
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | AGNIESZKA HEROSIMCZYK | -- | 2631 | 2024-01-30 12:12:00 | | | |
| 2 | Lindsay Dong | Meta information modification | 2631 | 2024-01-31 02:31:44 | | |
Video Upload Options
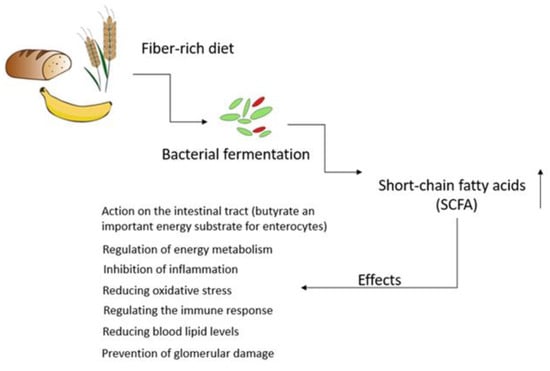
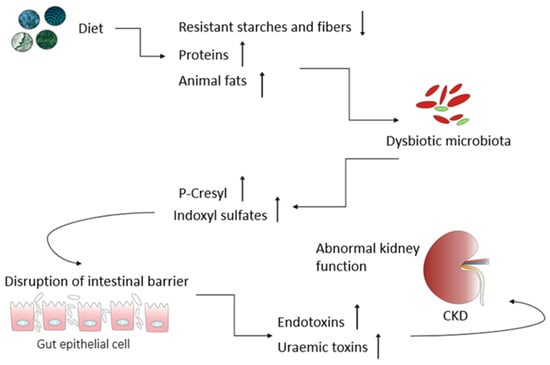
A well-balanced diet is integral for overall health, aiding in managing key risk factors for kidney damage like hypertension while supplying necessary precursors for metabolite production. Dietary choices directly influence the composition and metabolic patterns of the gut microbiota, showing promise as therapeutic tools for addressing various health conditions, including chronic kidney diseases (CKD). CKD pathogenesis involves a decline in the glomerular filtration rate and the retention of nitrogen waste, fostering gut dysbiosis and the excessive production of bacterial metabolites. These metabolites act as uremic toxins, contributing to inflammation, oxidative stress, and tissue remodeling in the kidneys. Dietary interventions hold significance in reducing oxidative stress and inflammation, potentially slowing CKD progression. Functional ingredients, nutrients, and nephroprotective phytoconstituents could modulate inflammatory pathways or impact the gut mucosa. The “gut–kidney axis” underscores the impact of gut microbes and their metabolites on health and disease, with dysbiosis serving as a triggering event in several diseases, including CKD.
1. Introduction
2. The Kidney–Gut Axis: A Potential Connection between Gut Dysbiosis and CKD
3. Nutritional Strategies Focusing on the Gut Microbiota as a Novel Treatment for Counteracting CKD Progression
4. Conclusions
References
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global impacts of western diet and its effects on metabolism and health: A narrative review. Nutrients 2023, 15, 2749.
- Dobrek, Ł. The kidney-gut axis in chronic kidney disease. Pol. Merkur. Lekarski 2022, 50, 323–327.
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as oxidative stress-and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752.
- Khan, K.N.M.; Hard, G.C.; Alden, C.L. Chapter 47—Kidney. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Academic Press: Cambridge, MA, USA, 2013; pp. 1667–1773.
- Bhat, P.V.; Manolescu, D.C. Role of vitamin A in determining nephron mass and possible relationship to hypertension. J. Nutr. 2008, 138, 1407–1410.
- Bonvalet, J.P.; Champion, M.; Courtalon, A.; Farman, N.; Vandewalle, A.; Wanstok, F. Number of glomeruli in normal and hypertrophied kidneys of mice and guinea pigs. J. Physiol. 1977, 269, 627–641.
- Wang, X.; Johnson, A.C.; Williams, J.M.; White, T.; Chade, A.R.; Zhang, J.; Liu, R.; Roman, R.J.; Lee, J.W.; Kyle, P.B. Nephron deficiency and predisposition to renal injury in a novel one-kidney genetic model. J. Am. Soc. Nephrol. 2015, 26, 1634–1646.
- van Vuuren, S.H.; Sol, C.M.; Broekhuizen, R.; Lilien, M.R.; Oosterveld, M.J.S.; Nguyen, T.Q.; Goldschmeding, R.; de Jong, T.P.V.M. Compensatory growth of congenital solitary kidneys in pigs reflects increased nephron numbers rather than hypertrophy. PLoS ONE 2012, 7, e49735.
- Wintour, E.M.; Moritz, K.M.; Johnson, K.; Ricardo, S.; Samuel, C.S.; Dodic, M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J. Physiol. 2003, 549, 929–935.
- Magliocca, G.; Mone, P.; Di Iorio, B.R.; Heidland, A.; Marzocco, S. Short-chain fatty acids in chronic kidney disease: Focus on inflammation and oxidative stress regulation. Int. J. Mol. Sci. 2022, 23, 5354.
- Lambert, K.; Rinninella, E.; Biruete, A.; Sumida, K.; Stanford, J.; Raoul, P.; Mele, M.C.; Wang, A.Y.; Mafra, D. Targeting the gut microbiota in kidney disease: The future in renal nutrition and metabolism. J. Ren. Nutr. 2023, 33, S30–S39.
- Hsu, C.N.; Tain, Y.L. Chronic kidney disease and gut microbiota: What is their connection in early life? Int. J. Mol. Sci. 2022, 23, 3954.
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135.
- Jeong, S. Factors influencing development of the infant microbiota: From prenatal period to early infancy. Clin. Exp. Pediatr. 2022, 65, 438–447.
- Turroni, F.; Milani, C.; Ventura, M.; van Sinderen, D. The human gut microbiota during the initial stages of life: Insights from bifidobacteria. Curr. Opin. Biotechnol. 2022, 73, 81–87.
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836.
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 2022, 13, 1106–1126.
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196.
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243.
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876.
- Li, L.Z.; Ma, L.; Fu, P. Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des. Devel. Ther. 2017, 11, 3531–3542.
- Tao, P.Y.; Ji, J.; Wang, Q.; Cui, M.M.; Cao, M.F.; Xu, Y.Z. The role and mechanism of gut microbiota-derived short-chain fatty in the prevention and treatment of diabetic kidney disease. Front. Immunol. 2022, 13, 1080456.
- Evenepoel, P.; Poesen, R.; Meijers, B. The gut-kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014.
- Huang, Y.H.; Xin, W.; Xiong, J.C.; Yao, M.Y.; Zhang, B.; Zhao, J.H. The intestinal microbiota and metabolites in the gut-kidney-heart axis of chronic kidney disease. Front. Pharmacol. 2022, 13, 837500.
- Filipska, I.; Winiarska, A.; Knysak, M.; Stompór, T. Contribution of gut microbiota-derived uremic toxins to the cardiovascular system mineralization. Toxins 2021, 13, 274.
- Naber, T.; Purohit, S. Chronic kidney disease: Role of diet for a reduction in the severity of the disease. Nutrients 2021, 13, 3277.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Yang, J.; Qin, S.; Zhang, H. Precise strategies for selecting probiotic bacteria in treatment of intestinal bacterial dysfunctional diseases. Front. Immunol. 2022, 13, 1034727.
- Tian, N.; Li, L.; Ng, J.K.-C.; Li, P.K.-T. The potential benefits and controversies of probiotics use in patients at different stages of chronic kidney disease. Nutrients 2022, 14, 4044.
- Soleimani, A.; Mojarrad, M.Z.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017, 91, 435–442.
- Yu, Z.X.; Zhao, J.; Qin, Y.L.; Wang, Y.W.; Zhang, Y.M.; Sun, S.R. Probiotics, prebiotics, and synbiotics improve uremic, inflammatory, and gastrointestinal symptoms in end-stage renal disease with dialysis: A network meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 850425.
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3.
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota-introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412.
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275.
- Fao, F.; Organisation, A. FAO technical meeting on prebiotics. In Food Quality and Standards Services (AGNIS); Food and Agricultural Organisation of the United Nations: San Francisco, CA, USA, 2007.
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502.
- Zirker, L. Benefit and Use of Prebiotics in Patients with Chronic Kidney Disease. J. Ren. Nutr. 2015, 25, e9–e10.
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553.
- Zheng, H.J.; Guo, J.; Wang, Q.H.; Wang, L.S.; Wang, Y.H.; Zhang, F.; Huang, W.J.; Zhang, W.T.; Liu, W.J.; Wang, Y.X. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598.
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as medicine: Targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 2021, 17, 153–171.
- Tienda-Vazquez, M.A.; Morreeuw, Z.P.; Sosa-Hernandez, J.E.; Cardador-Martinez, A.; Sabath, E.; Melchor-Martinez, E.M.; Iqbal, H.M.N.; Parra-Saldivar, R. Nephroprotective plants: A review on the use in pre-renal and post-renal diseases. Plants 2022, 11, 818.
- Basist, P.; Parveen, B.; Zahiruddin, S.; Gautam, G.; Parveen, R.; Khan, M.A.; Krishnan, A.; Shahid, M.; Ahmad, S. Potential nephroprotective phytochemicals: Mechanism and future prospects. J. Ethnopharmacol. 2022, 283, 114743.
- Abdel-Rahman, A.; Anyangwe, N.; Carlacci, L.; Casper, S.; Danam, R.P.; Enongene, E.; Erives, G.; Fabricant, D.; Gudi, R.; Hilmas, C.J. The safety and regulation of natural products used as foods and food ingredients. Toxicol. Sci. 2011, 123, 333–348.
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688.
- Sansores-España, D.; Pech-Aguilar, A.G.; Cua-Pech, K.G.; Medina-Vera, I.; Guevara-Cruz, M.; Gutiérrez-Solis, A.L.; Reyes-García, J.G.; Avila-Nava, A. plants used in mexican traditional medicine for the management of urolithiasis: A review of preclinical evidence, bioactive compounds, and molecular mechanisms. Molecules 2022, 27, 2008.




