
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xinyu Zhou | -- | 2393 | 2024-01-29 15:44:20 | | | |
| 2 | Xinyu Zhou | + 924 word(s) | 3317 | 2024-01-30 12:20:53 | | | | |
| 3 | Lindsay Dong | -1 word(s) | 3316 | 2024-01-31 02:04:04 | | | | |
| 4 | Lindsay Dong | -38 word(s) | 3278 | 2024-02-07 02:26:25 | | | | |
| 5 | Lindsay Dong | Meta information modification | 3278 | 2024-03-08 06:18:35 | | |
Video Upload Options
The mortality rate of acute respiratory distress syndrome (ARDS) is still very high, and the remission and treatment of ARDS are still the focus of research. The causes of acute respiratory distress syndrome are varied, with pneumonia and non-pulmonary sepsis being the most common. Trauma and blood transfusion can also cause acute respiratory distress syndrome. In ARDS, the accumulation and infiltration of neutrophils in the lungs have a great influence on the development of the disease. Neutrophils regulate inflammatory responses through various pathways, and neutrophils release via neutrophilic extracellular traps (NETs) is considered to be one of the most important mechanisms.
1. Introduction
Acute respiratory distress syndrome (ARDS) is the acute onset of respiratory disease characterized by bilateral pulmonary edema and hypoxemia of noncardiac origin caused by damage to the pulmonary endothelial barrier and excessive permeability of alveolar capillaries, which is clinically manifested as bilateral pulmonary infiltrates and respiratory failure [1]. The Berlin definition classifies ARDS as mild, moderate, or severe based on the level of pulmonary oxygenation, and removes the definition of acute lung injury (ALI) [2]. The most common causes of ARDS include pneumonia and non-pulmonary sepsis, as well as the aspiration of the stomach contents, trauma, pancreatitis, burns, inhalation injury, drug overdose, multiple blood transfusions or shock, e-cigarettes, chemotherapy and immunotherapy (including checkpoint inhibitors), etc. [3][4]. A 2016 observational study covering 459 intensive care units (ICU) in 50 countries showed that 10% of ICU patients and 23% of mechanically ventilated patients had ARDS, with a 28-day mortality rate of 35% and a mortality rate of more than 40% for patients with severe ARDS [5]. In addition to a high incidence and mortality, patients with ARDS may have a sequelae of physical, cognitive, and mental health conditions after recovery [3]. Alveoli neutrophil infiltration, which causes persistent inflammation, is one of the ARDS [6] (Figure 1).
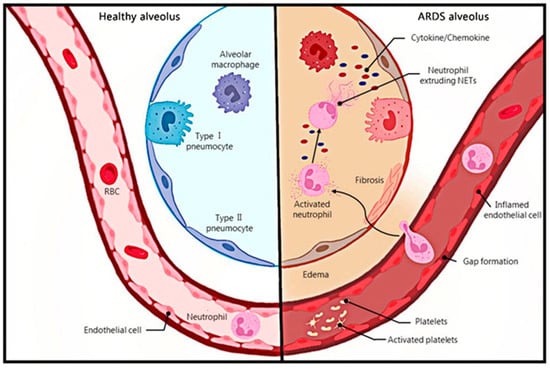
Neutrophils, a major component of innate immunity and the host’s first line of defense against infectious pathogens, are recruited at the onset of infection and destroy pathogens through mechanisms such as phagocytosis, degranulation, production of reactive oxygen species, release of antimicrobial peptides, and the recently discovered formation of neutrophil extracellular traps (NET) [7]. NETs are produced by activated neutrophils and consist of DNA, histones, granular proteins such as neutrophil elastase, cathepsin G, and myeloperoxidase [8]. The process of NET formation by neutrophils, known as NETosis, is a novel mechanism of programmed cell death of neutrophils, and can be divided into NADPH oxidase 2 (NOX)-dependent and independent NETosis [9] (Figure 2). NETs can be induced by IL-8, LPS, etc., and have strong antibacterial effects [8]. In addition, NETs can also mediate tissue damage [10], cancer [11], inflammation [12], and autoimmune diseases [13]. Recently, the association between NETs and ARDS has been confirmed by a number of studies.
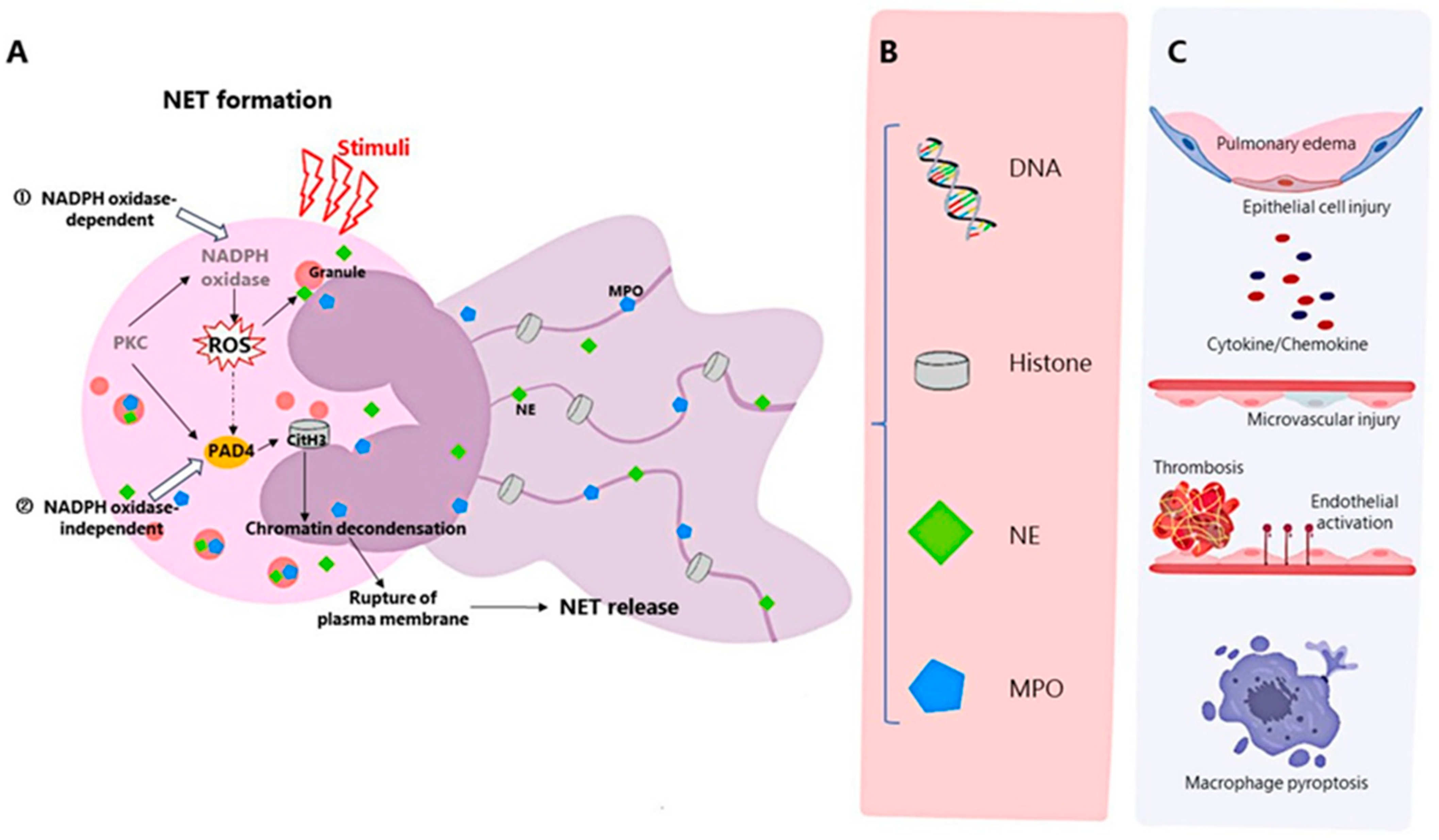
2. Evidence on the Influence of NETs on ARDS
3. The Mechanism of NETs Influencing ARDS
3.1. Histones
3.2. NE
3.3. DNA
3.4. MPO
4. The Mechanism of NETs Production in ARDS and the Substances That Affect the Production
5. Relationship between the Inflammatory Storm and NETs
6. Correlation between Immune Balance and Regulation in ARDS and NETs
7. Production and Role of NETs in COVID-19 ARDS
8. Clinical Research
9. Treatment
10. Conclusions
References
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637.
- ARDS Definition of Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533.
- Gorman, E.A.; O’kane, C.M.; McAuley, D.F. Acute respiratory distress syndrome in adults: Diagnosis, outcomes, long-term sequelae, and management. Lancet 2022, 400, 1157–1170.
- Bos, L.D.J.; Ware, L.B. Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156.
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800, Erratum in JAMA 2016, 316, 350.
- Steinberg, K.P.; A Milberg, J.; Martin, T.R.; Maunder, R.J.; A Cockrill, B.; Hudson, L.D. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1994, 150, 113–122.
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218.
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535.
- Jo, A.; Kim, D.W. Neutrophil Extracellular Traps in Airway Diseases: Pathological Roles and Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 5034.
- Liu, L.; Mao, Y.; Xu, B.; Zhang, X.; Fang, C.; Ma, Y.; Men, K.; Qi, X.; Yi, T.; Wei, Y.; et al. Induction of neutrophil extracellular traps during tissue injury: Involvement of STING and Toll-like receptor 9 pathways. Cell Prolif. 2019, 52, e12579, Erratum in Cell Prolif. 2020, 53, e12775.
- Ronchetti, L.; Boubaker, N.S.; Barba, M.; Vici, P.; Gurtner, A.; Piaggio, G. Neutrophil extracellular traps in cancer: Not only catching microbes. J. Exp. Clin. Cancer Res. 2021, 40, 231.
- Twaddell, S.H.; Baines, K.J.; Grainge, C.; Gibson, P.G. The Emerging Role of Neutrophil Extracellular Traps in Respiratory Disease. Chest 2019, 156, 774–782.
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2017, 18, 134–147.
- Yang, S.-C.; Tsai, Y.-F.; Pan, Y.-L.; Hwang, T.-L. Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed. J. 2020, 44, 439–446.
- Scozzi, D.; Liao, F.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The role of neutrophil extracellular traps in acute lung injury. Front. Immunol. 2022, 13, 953195.
- Grégoire, M.; Uhel, F.; Lesouhaitier, M.; Gacouin, A.; Guirriec, M.; Mourcin, F.; Dumontet, E.; Chalin, A.; Samson, M.; Berthelot, L.-L.; et al. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur. Respir. J. 2018, 52, 1702590.
- Ojima, M.; Yamamoto, N.; Hirose, T.; Hamaguchi, S.; Tasaki, O.; Kojima, T.; Tomono, K.; Ogura, H.; Shimazu, T. Serial change of neutrophil extracellular traps in tracheal aspirate of patients with acute respiratory distress syndrome: Report of three cases. J. Intensiv. Care 2020, 8, 25.
- Song, C.; Li, H.; Mao, Z.; Peng, L.; Liu, B.; Lin, F.; Li, Y.; Dai, M.; Cui, Y.; Zhao, Y.; et al. Delayed neutrophil apoptosis may enhance NET formation in ARDS. Respir. Res. 2022, 23, 155.
- Lefrançais, E.; Mallavia, B.; Zhuo, H.; Calfee, C.S.; Looney, M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. J. Clin. Investig. 2018, 3, e98178.
- Ode, Y.; Aziz, M.; Jin, H.; Arif, A.; Nicastro, J.G.; Wang, P. Cold-inducible RNA-binding Protein Induces Neutrophil Extracellular Traps in the Lungs during Sepsis. Sci. Rep. 2019, 9, 6252.
- Song, C.; Li, H.; Li, Y.; Dai, M.; Zhang, L.; Liu, S.; Tan, H.; Deng, P.; Liu, J.; Mao, Z.; et al. NETs promote ALI/ARDS inflammation by regulating alveolar macrophage polarization. Exp. Cell Res. 2019, 382, 111486.
- Li, H.; Li, Y.; Song, C.; Hu, Y.; Dai, M.; Liu, B.; Pan, P. Neutrophil Extracellular Traps Augmented Alveolar Macrophage Pyroptosis via AIM2 Inflammasome Activation in LPS-Induced ALI/ARDS. J. Inflamm. Res. 2021, 14, 4839–4858.
- Zhang, H.; Liu, J.; Zhou, Y.; Qu, M.; Wang, Y.; Guo, K.; Shen, R.; Sun, Z.; Cata, J.P.; Yang, S.; et al. Neutrophil extracellular traps mediate m6A modification and regulates sepsis-associated acute lung injury by activating ferroptosis in alveolar epithelial cells. Int. J. Biol. Sci. 2022, 18, 3337–3357.
- Toy, P.; Lowell, C. TRALI—Definition, mechanisms, incidence and clinical relevance. Best Pract. Res. Clin. Anaesthesiol. 2007, 21, 183–193.
- Hayase, N.; Doi, K.; Hiruma, T.; Matsuura, R.; Hamasaki, Y.; Noiri, E.; Nangaku, M.; Morimura, N. Recombinant thrombomodulin prevents acute lung injury induced by renal ischemia-reperfusion injury. Sci. Rep. 2020, 10, 289.
- Surolia, R.; Li, F.J.; Wang, Z.; Kashyap, M.; Srivastava, R.K.; Traylor, A.M.; Singh, P.; Dsouza, K.G.; Kim, H.; Pittet, J.-F.; et al. NETosis in the pathogenesis of acute lung injury following cutaneous chemical burns. J. Clin. Investig. 2021, 6, e147564.
- Zhan, Y.; Ling, Y.; Deng, Q.; Qiu, Y.; Shen, J.; Lai, H.; Chen, Z.; Huang, C.; Liang, L.; Li, X.; et al. HMGB1-Mediated Neutrophil Extracellular Trap Formation Exacerbates Intestinal Ischemia/Reperfusion-Induced Acute Lung Injury. J. Immunol. 2022, 208, 968–978.
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639.
- Hoeksema, M.; van Eijk, M.; Haagsman, H.P.; Hartshorn, K.L. Histones as mediators of host defense, inflammation and thrombosis. Futur. Microbiol. 2016, 11, 441–453.
- Sørensen, O.E.; Borregaard, N. Neutrophil extracellular traps—The dark side of neutrophils. J. Clin. Investig. 2016, 126, 1612–1620.
- Rohrbach, A.S.; Slade, D.J.; Thompson, P.R.; Mowen, K.A. Activation of PAD4 in NET formation. Front. Immunol. 2012, 3, 360.
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862.
- Neeli, I.; Khan, S.N.; Radic, M. Histone Deimination As a Response to Inflammatory Stimuli in Neutrophils. J. Immunol. 2008, 180, 1895–1902.
- Tatsiy, O.; McDonald, P.P. Physiological Stimuli Induce PAD4-Dependent, ROS-Independent NETosis, With Early and Late Events Controlled by Discrete Signaling Pathways. Front. Immunol. 2018, 9, 2036.
- Biron, B.M.; Chung, C.-S.; O’Brien, X.M.; Chen, Y.; Reichner, J.S.; Ayala, A. Cl-Amidine Prevents Histone 3 Citrullination and Neutrophil Extracellular Trap Formation, and Improves Survival in a Murine Sepsis Model. J. Innate Immun. 2016, 9, 22–32.
- Kolaczkowska, E.; Jenne, C.N.; Surewaard, B.G.J.; Thanabalasuriar, A.; Lee, W.-Y.; Sanz, M.-J.; Mowen, K.; Opdenakker, G.; Kubes, P. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat. Commun. 2015, 6, 6673.
- Guiducci, E.; Lemberg, C.; Küng, N.; Schraner, E.; Theocharides, A.P.A.; LeibundGut-Landmann, S. Candida albicans-Induced NETosis Is Independent of Peptidylarginine Deiminase 4. Front. Immunol. 2018, 9, 1573.
- Claushuis, T.A.M.; van der Donk, L.E.H.; Luitse, A.L.; van Veen, H.A.; van der Wel, N.N.; van Vught, L.A.; Roelofs, J.J.T.H.; de Boer, O.J.; Lankelma, J.M.; Boon, L.; et al. Role of Peptidylarginine Deiminase 4 in Neutrophil Extracellular Trap Formation and Host Defense during Klebsiella pneumoniae–Induced Pneumonia-Derived Sepsis. J. Immunol. 2018, 201, 1241–1252.
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320.
- Caudrillier, A.; Kessenbrock, K.; Gilliss, B.M.; Nguyen, J.X.; Marques, M.B.; Monestier, M.; Toy, P.; Werb, Z.; Looney, M.R. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Investig. 2012, 122, 2661–2671.
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321.
- Abrams, S.T.; Zhang, N.; Manson, J.; Liu, T.; Dart, C.; Baluwa, F.; Wang, S.S.; Brohi, K.; Kipar, A.; Yu, W.; et al. Circulating Histones Are Mediators of Trauma-associated Lung Injury. Am. J. Respir. Crit. Care Med. 2013, 187, 160–169.
- O’donoghue, A.J.; Jin, Y.; Knudsen, G.M.; Perera, N.C.; Jenne, D.E.; Murphy, J.E.; Craik, C.S.; Hermiston, T.W. Global Substrate Profiling of Proteases in Human Neutrophil Extracellular Traps Reveals Consensus Motif Predominantly Contributed by Elastase. PLoS ONE 2013, 8, e75141.
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175.
- Suzuki, K.; Okada, H.; Takemura, G.; Takada, C.; Kuroda, A.; Yano, H.; Zaikokuji, R.; Morishita, K.; Tomita, H.; Oda, K.; et al. Neutrophil Elastase Damages the Pulmonary Endothelial Glycocalyx in Lipopolysaccharide-Induced Experimental Endotoxemia. Am. J. Pathol. 2019, 189, 1526–1535.
- Perl, M.; Lomas-Neira, J.; Chung, C.-S.; Ayala, A. Epithelial Cell Apoptosis and Neutrophil Recruitment in Acute Lung Injury—A Unifying Hypothesis? What We Have Learned from Small Interfering RNAs. Mol. Med. 2008, 14, 465–475.
- Frantzeskaki, F.; Armaganidis, A.; E Orfanos, S. Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks between Inflammation and Coagulation. Respiration 2017, 93, 212–225.
- Hermant, B.; Bibert, S.; Concord, E.; Dublet, B.; Weidenhaupt, M.; Vernet, T.; Gulino-Debrac, D. Identification of Proteases Involved in the Proteolysis of Vascular Endothelium Cadherin during Neutrophil Transmigration. J. Biol. Chem. 2003, 278, 14002–14012.
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885.
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE 2012, 7, e32366.
- Nishinaka, Y.; Arai, T.; Adachi, S.; Takaori-Kondo, A.; Yamashita, K. Singlet oxygen is essential for neutrophil extracellular trap formation. Biochem. Biophys. Res. Commun. 2011, 413, 75–79.
- Li, H.; Pan, P.; Su, X.; Liu, S.; Zhang, L.; Wu, D.; Li, H.; Dai, M.; Li, Y.; Hu, C.; et al. Neutrophil Extracellular Traps Are Pathogenic in Ventilator-Induced Lung Injury and Partially Dependent on TLR4. BioMed Res. Int. 2017, 2017, 8272504.
- Chen, W.; Chen, H.; Yang, Z.-T.; Mao, E.-Q.; Chen, Y.; Chen, E.-Z. Free fatty acids-induced neutrophil extracellular traps lead to dendritic cells activation and T cell differentiation in acute lung injury. Aging 2021, 13, 26148–26160.
- Thomas, G.M.; Carbo, C.; Curtis, B.R.; Martinod, K.; Mazo, I.B.; Schatzberg, D.; Cifuni, S.M.; Fuchs, T.A.; von Andrian, U.H.; Hartwig, J.H.; et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood 2012, 119, 6335–6343.
- Szturmowicz, M.; Demkow, U. Neutrophil Extracellular Traps (NETs) in Severe SARS-CoV-2 Lung Disease. Int. J. Mol. Sci. 2021, 22, 8854.
- Hamam, H.J.; Palaniyar, N. Post-Translational Modifications in NETosis and NETs-Mediated Diseases. Biomolecules 2019, 9, 369.
- Liang, Y.; Pan, B.; Alam, H.B.; Deng, Q.; Wang, Y.; Chen, E.; Liu, B.; Tian, Y.; Williams, A.M.; Duan, X.; et al. Inhibition of peptidylarginine deiminase alleviates LPS-induced pulmonary dysfunction and improves survival in a mouse model of lethal endotoxemia. Eur. J. Pharmacol. 2018, 833, 432–440.
- Liu, S.; Yue, Y.; Pan, P.; Zhang, L.; Su, X.; Li, H.; Li, H.; Li, Y.; Dai, M.; Li, Q.; et al. IRF-1 Intervention in the Classical ROS-Dependent Release of NETs during LPS-Induced Acute Lung Injury in Mice. Inflammation 2018, 42, 387–403.
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77.
- Chen, C.-M.; Lu, H.-C.; Tung, Y.-T.; Chen, W. Antiplatelet Therapy for Acute Respiratory Distress Syndrome. Biomedicines 2020, 8, 230.
- Fuchs, T.A.; Brill, A.; Wagner, D.D. Neutrophil Extracellular Trap (NET) Impact on Deep Vein Thrombosis. Arter. Thromb. Vasc. Biol. 2012, 32, 1777–1783.
- Burkard, P.; Schonhart, C.; Vögtle, T.; Köhler, D.; Tang, L.; Johnson, D.; Hemmen, K.; Heinze, K.G.; Zarbock, A.; Hermanns, H.M.; et al. A key role for platelet GPVI in neutrophil recruitment, migration and NETosis in the early stages of acute lung injury. Blood 2023, 142, 1463–1477.
- Rossaint, J.; Herter, J.M.; Van Aken, H.; Napirei, M.; Döring, Y.; Weber, C.; Soehnlein, O.; Zarbock, A. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap–mediated sterile inflammation. Blood J. Am. Soc. Hematol. 2014, 123, 2573–2584.
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469.
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255, Erratum in Signal Transduct. Target. Ther. 2021, 6, 326.
- Montazersaheb, S.; Khatibi, S.M.H.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Sorbeni, F.G.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92.
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652.
- Veras, F.P.; Pontelli, M.C.; Silva, C.M.; Toller-Kawahisa, J.E.; de Lima, M.; Nascimento, D.C.; Schneider, A.H.; Caetité, D.; Tavares, L.A.; Paiva, I.M.; et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020, 217, e20201129.
- Ventura-Santana, E.; Ninan, J.R.; Snyder, C.M.; Okeke, E.B. Neutrophil Extracellular Traps, Sepsis and COVID-19—A Tripod Stand. Front. Immunol. 2022, 13, 902206.
- Blanch-Ruiz, M.A.; Ortega-Luna, R.; Gómez-García, G.; Martínez-Cuesta, M.Á.; Álvarez, Á. Role of Neutrophil Extracellular Traps in COVID-19 Progression: An Insight for Effective Treatment. Biomedicines 2021, 10, 31.
- Obermayer, A.; Jakob, L.-M.; Haslbauer, J.D.; Matter, M.S.; Tzankov, A.; Stoiber, W. Neutrophil Extracellular Traps in Fatal COVID-19-Associated Lung Injury. Dis. Markers 2021, 2021, 5566826.
- Rudd, J.M.; Pulavendran, S.; Ashar, H.K.; Ritchey, J.W.; Snider, T.A.; Malayer, J.R.; Marie, M.; Chow, V.T.K.; Narasaraju, T. Neutrophils Induce a Novel Chemokine Receptors Repertoire during Influenza Pneumonia. Front. Cell. Infect. Microbiol. 2019, 9, 108.
- Tomar, B.; Anders, H.-J.; Desai, J.; Mulay, S.R. Neutrophils and Neutrophil Extracellular Traps Drive Necroinflammation in COVID-19. Cells 2020, 9, 1383.
- Silva, B.M.; Gomes, G.F.; Veras, F.P.; Cambier, S.; Silva, G.V.; Quadros, A.U.; Caetité, D.B.; Nascimento, D.C.; Silva, C.M.; Silva, J.C.C.; et al. C5aR1 signaling triggers lung immunopathology in COVID-19 through neutrophil extracellular traps. J. Clin. Investig. 2023, 133, e163105.
- Liu, C.; Xi, L.; Liu, Y.; Mak, J.C.W.; Mao, S.; Wang, Z.; Zheng, Y. An Inhalable Hybrid Biomimetic Nanoplatform for Sequential Drug Release and Remodeling Lung Immune Homeostasis in Acute Lung Injury Treatment. ACS Nano 2023, 17, 11626–11644.
- McKenna, E.; Wubben, R.; Isaza-Correa, J.M.; Melo, A.M.; Mhaonaigh, A.U.; Conlon, N.; O’donnell, J.S.; Cheallaigh, C.N.; Hurley, T.; Stevenson, N.J.; et al. Neutrophils in COVID-19: Not Innocent Bystanders. Front. Immunol. 2022, 13, 864387.
- Panda, R.; Castanheira, F.V.; Schlechte, J.M.; Surewaard, B.G.; Shim, H.B.; Zucoloto, A.Z.; Slavikova, Z.; Yipp, B.G.; Kubes, P.; McDonald, B. A functionally distinct neutrophil landscape in severe COVID-19 reveals opportunities for adjunctive therapies. J. Clin. Investig. 2021, 7, e152291.
- Florence, J.M.; Krupa, A.; Booshehri, L.M.; Davis, S.A.; Matthay, M.A.; Kurdowska, A.K. Inhibiting Bruton’s tyrosine kinase rescues mice from lethal influenza-induced acute lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2018, 315, L52–L58.
- Pedersen, S.F.; Ho, Y.-C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205.
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999.
- Ouwendijk, W.J.D.; Raadsen, M.P.; A van Kampen, J.J.; Verdijk, R.M.; von der Thusen, J.H.; Guo, L.; Hoek, R.A.S.; van den Akker, J.P.C.; Endeman, H.; Langerak, T.; et al. High Levels of Neutrophil Extracellular Traps Persist in the Lower Respiratory Tract of Critically Ill Patients with Coronavirus Disease 2019. J. Infect. Dis. 2021, 223, 1512–1521.
- Arcanjo, A.; Logullo, J.; Menezes, C.C.B.; de Souza Carvalho Giangiarulo, T.C.; dos Reis, M.C.; de Castro, G.M.M.; Fontes, Y.d.S.; Todeschini, A.R.; Freire-De-Lima, L.; Decoté-Ricardo, D.; et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci. Rep. 2020, 10, 19630.
- Ng, H.; Havervall, S.; Rosell, A.; Aguilera, K.; Parv, K.; von Meijenfeldt, F.A.; Lisman, T.; Mackman, N.; Thålin, C.; Phillipson, M. Circulating Markers of Neutrophil Extracellular Traps Are of Prognostic Value in Patients with COVID-19. Arter. Thromb. Vasc. Biol. 2021, 41, 988–994, Erratum in Arter. Thromb. Vasc. Biol. 2021, 41, e384.
- Prével, R.; Dupont, A.; Labrouche-Colomer, S.; Garcia, G.; Dewitte, A.; Rauch, A.; Goutay, J.; Caplan, M.; Jozefowicz, E.; Lanoix, J.-P.; et al. Plasma Markers of Neutrophil Extracellular Trap Are Linked to Survival but Not to Pulmonary Embolism in COVID-19-Related ARDS Patients. Front. Immunol. 2022, 13, 851497.
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Gandet, F.F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098.
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179.
- Morris, G.; Bortolasci, C.C.; Puri, B.K.; Olive, L.; Marx, W.; O’Neil, A.; Athan, E.; Carvalho, A.; Maes, M.; Walder, K.; et al. Preventing the development of severe COVID-19 by modifying immunothrombosis. Life Sci. 2020, 264, 118617.
- Cesta, M.C.; Zippoli, M.; Marsiglia, C.; Gavioli, E.M.; Cremonesi, G.; Khan, A.; Mantelli, F.; Allegretti, M.; Balk, R. Neutrophil activation and neutrophil extracellular traps (NETs) in COVID-19 ARDS and immunothrombosis. Eur. J. Immunol. 2022, 53, e2250010.
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613.
- Yaqinuddin, A.; Kashir, J. Novel therapeutic targets for SARS-CoV-2-induced acute lung injury: Targeting a potential IL-1β/neutrophil extracellular traps feedback loop. Med. Hypotheses 2020, 143, 109906.
- Kaiser, R.; Leunig, A.; Pekayvaz, K.; Popp, O.; Joppich, M.; Polewka, V.; Escaig, R.; Anjum, A.; Hoffknecht, M.-L.; Gold, C.; et al. Self-sustaining IL-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19. J. Clin. Investig. 2021, 6, e150862.
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines Induced Neutrophil Extracellular Traps Formation: Implication for the Inflammatory Disease Condition. PLoS ONE 2012, 7, e48111.
- Weber, A.G.; Chau, A.S.; Egeblad, M.; Barnes, B.J.; Janowitz, T. Nebulized in-line endotracheal dornase alfa and albuterol administered to mechanically ventilated COVID-19 patients: A case series. Mol. Med. 2020, 26, 91.
- Holliday, Z.M.; Earhart, A.P.; Alnijoumi, M.M.; Krvavac, A.; Allen, L.-A.H.; Schrum, A.G. Non-Randomized Trial of Dornase Alfa for Acute Respiratory Distress Syndrome Secondary to COVID-19. Front. Immunol. 2021, 12, 714833.
- Clemente-Moragón, A.; Martínez-Milla, J.; Oliver, E.; Santos, A.; Flandes, J.; Fernández, I.; Rodríguez-González, L.; del Castillo, C.S.; Ioan, A.-M.; López-Álvarez, M.; et al. Metoprolol in Critically Ill Patients With COVID-19. J. Am. Coll. Cardiol. 2021, 78, 1001–1011.
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287.
- Panka, B.A.; de Grooth, H.-J.; Spoelstra-de Man, A.M.E.; Looney, M.R.; Tuinman, P.-R. Prevention or Treatment of Ards with Aspirin: A Review of Preclinical Models and Meta-Analysis of Clinical Studies. Shock 2017, 47, 13–21.
- Lasky, J.A.; Fuloria, J.; Morrison, M.E.; Lanier, R.; Naderer, O.; Brundage, T.; Melemed, A. Design and Rationale of a Randomized, Double-Blind, Placebo-Controlled, Phase 2/3 Study Evaluating Dociparstat in Acute Lung Injury Associated with Severe COVID-19. Adv. Ther. 2020, 38, 782–791.
- Du, M.; Yang, L.; Gu, J.; Wu, J.; Ma, Y.; Wang, T. Inhibition of Peptidyl Arginine Deiminase-4 Prevents Renal Ischemia-Reperfusion-Induced Remote Lung Injury. Mediat. Inflamm. 2020, 2020, 1724206.
- Yi, T.; Ding, W.; Hao, Y.; Cen, L.; Li, J.; Shi, X.; Wang, T.; Chen, D.; Zhu, H. Neutrophil extracellular traps mediate severe lung injury induced by influenza A virus H1N1 in mice coinfected with Staphylococcus aureus. Microb. Pathog. 2022, 166, 105558.
- Okeke, E.B.; Louttit, C.; Fry, C.; Najafabadi, A.H.; Han, K.; Nemzek, J.; Moon, J.J. Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials 2020, 238, 119836.
- Gan, T.; Yang, Y.; Hu, F.; Chen, X.; Zhou, J.; Li, Y.; Xu, Y.; Wang, H.; Chen, Y.; Zhang, M. TLR3 Regulated Poly I:C-Induced Neutrophil Extracellular Traps and Acute Lung Injury Partly through p38 MAP Kinase. Front. Microbiol. 2018, 9, 3174.
- Zhu, C.-L.; Xie, J.; Zhao, Z.-Z.; Li, P.; Liu, Q.; Guo, Y.; Meng, Y.; Wan, X.-J.; Bian, J.-J.; Deng, X.-M.; et al. PD-L1 maintains neutrophil extracellular traps release by inhibiting neutrophil autophagy in endotoxin-induced lung injury. Front. Immunol. 2022, 13, 949217, Erratum in Front. Immunol. 2022, 13, 1038083.
- Wu, Z.; Zhang, L.; Zhao, X.; Li, Z.; Lu, H.; Bu, C.; Wang, R.; Wang, X.; Cai, T.; Wu, D. Protectin D1 protects against lipopolysaccharide-induced acute lung injury through inhibition of neutrophil infiltration and the formation of neutrophil extracellular traps in lung tissue. Exp. Ther. Med. 2021, 22, 1074.
- Kono, M.; Matsuhiroya, S.; Obuchi, A.; Takahashi, T.; Imoto, S.; Kawano, S.; Saigo, K. Deferasirox, an iron-chelating agent, alleviates acute lung inflammation by inhibiting neutrophil activation and extracellular trap formation. J. Int. Med. Res. 2020, 48, 300060520951015.
- Pedrazza, L.; Cunha, A.A.; Luft, C.; Nunes, N.K.; Schimitz, F.; Gassen, R.B.; Breda, R.V.; Donadio, M.V.F.; de Souza Wyse, A.T.; Pitrez, P.M.C.; et al. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. J. Cell. Physiol. 2017, 232, 3552–3564.
- Kasetty, G.; Papareddy, P.; Bhongir, R.K.V.; Ali, M.N.; Mori, M.; Wygrecka, M.; Erjefält, J.S.; Hultgårdh-Nilsson, A.; Palmberg, L.; Herwald, H.; et al. Osteopontin protects against lung injury caused by extracellular histones. Mucosal Immunol. 2019, 12, 39–50.
- Dupuis, J.; Sirois, M.G.; Rhéaume, E.; Nguyen, Q.T.; Clavet-Lanthier, M.-É.; Brand, G.; Mihalache-Avram, T.; Théberge-Julien, G.; Charpentier, D.; Rhainds, D.; et al. Colchicine reduces lung injury in experimental acute respiratory distress syndrome. PLoS ONE 2020, 15, e0242318.
- Adrover, J.M.; Carrau, L.; Daßler-Plenker, J.; Bram, Y.; Chandar, V.; Houghton, S.; Redmond, D.; Merrill, J.R.; Shevik, M.; Tenoever, B.R.; et al. Disulfiram inhibits neutrophil extracellular trap formation and protects rodents from acute lung injury and SARS-CoV-2 infection. J. Clin. Investig. 2022, 7, e157342.
- Antunes, G.L.; Matzenbacher, L.S.; Costa, B.P.; Basso, B.d.S.; Levorse, V.G.S.; Antunes, K.H.; Costa-Ferro, Z.S.M.; de Oliveira, J.R. Methoxyeugenol Protects Against Lung Inflammation and Suppresses Neutrophil Extracellular Trap Formation in an LPS-Induced Acute Lung Injury Model. Inflammation 2022, 45, 1534–1547.
- Sung, P.-S.; Peng, Y.-C.; Yang, S.-P.; Chiu, C.-H.; Hsieh, S.-L. CLEC5A is critical in Pseudomonas aeruginosa-induced NET formation and acute lung injury. J. Clin. Investig. 2022, 7, e156613.
- Baron, S.; Rashal, T.; Vaisman, D.; Elhasid, R.; Shukrun, R. Selinexor, a selective inhibitor of nuclear export, inhibits human neutrophil extracellular trap formation in vitro. Front. Pharmacol. 2022, 13, 1030991.
- Zhang, L.; Lu, J.; Wu, Z. Auricular Vagus Nerve Stimulation Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting Neutrophil Infiltration and Neutrophil Extracellular Traps Formation. Shock 2021, 57, 427–434.
- Qiao, X.; Kashiouris, M.G.; L’heureux, M.; Fisher, B.J.; Leichtle, S.W.; Truwit, J.D.; Nanchal, R.; Hite, R.D.; Morris, P.E.; Martin, G.S.; et al. Biological Effects of Intravenous Vitamin C on Neutrophil Extracellular Traps and the Endothelial Glycocalyx in Patients with Sepsis-Induced ARDS. Nutrients 2022, 14, 4415.




