.; Fortini, E.A.; Chagas, K.; et al. Light quality in plant tissue culture: Does it matter? In Vitro Cell. Dev. Biol.-Plant 2018, 54, 195–215.
- Cioć, M.; Dziurka, M.; Pawłowska, B. Changes in Endogenous phytohormones of Gerbera jamesonii axillary shoots multiplied under different light emitting diodes light quality. Molecules 2022, 27, 1804.
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105.
- Puijalon, S.; Piola, F.; Bornette, G. Abiotic stresses increase plant regeneration ability. Evol. Ecol. 2008, 22, 493–506.
- Qin, Q. ROS: Important factor in plant stem cell fate regulation. J. Plant Physiol. 2023, 289, 154082.
- Szechyńska-Hebda, M.; Skrzypek, E.; Dąbrowska, G.; Wędzony, M.; van Lammeren, A. The effect of endogenous hydrogen peroxide induced by cold treatment in the improvement of tissue regeneration efficiency. Acta Physiol. Plant. 2012, 34, 547–560.
- Chinnusamy, V.; Zhu, J.K. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009, 12, 133–139.
- Pasternak, T.P.; Ötvös, K.; Domoki, M.; Fehér, A. Linked activation of cell division and oxidative stress defense in alfalfa leaf protoplast-derived cells is dependent on exogenous auxin. Plant Growth Regul. 2007, 51, 109–117.
- Pereira, C.; Castander-Olarieta, A.; Sales, E.; Montalbán, I.A.; Canhoto, J.; Moncaleán, P. Heat stress in Pinus halepensis somatic embryogenesis induction: Effect in DNA methylation and differential expression of stress-related genes. Plants 2021, 10, 2333.
- Lee, K.; Seo, P.J. Dynamic epigenetic changes during plant regeneration. Trends Plant Sci. 2018, 23, 235–247.
- Cordeiro, D.; Pérez-Pérez, Y.; Canhoto, J.; Testillano, P.S.; Correia, S. H3K9 methylation patterns during somatic embryogenic competence expression in tamarillo (Solanum betaceum Cav.). Sci. Hortic. 2023, 321, 112259.
- Pasternak, T.; Dudits, D. Epigenetic Clues to Better Understanding of the Asexual Embryogenesis in planta and in vitro. Front. Plant Sci. 2019, 10, 778.
- Smulders, M.J.M.; De Klerk, G.J. Epigenetics in plant tissue culture. Plant Growth Regul. 2011, 63, 137–146.
- Ramakrishnan, M.; Zhou, M.; Ceasar, S.A.; Ali, D.J.; Maharajan, T.; Vinod, K.K.; Sharma, A.; Ahmad, Z.; Wei, Q. Epigenetic modifications and miRNAs determine the transition of somatic cells into somatic embryos. Plant Cell Rep. 2023, 42, 1845–1873.
- Chen, Y.; Hung, F.Y.; Sugimoto, K. Epigenomic reprogramming in plant regeneration: Locate before you modify. Curr. Opin. Plant Biol. 2023, 75, 102415.
- Temman, H.; Sakamoto, T.; Ueda, M.; Sugimoto, K.; Migihashi, M.; Yamamoto, K.; Tsujimoto-Inui, Y.; Sato, H.; Shibuta, M.K.; Nishino, N.; et al. Histone deacetylation regulates de novo shoot regeneration. PNAS Nexus 2023, 2, pgad002.
- Valero-Rubira, I.; Castillo, A.M.; Burrell, M.Á.; Valles, M.P. Microspore embryogenesis induction by mannitol and TSA results in a complex regulation of epigenetic dynamics and gene expression in bread wheat. Front. Plant Sci. 2023, 13, 1058421.
- Osorio-Montalvo, P.; Sáenz-Carbonell, L.; De-la-Peña, C. 5-Azacytidine: A promoter of epigenetic changes in the quest to improve plant somatic embryogenesis. Int. J. Mol. Sci. 2018, 19, 3182.
- Pasternak, T.; Miskolczi, P.; Ayaydin, F.; Mészáros, T.; Dudits, D.; Fehér, A. Exogenous auxin and cytokinin dependent activation of CDKs and cell division in leaf protoplast-derived cells of alfalfa. Plant Growth Regul. 2000, 32, 129–141.
- Fehér, A.; Ötvös, K.; Pasternak, T.P.; Pettkó-Szandtner, A. The involvement of reactive oxygen species (ROS) in the cell cycle activation (G0-to-G1 transition) of plant cells. Plant Signal. Behav. 2008, 3, 823–826.
- Shi, X.; Yang, L.; Yan, G.; Du, G. Medium pH between 5.5 and 7.5 has minimal effects on tissue culture of apple. HortScience 2017, 52, 475–478.
- Gunay, A.L.; Rao, P.S. In vitro plant regeneration from hypocotyl and cotyledon explants of red pepper (Capsicum). Plant Sci. Lett. 1978, 11, 365–372.
- Gunay, A.L.; Rao, P.S. In vitro propagation of hybrid tomato plants (Lycopersicon esculentum L.) using hypocotyl and cotyledon explants. Ann. Bot. 1980, 45, 205–207.
- Zheng, Z.; Guo, Y.; Novák, O.; Chen, W.; Ljung, K.; Noel, J.P.; Chory, J. Local auxin metabolism regulates environment-induced hypocotyl elongation. Nat. Plants 2016, 2, 16025.
- Galán-Ávila, A.; García-Fortea, E.; Prohens, J.; Herraiz, F.J. Development of a direct in vitro plant regeneration protocol from Cannabis sativa L. seedling explants: Developmental morphology of shoot regeneration and ploidy level of regenerated plants. Front. Plant Sci. 2020, 11, 645.
- Thakur, P.; Kumari, N.; Chadha, S. Development of an efficient in vitro regeneration system in broccoli (Brassica oleracea L. var. italica), a highly recommended vegetable crop. Plant Physiol. Rep. 2023, 28, 459–465.
- Larriba, E.; Sánchez-García, A.B.; Martínez-Andújar, C.; Albacete, A.; Pérez-Pérez, J.M. Tissue-specific metabolic reprogramming during wound-induced organ formation in tomato hypocotyl explants. Int. J. Mol. Sci. 2021, 22, 10112.
- Zhu, X.; Xu, Z.; Wang, G.; Cong, Y.; Yu, L.; Jia, R.; Qin, Y.; Zhang, G.; Li, B.; Yuan, D.; et al. Single-cell resolution analysis reveals the preparation for reprogramming the fate of stem cell niche in cotton lateral meristem. Genome Biol. 2023, 24, 194.
- Kabir, M.R.; Nonhebel, H.M.; Backhouse, D.; Winter, G. Expression of key auxin biosynthesis genes correlates with auxin and starch content of developing wheat (Triticum aestivum) grains. Funct. Plant Biol. 2021, 48, 802–814.
- Liu, X.; Bie, X.M.; Lin, X.; Li, M.; Wang, H.; Zhang, X.; Yang, Y.; Zhang, C.; Zhang, X.S.; Xiao, J. Uncovering the transcriptional regulatory network involved in boosting wheat regeneration and transformation. Nat. Plants 2023, 9, 908–925.
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173.
- Kaeppler, S.M.; Kaeppler, H.F.; Rhee, Y. Epigenetic aspects of somaclonal variation in plants. In Plant Gene Silencing; Springer: Berlin/Heidelberg, Germany, 2000; pp. 59–68.
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59.
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450.
- Hesami, M.; Pepe, M.; Baiton, A.; Jones, A.M.P. Current status and future prospects in cannabinoid production through in vitro culture and synthetic biology. Biotechnol. Adv. 2022, 62, 108074.
- Šenkyřík, J.B.; Křivánková, T.; Kaczorová, D.; Štefelová, N. Investigation of the Effect of the Auxin Antagonist PEO-IAA on Cannabinoid Gene Expression and Content in Cannabis sativa L. Plants under In Vitro Conditions. Plants 2023, 12, 1664.
- Nemeth, G. Induction of rooting. In Trees I; Springer: Berlin/Heidelberg, Germany, 1986; pp. 49–64.
- Quambusch, M.; Gruß, S.; Pscherer, T.; Winkelmann, T.; Bartsch, M. Improved in vitro rooting of Prunus avium microshoots using a dark treatment and an auxin pulse. Sci. Hortic. 2017, 220, 52–56.
- Polivanova, O.B.; Bedarev, V.A. Hyperhydricity in plant tissue culture. Plants 2022, 11, 3313.
- de Vasconcelos, A.G.V.; Tomas, L.F.; Willadino, T.R.C.L. Hyperhydricity: A metabolic disorder/Hiperidricidade: Uma desordem metabolica. Ciênc. Rural 2012, 42, 837–845.
- Lee, J.; Naing, A.H.; Park, K.I.; Kim, C.K. Silver nitrate reduces hyperhydricity in shoots regenerated from the hypocotyl of snapdragon cv. Maryland Apple Blossom. Sci. Hortic. 2023, 308, 111593.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
Environmental Sciences
Revisions:
3 times
(View History)
Update Date:
05 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




