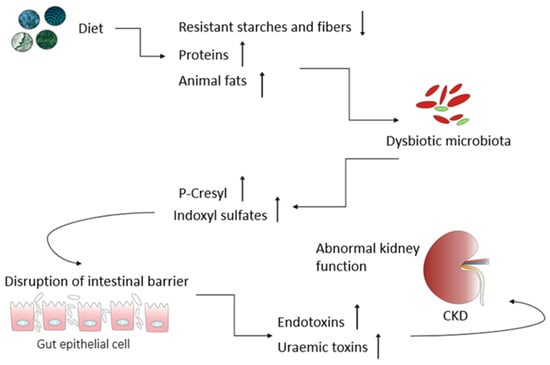
A well-balanced diet is integral for overall health, aiding in managing key risk factors for kidney damage like hypertension while supplying necessary precursors for metabolite production. Dietary choices directly influence the composition and metabolic patterns of the gut microbiota, showing promise as therapeutic tools for addressing various health conditions, including chronic kidney diseases (CKD). CKD pathogenesis involves a decline in the glomerular filtration rate and the retention of nitrogen waste, fostering gut dysbiosis and the excessive production of bacterial metabolites. These metabolites act as uremic toxins, contributing to inflammation, oxidative stress, and tissue remodeling in the kidneys. Dietary interventions hold significance in reducing oxidative stress and inflammation, potentially slowing CKD progression. Functional ingredients, nutrients, and nephroprotective phytoconstituents could modulate inflammatory pathways or impact the gut mucosa. The “gut–kidney axis” underscores the impact of gut microbes and their metabolites on health and disease, with dysbiosis serving as a triggering event in several diseases, including CKD.
- chronic kidney disease
- gut microbiota
- gut–kidney axis
1. Introduction
2. The Kidney–Gut Axis: A Potential Connection between Gut Dysbiosis and CKD
The kidney plays a critical role in maintaining plasma osmolarity by intricately regulating water, solute, and electrolyte levels in the bloodstream. Beyond this, they also maintain an acid-base balance, produce essential hormones, and participate in specific metabolic functions. Of notable significance, the kidneys are indispensable in excreting nitrogenous waste products, including urea, creatinine, and ammonia ions. Consequently, any substantial alterations in renal function lead to the accumulation of these waste products within the body [6][4]. It should be emphasized that the kidneys’ ability to perform their functions is predominantly determined during fetal development. Throughout this phase, the formation of nephrons occurs, and the final number that is established before birth becomes the lifelong kidney endowment. A literature analysis reveals considerable variability in the number of nephrons, observed in both humans [7][5] and various animals, such as mice [8][6], rats [9][7], pigs [10][8], and sheep [11][9]. Recent findings strongly indicate that the gut microbiota (GM) have emerged as a key player in CKD pathogenesis, and the interaction between GM and CKD is reciprocal. CKD can influence the composition of the gut microbiota, leading to gut dysbiosis. Conversely, dysbiosis in CKD patients may elevate uremic toxin levels, thereby contributing to the progression of CKD [18,19,20][10][11][12]. Microorganisms, including bacteria, yeasts, and viruses, residing within the gastrointestinal tract, collectively referred to as the GM, play a crucial role in maintaining the overall balance and well-being of the host, although they can also act as a potential source of disease [21][13]. From the moment of birth, the establishment of the microbiome is an extremely dynamic process, characterized by continuous changes in its composition, which are greatly influenced by a wide range of external factors, especially during the early stages of life. Elements like the delivery method, dietary preferences, hygiene practices, and medication use, particularly antibiotics, all play a significant role in shaping the final composition and diversity of the gut microbiota [23][14]. During the first 2–3 years of human life, the gut microbiome starts to develop, eventually stabilizing into a configuration that closely resembles the typical microbial taxonomy found in adults [24][15]. This intestinal microbial species exert a remarkable influence on the absorption, metabolism, and storage of nutrients [4][16]. Most importantly, GM also play a critical role in facilitating the fermentation of a diverse array of compounds, especially those that that are resistant to digestion by human enzymes. This results in the generation of a diverse array of metabolites that can affect host cells, tissues, and organs [26][17]. Gut-microbiota-derived products encompass both intermediates and the end products of bacterial metabolic processes, and their ultimate composition in the gut is greatly influenced by the dietary intake of specific nutrients. The microbial fermentation of complex non-digestible dietary carbohydrates primarily occurs in the cecum and proximal regions of the colon. The large intestines also serve as a site for the fermentation of dietary proteins that escaped digestion in the upper regions of the gastrointestinal tract. Residual proteins and peptides are subjected to hydrolysis, breaking down into amino acids through the action of extracellular proteases and peptidases produced by intestinal microorganisms [27][18]. It should be emphasized that the non-digestible carbohydrate fermentation is much more favorable as it results in the production of short-chain fatty acids (SCFAs) and gases such as carbon dioxide, hydrogen, methane, and hydrogen sulfide [28,29][19][20]. SCFAs, particularly acetate, propionate, and butyrate, are recognized for their crucial roles in regulating the energy metabolism, preserving the integrity and functionality of the gut barrier, inhibiting inflammation and oxidative stress, and modulating the immune response (Figure 1) [30,31][21][22].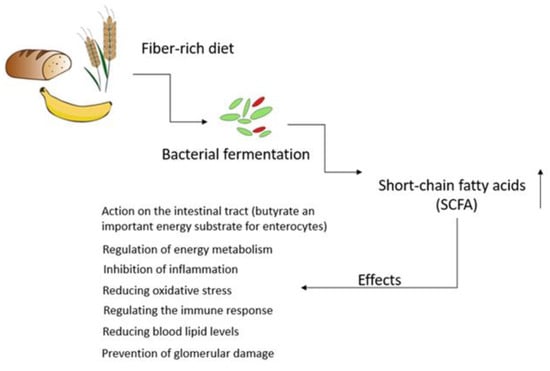
3. Nutritional Strategies Focusing on the Gut Microbiota as a Novel Treatment for Counteracting CKD Progression
4. Conclusions
References
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global impacts of western diet and its effects on metabolism and health: A narrative review. Nutrients 2023, 15, 2749.
- Dobrek, Ł. The kidney-gut axis in chronic kidney disease. Pol. Merkur. Lekarski 2022, 50, 323–327.
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as oxidative stress-and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752.
- Khan, K.N.M.; Hard, G.C.; Alden, C.L. Chapter 47—Kidney. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Academic Press: Cambridge, MA, USA, 2013; pp. 1667–1773.
- Bhat, P.V.; Manolescu, D.C. Role of vitamin A in determining nephron mass and possible relationship to hypertension. J. Nutr. 2008, 138, 1407–1410.
- Bonvalet, J.P.; Champion, M.; Courtalon, A.; Farman, N.; Vandewalle, A.; Wanstok, F. Number of glomeruli in normal and hypertrophied kidneys of mice and guinea pigs. J. Physiol. 1977, 269, 627–641.
- Wang, X.; Johnson, A.C.; Williams, J.M.; White, T.; Chade, A.R.; Zhang, J.; Liu, R.; Roman, R.J.; Lee, J.W.; Kyle, P.B. Nephron deficiency and predisposition to renal injury in a novel one-kidney genetic model. J. Am. Soc. Nephrol. 2015, 26, 1634–1646.
- van Vuuren, S.H.; Sol, C.M.; Broekhuizen, R.; Lilien, M.R.; Oosterveld, M.J.S.; Nguyen, T.Q.; Goldschmeding, R.; de Jong, T.P.V.M. Compensatory growth of congenital solitary kidneys in pigs reflects increased nephron numbers rather than hypertrophy. PLoS ONE 2012, 7, e49735.
- Wintour, E.M.; Moritz, K.M.; Johnson, K.; Ricardo, S.; Samuel, C.S.; Dodic, M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J. Physiol. 2003, 549, 929–935.
- Magliocca, G.; Mone, P.; Di Iorio, B.R.; Heidland, A.; Marzocco, S. Short-chain fatty acids in chronic kidney disease: Focus on inflammation and oxidative stress regulation. Int. J. Mol. Sci. 2022, 23, 5354.
- Lambert, K.; Rinninella, E.; Biruete, A.; Sumida, K.; Stanford, J.; Raoul, P.; Mele, M.C.; Wang, A.Y.; Mafra, D. Targeting the gut microbiota in kidney disease: The future in renal nutrition and metabolism. J. Ren. Nutr. 2023, 33, S30–S39.
- Hsu, C.N.; Tain, Y.L. Chronic kidney disease and gut microbiota: What is their connection in early life? Int. J. Mol. Sci. 2022, 23, 3954.
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135.
- Jeong, S. Factors influencing development of the infant microbiota: From prenatal period to early infancy. Clin. Exp. Pediatr. 2022, 65, 438–447.
- Turroni, F.; Milani, C.; Ventura, M.; van Sinderen, D. The human gut microbiota during the initial stages of life: Insights from bifidobacteria. Curr. Opin. Biotechnol. 2022, 73, 81–87.
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836.
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 2022, 13, 1106–1126.
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196.
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243.
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876.
- Li, L.Z.; Ma, L.; Fu, P. Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des. Devel. Ther. 2017, 11, 3531–3542.
- Tao, P.Y.; Ji, J.; Wang, Q.; Cui, M.M.; Cao, M.F.; Xu, Y.Z. The role and mechanism of gut microbiota-derived short-chain fatty in the prevention and treatment of diabetic kidney disease. Front. Immunol. 2022, 13, 1080456.
- Evenepoel, P.; Poesen, R.; Meijers, B. The gut-kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014.
- Huang, Y.H.; Xin, W.; Xiong, J.C.; Yao, M.Y.; Zhang, B.; Zhao, J.H. The intestinal microbiota and metabolites in the gut-kidney-heart axis of chronic kidney disease. Front. Pharmacol. 2022, 13, 837500.
- Filipska, I.; Winiarska, A.; Knysak, M.; Stompór, T. Contribution of gut microbiota-derived uremic toxins to the cardiovascular system mineralization. Toxins 2021, 13, 274.
- Naber, T.; Purohit, S. Chronic kidney disease: Role of diet for a reduction in the severity of the disease. Nutrients 2021, 13, 3277.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Yang, J.; Qin, S.; Zhang, H. Precise strategies for selecting probiotic bacteria in treatment of intestinal bacterial dysfunctional diseases. Front. Immunol. 2022, 13, 1034727.
- Tian, N.; Li, L.; Ng, J.K.-C.; Li, P.K.-T. The potential benefits and controversies of probiotics use in patients at different stages of chronic kidney disease. Nutrients 2022, 14, 4044.
- Soleimani, A.; Mojarrad, M.Z.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017, 91, 435–442.
- Yu, Z.X.; Zhao, J.; Qin, Y.L.; Wang, Y.W.; Zhang, Y.M.; Sun, S.R. Probiotics, prebiotics, and synbiotics improve uremic, inflammatory, and gastrointestinal symptoms in end-stage renal disease with dialysis: A network meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 850425.
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3.
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota-introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412.
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275.
- Fao, F.; Organisation, A. FAO technical meeting on prebiotics. In Food Quality and Standards Services (AGNIS); Food and Agricultural Organisation of the United Nations: San Francisco, CA, USA, 2007.
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502.
- Zirker, L. Benefit and Use of Prebiotics in Patients with Chronic Kidney Disease. J. Ren. Nutr. 2015, 25, e9–e10.
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553.
- Zheng, H.J.; Guo, J.; Wang, Q.H.; Wang, L.S.; Wang, Y.H.; Zhang, F.; Huang, W.J.; Zhang, W.T.; Liu, W.J.; Wang, Y.X. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598.
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as medicine: Targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 2021, 17, 153–171.
- Tienda-Vazquez, M.A.; Morreeuw, Z.P.; Sosa-Hernandez, J.E.; Cardador-Martinez, A.; Sabath, E.; Melchor-Martinez, E.M.; Iqbal, H.M.N.; Parra-Saldivar, R. Nephroprotective plants: A review on the use in pre-renal and post-renal diseases. Plants 2022, 11, 818.
- Basist, P.; Parveen, B.; Zahiruddin, S.; Gautam, G.; Parveen, R.; Khan, M.A.; Krishnan, A.; Shahid, M.; Ahmad, S. Potential nephroprotective phytochemicals: Mechanism and future prospects. J. Ethnopharmacol. 2022, 283, 114743.
- Abdel-Rahman, A.; Anyangwe, N.; Carlacci, L.; Casper, S.; Danam, R.P.; Enongene, E.; Erives, G.; Fabricant, D.; Gudi, R.; Hilmas, C.J. The safety and regulation of natural products used as foods and food ingredients. Toxicol. Sci. 2011, 123, 333–348.
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688.
- Sansores-España, D.; Pech-Aguilar, A.G.; Cua-Pech, K.G.; Medina-Vera, I.; Guevara-Cruz, M.; Gutiérrez-Solis, A.L.; Reyes-García, J.G.; Avila-Nava, A. plants used in mexican traditional medicine for the management of urolithiasis: A review of preclinical evidence, bioactive compounds, and molecular mechanisms. Molecules 2022, 27, 2008.
