
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victor Pike | -- | 1414 | 2024-01-30 20:09:34 | | | |
| 2 | Fanny Huang | Meta information modification | 1414 | 2024-01-31 10:17:48 | | |
Video Upload Options
The short-lived positron-emitter carbon-11 (t1/2 = 20.4 min; β+, 99.8%) is prominent for labeling tracers for use in biomedical research with positron emission tomography (PET). Carbon-11 is produced for this purpose with a cyclotron, by the 14N(p,α)11C nuclear reaction, either on nitrogen containing a low concentration of oxygen (0.1–0.5%) or hydrogen (~5%) to produce [11C]carbon dioxide or [11C]methane, respectively. These primary radioactive products can be produced in high yields and with high molar activities. However, only [11C]carbon dioxide has some utility for directly labeling PET tracers. Primary products are required to be converted rapidly and efficiently into secondary labeling synthons to provide versatile radiochemistry for labeling diverse tracer chemotypes at molecular positions of choice. Because of their simplicity, reliability, re-usability, and amenability for automation, gas phase transformations play a major part in carbon-11 chemistry and in PET tracer development.
1. Introduction
2. Carbon-11 Production and Gas Phase Transformation
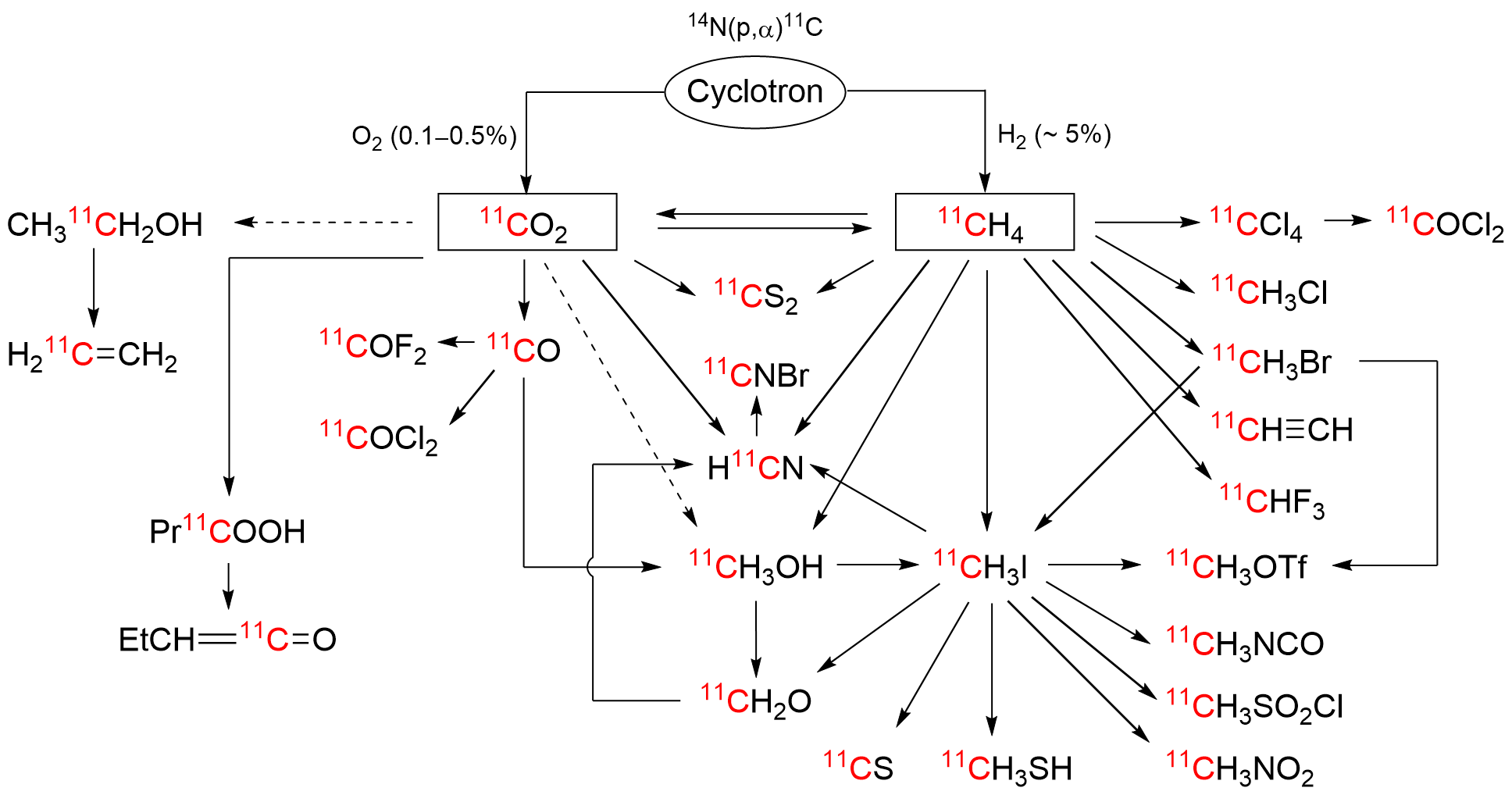
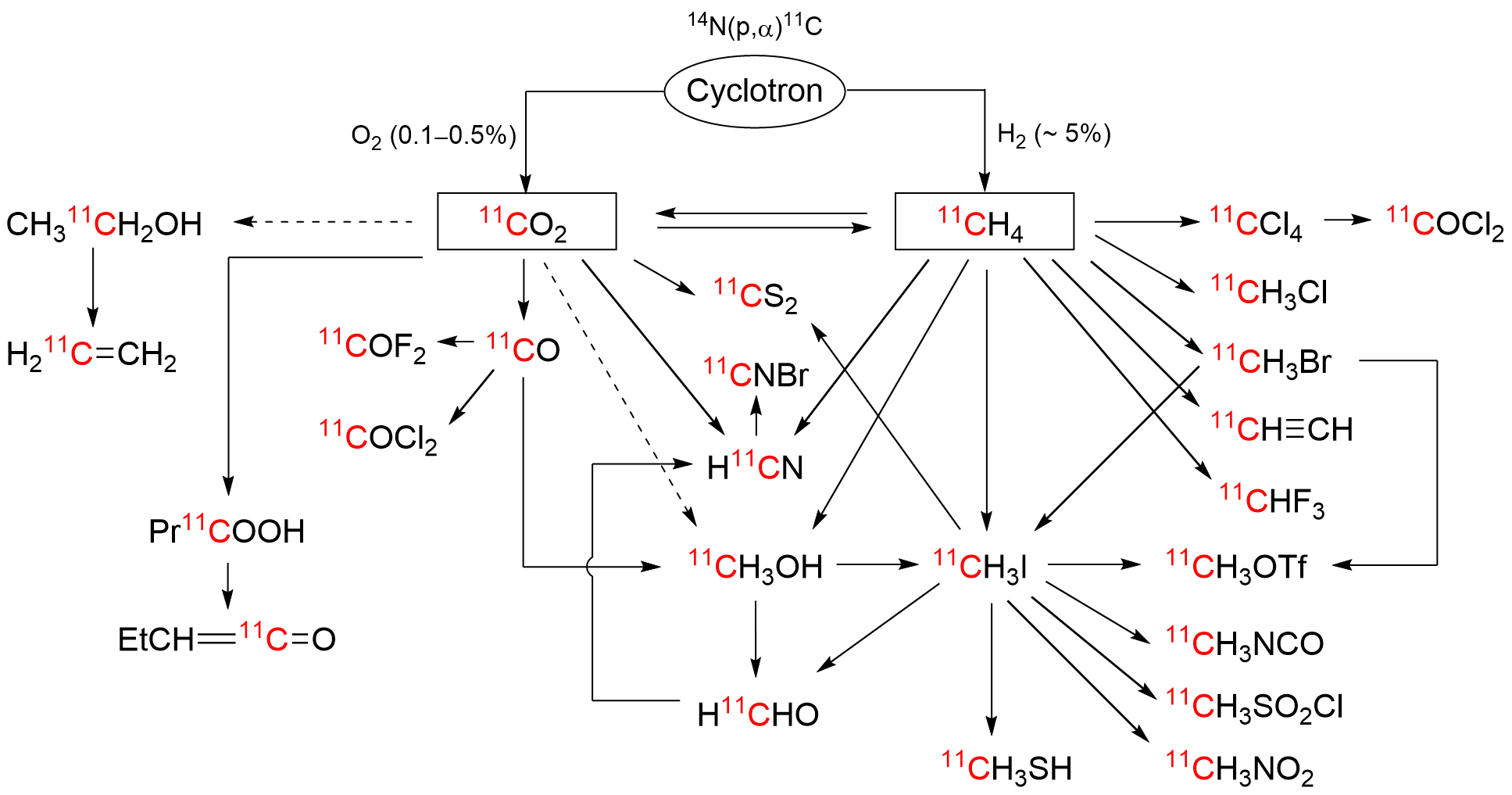
References
- Phelps, M.E. Positron emission tomography provides molecular imaging of biological processes. Proc. Natl. Acad. Sci. USA 2000, 97, 9226–9233.
- Cherry, S.R.; Badawi, R.D.; Karp, J.S.; Moses, W.W.; Price, P.; Jones, T. Total-body imaging: Transforming the role of positron emission tomography. Sci. Transl. Med. 2017, 9, eaaf6169.
- Casella, V.R.; Christman, D.R.; Ido, T.; Wolf, A.P. Excitation-function for 14N(p,a)11C reaction up to 15-MeV. Radiochim. Acta 1978, 25, 17–20.
- Bida, G.T.; Ruth, T.J.; Wolf, A.P. Experimentally determined thick target yields for the 14N (p,α)11C reaction. Radiochim. Acta 1980, 27, 181–186.
- Christman, D.R.; Finn, R.D.; Karlstrom, K.I.; Wolf, A.P. Production of ultra high activity 11C-labeled hydrogen cyanide, carbon dioxide, carbon monoxide and methane via 14N(p,a)11C reaction (XV). Int. J. Appl. Radiat. Isot. 1975, 26, 435–442.
- IAEA. Appendix 1: PET Cyclotron Comparison. In Cyclotron Produced Radionuclides: Principles and Practice; Technical Reports Series No. 465; IAEA: Vienna, Austria, 2008; Available online: https://www.iaea.org/publications/7849/cyclotron-produced-radionuclides-principles-and-practice (accessed on 5 January 2024).
- Ruth, T.J. Accelerators available for isotope production. In Handbook of Radiopharmaceuticals—Radiochemistry and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2002; pp. 71–85.
- Ache, H.J.; Wolf, A.P. Effect of radiation on the reactions of recoil carbon-11 in the nitrogen-oxygen system. J. Phys. Chem. 1968, 72, 1988–1993.
- Mock, B.H.; Vavrek, M.T.; Mulholland, G.K. Solid-phase reversible trap for carbon dioxide using carbon molecular sieves. Nucl. Med. Biol. 1995, 22, 667–670.
- Landais, P.; Crouzel, C. A new synthesis of carbon-11 labelled phosgene. Int. J. Radiat. Appl. Instrum. Part AAppl. Radiat. Isot. 1987, 38, 297–300.
- Suzuki, K.; Yamazaki, T.; Sasaki, M.; Kubodera, A. Specific activity of CO2 generated in a N2 gas target: Effect of irradiation dose, irradiation history, oxygen content and beam energy. Radiochim. Acta 2000, 88, 211–216.
- Gómez-Vallejo, V.; Gaja, V.; Koziorowski, J.; Llop, J. Specific activity of 11C-labelled radiotracers: A big challenge for PET chemists. In Positron Emission Tomography—Current Clinical and Research Aspects; Hsieh, C.-H., Ed.; Intechopen.com: London, UK, 2012; pp. 183–210.
- Zhang, M.-R.; Suzuki, K. Sources of carbon which decrease the specific activity of CH3I synthesized by the single pass I2 method. Appl. Radiat. Isot. 2005, 62, 447–450.
- Pichler, V.; Zenz, T.; Philippe, C.; Vraka, C.; Berrotéran-Infante, N.; Pfaff, S.; Nics, L.; Ozenil, M.; Langer, O.; Willeit, M.; et al. Molar activity—The keystone in 11C-radiochemistry: An explorative study using the gas phase method. Nucl. Med. Biol. 2018, 67, 21–26.
- Andersson, J.; Truong, P.; Halldin, C. In-target produced methane: Increased specific radioactivity. Appl. Radiat. Isot. 2009, 67, 106–110.
- Zeisler, S.K.; Nader, M.; Theobald, A.; Oberdorfer, F. Conversion of no-carrier-added carbon dioxide to carbon monoxide on molybdenum for the synthesis of 11C-labelled aromatic ketones. Appl. Radiat. Isot. 1997, 48, 1091–1095.
- Finn, R.D.; Christman, D.R.; Ache, H.J.; Wolf, A.P. The preparation of cyanide-11C for use in the synthesis of organic radiopharmaceuticals II. Int. J. Appl. Radiat. Isot. 1971, 22, 735–744.
- Larsen, P.; Ulin, J.; Dahlstrom, K.; Jensen, M. Synthesis of iodomethane by iodination of methane. Appl. Radiat. Isot. 1997, 48, 153–157.
- Någren, K.; Müller, L.; Halldin, C.; Swahn, C.-G.; Lehikoinen, P. Improved synthesis of some commonly used PET radioligands by the use of methyl triflate. Nucl. Med. Biol. 1995, 22, 235–239.
- Vaalburg, W.; Feenstra, A.; Wiegman, T.; Beerling, H.D.; Reiffers, S.; Talma, A.; Woldring, M.G.; Wynberg, H. Carbon-11 labelled Moxestrol and 17α-methylestradiol as receptor binding radiopharmaceuticals. J. Label. Compd. Radiopharm. 1981, 18, 100–101.
- Roeda, D.; Zanten, B.V.; Crouzel, C. The production of 11C-phosgene without added carrier. Radiochem. Radioanal. Lett. 1978, 33, 175–178.





