3. The Mechanism of NETs影响 Influencing ARDS的机制
3.1. 组蛋白Histones
组蛋白通常位于细胞核中,是染色质的成分,可结合并调节Histones are usually located in the nucleus and are components of chromatin that bind and regulate the expression of DNA
的表达;同时,组蛋白是NETs的重要组成部分,占所有NET相关蛋白的70%,具有宿主防御功能,可促进炎症反应; At the same time, histones are an important component of NETs, accounting for 70% of all NET-related proteins, which have host defense functions and promote inflammatory response[
39,40]
。肽基精氨酸脱亚胺酶. Peptidyl arginine deiminase 4
(PAD4)介导的组蛋白精氨酸催化转化为瓜氨酸可改变组蛋白分子之间的相互作用,降低组蛋白的稳定性,促进异染色质的解缩,是NETs形成的必要条件 (PAD4) mediated catalytic conversion of histone arginine to citrulline can change the interaction between histone molecules, reduce histone stability, promote heterochromatin decompression, It is a necessary condition for the formation of NETs[41,42,43,44,45].
PAD4抑制剂治疗或PAD4基因缺陷导致的NET减少和肺损伤也证明了PAD4的关键作用 Reduced NET and lung damage caused by PAD4 inhibitor treatment or PAD4 gene defects have also demonstrated the key role of PAD4 [
36,43,46,47]
。然而,其他研究表明,. However, other studies have shown that NETs
在PAD4失活的情况下释放 are released in the presence of PAD4 inactivation[
48,49,50]
。.
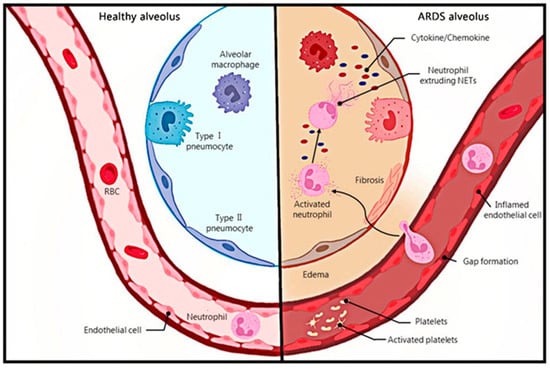
Histones can cause endothelial cell damage, alveolar bleeding, and thrombosis [
29,
52,
53]. Axelle Caudrillier et al. found that NETs were present in large numbers in TRALI patients and mouse models, and that NETs damaged endothelial cells, increasing their permeability, while histone-blocking antibodies were protective against pulmonary endothelial injury in TRALI [
29]. The primary mechanism of histone cytotoxicity to endothelial cells is calcium influx due to plasma membrane disruption, which has been reported by Simon T. Abrams et al. [
53]. The pathological examination in the experiments by Abrams et al. showed that extracellular histones can cause intra-alveolar hemorrhages and microvascular thrombosis, which is also reflected in a study by Jun Xu and colleagues [
52,
53].
3.2. NE
Neutrophil elastin (NE) is the highest non-histone protein in NETs [
39]. As a class of serine proteases, NE is capable of directly degrading virulence factors and participating in the proteolytic process of chemokines, cytokines, and receptors, and is the major source of proteolytic activity of NETs [
57]. Just like histones, NE is also associated with the endothelial cytotoxicity of NETs [
37,
58]. NE mediates neutrophil-induced tissue damage and effectively degrades the extracellular matrix [
59]. NE also degrades the endothelial cytoskeleton by affecting the function of E-cadherin and VE-cadherin, thereby undermining the integrity of the alveolar–capillary barrier [
60,
61].
3.3. DNA
DNA is the major structural component of NETs, and transient treatment with deoxyribonuclease (DNase) can cause the disintegration of NETs, indicating that DNA maintains the integrity of NETs [
8]. In addition to this, the DNA in NETs has been shown to be associated with coagulation. NETs provide a scaffold for platelet binding and stimulate platelet aggregation. Interactions between NETs and platelets are regulated in a number of ways, including the direct interaction of platelets with the DNA in NETs [
64].
3.4. MPO
Myeloperoxidase (MPO), another component of NETs, is also a cause of NET-mediated cytotoxicity [
8,
37,
38,
39]. MPO converts hydrogen peroxide into hypochlorous acid and produces ROS, which can help kill bacteria or cause tissue damage [
38,
74]. One study showed that MPO released after secondary necrosis of neutrophils induced DNA strand breaks in lung epithelial cells, resulting in lung epithelial cell damage [
8].
In conclusion, NETs damage lung tissue in various forms through their components and promote the occurrence and development of ARDS. Histone, NE, and MPO can damage lung endothelial cells and epithelial cells, and destroy the barrier. Histones and DNA also promote coagulation and mediate the formation of immune thrombus interacting with inflammatory responses, becoming key events in the development of ARDS [
60]. In addition, DNA can activate STING-IFN, TLR9, NLRP3, and other pathways, playing a pro-inflammatory role. Other studies have shown that LPs-mediated NETs can induce the pro-death of macrophages and thus regulate the inflammation of ARDS [
33]. NETs mediated by free fatty acids can induce ALI by mediating dendritic cell activation and T cell differentiation [
76]. NETs play a key role in sepsis-associated lung injury by inducing the m6A modification of GPX4 through the up-regulation of methyltransferase-like 3 and subsequently inducing ferroptosis in alveolar epithelial cells [
24].
4. The Mechanism of NETs Production in ARDS and the Substances That Affect the Production
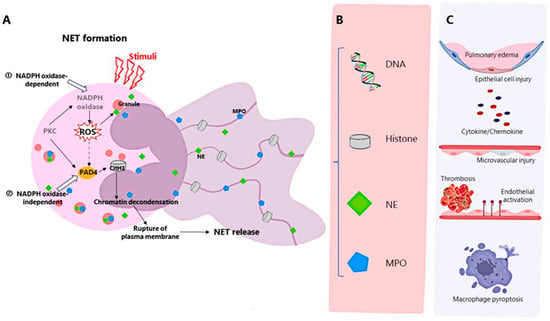
NETosis can be divided into NADPH oxidase 2 (NOX)-dependent and independent NETosis [
9]. PKC can activate NOX through the Raf-MEK-ERK signal transduction pathway to produce reactive oxygen species (ROS) [
28,
77]. ROS triggers the separation, release, and migration of neutrophil elastase (NE) and myeloperoxidase (MPO) to the neutrophil nucleus. NE triggers histone degradation, and MPO collaborates with NE to promote chromatin decondensation. Activated peptidylarginine deiminase 4 (PAD 4)-mediated histone citrullination leads to chromatin decondensation [
41,
77]. The process of the formation and release of NETs includes nuclear and granule membrane dissolution, chromatin decondensation, and plasma membrane breakdown [
9]. However, there is also NETosis in which neutrophils remain intact, and calcium-mediated and platelet-stimulated Toll-like receptor 4 (TLR4)-mediated NETosis have also been demonstrated to be ROS-independent [
77].
PAD4 is essential for mediating NET formation [
41], and PAD4 activated by PKC or the influx of Ca
2+ is capable of citrullinating histones [
9,
78], so it is possible to regulate the production of NETs by regulating PAD4. A study in a mouse model of LPS-induced endotoxin reduced NET formation by treatment with the PAD2/PAD4 inhibitor YW356, thereby attenuating acute lung injury in mice [
79]. PAD4 inhibitors also prevent NETosis in ALI caused by skin chemical burns or intestinal ischemia/reperfusion [
35,
36].
Existing studies have shown that ROS plays an important role in the formation of NETs. LPS activates platelets and then promotes NET release through ROS-dependent classical NETosis or ROS-independent early/rapid NETosis, in which the classical pathway plays a dominant role and is regulated by IRF-1 [
84]. Free fatty acids (FFA) have also been shown to activate the NOX pathway to promote ROS production and thus induce NETs production by neutrophils, while FFA can also induce NET formation via the p38 and JNK pathways [
36]. The Raf-MEK-ERK pathway, which activates NADPH oxidase, is also involved in the formation of NETs through the up-regulation of antiapoptotic proteins [
30].
Platelets are also associated with the formation of NETs [
60,
86]. Activated platelets induce neutrophil-forming NETs [
87]. A recent study demonstrated that platelet-specific receptor glycoprotein (GP)VI, which mediates deleterious platelet activation, is required for NETosis in an LPS-induced ALI model, and is located early in thromboinflammation-driven ARDS/ALI [
88]. Targeting platelet activation reduces the production of NETs in the TRALI model [
29], and Jan Rossaint et al. demonstrated the platelet-dependent formation of NETs during VILI [
31]. The mechanism of platelet-induced NETs is through TLR4. TLR4 on platelets can recognize and bind the TLR4 ligands of neutrophils, enabling neutrophils to be activated and release NETs [
89].
5. Relationship between the Inflammatory Storm and NETs
Cytokine storms are a syndrome involving the overproduction of inflammatory cytokines and overactivation of immune cells, covering various events that may eventually lead to multiple organ failure and death, such as acute respiratory distress syndrome (ARDS) [
96,
97]. The link between cytokine storms and ARDS has been established, and NETs also play a role. What is more, the formation of NETs can induce macrophages to secrete IL-1β, which can further induce the formation of NETs [
98]. Several studies have suggested that cytokines are one of the triggers of NETosis [
99,
100], and cytokine storms can induce the production of NETs [
101,
102]. Proinflammatory cytokines, including IFN-γ, TNF-α, and GM-CSF, can regulate the expression of chemokine receptors (CRs), and CRs induced in vitro can regulate the release of NETs from lung-recruited neutrophils [
103], which may be one of the pathways by which cytokines regulate the formation of NETs. At the same time, NETs can also trigger cytokine storm formation [
77]. The formation of NETs can induce the production of pro-inflammatory cytokines, and NETs can amplify inflammation by promoting the production of cytokines/chemokines, thus causing cytokine storms [
104,
105].
6. Correlation between Immune Balance and Regulation in ARDS and NETs
In ALI, NETs can promote the activation and transformation of lung macrophages into pro-inflammatory M1 types, while M1 type macrophages can promote the infiltration of neutrophils in the lungs and exacerbate the production of NETs. The two cooperate to cause the disorder of immune cells and aggravate tissue injury [
107]. During influenza virus infections, the recruitment and activation of innate immune cells such as neutrophils and macrophages are out of control, and a large number of neutrophils accumulate and release NETs in the lungs, causing damage and leading to ARDS [
103]. Similarly, the ratio of neutrophils to lymphocytes increased in patients with severe COVID-19, and the neutrophil subsets of patients with severe COVID-19 were more active in producing NETs, which would aggravate the inflammation of ARDS [
108,
109]. In line with this, multiple studies have shown that immunomodulatory therapy is effective against COVID-19 [
105], and targeting innate immune-related factor Bruton’s tyrosine kinase can reduce centrocyte infiltration and NET secretion, and alleviate lung injury [
110]. These findings once again confirm the contribution of inflammation and immune disorders in the formation and development of ARDS, in which NETs are an important factor.
7. Production and Role of NETs in COVID-19 ARDS
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global pandemic infectious disease that can affect multiple systems, mainly the respiratory system. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are its most severe pulmonary manifestations [
111]. Elevated levels of NETs have been found in patients with COVID-19 ARDS [
109]. Serum from patients with COVID-19 triggered NET release from control neutrocytes in vitro, and NETs released from SARS-CoV-2-activated neutrocytes promoted lung epithelial cell death in vitro, suggesting that NETs may mediate SARS-CoV-2-induced ARDS [
99,
112,
113]. Angiotensin-converting enzyme 2, serine protease, viral replication, PAD4, and ROS were found to be associated with the activation of neutrophil-releasing NETs by SARS-CoV-2 [
99,
114]. Meanwhile, NETs can be used as a prognostic indicator for COVID-19. Multiple clinical studies have shown that circulating NET levels are associated with disease severity and affect clinical outcomes in patients with COVID-19, while NET levels are not associated with thrombosis development in patients, even though an association between NET formation and immunothrombosis is a well-established fact [
113,
115,
116]. Similar to lung injury caused by other factors, NETs and their components contribute to the development of COVID-19 ARDS by activating downstream inflammatory pathways, damaging endothelial and pulmonary epithelial cells, and disrupting barrier function [
77]. There is a higher rate of venous thromboembolism in severe COVID-19 ARDS than in other forms of ARDS [
117]. NETs interact with platelets to cause a thrombose-inflammatory cascade, forming an immune thrombus that promotes lung damage in COVID-19, and NETs released from recruited neutrophils against SARS-CoV-2 do more harm than good in the lungs of patients with COVID-19 [
118]. Neutrophils, which form NETs, also participate in the cytokine storm of COVID-19 [
119,
120]. The formation of NETs can lead to the production of excessive cytokines and chemokines, such as IL1β, IL6, IL8, IL10, TNF-α, and IFN-γ, which may trigger a cytokine storm leading to ALI, ARDS, and death [
98,
104,
121]. At the same time, IL1β and IL8 also mediate the production of NETs, and thus may lead to uncontrolled progressive inflammation during cytokine storms [
122,
123,
124]. The release of a large number of proinflammatory cytokines and chemokines can amplify the coagulation response and promote the formation of immune thrombosis [
120].
8. Clinical Research
At present, most clinical trials have been conducted on COVID-19 ARDS. Dornase alfa can act as a mucolytic agent and reduce NET levels in the lungs, thereby improving oxygenation and ventilation [
126]. Andrew G. Weber et al. applied nebulized dornase alfa combined with salbutamol in five patients with ARDS who required mechanical ventilation due to SARS-CoV-2. After 7 days of treatment, FiO2 demand decreased, the condition improved in all five patients, and there were no deaths. The therapeutic effect of dornase alfa on inhibiting NETs and improving lung function was confirmed [
126]. Similarly, the non-randomized trial of dornase alfa by Zachary M. Holliday’s group in the treatment of COVID-19 secondary ARDS also demonstrated the benefits of inhaled α-dornase, although the positive effect was limited to the time of administration [
127]. In addition, the results of a clinical trial involving 20 patients with ARDS from COVID-19 treated with invasive mechanical ventilation suggest that metoprolol can also reduce neutrophil extracellular trap content and other markers of lung inflammation, reduce aggravated lung inflammation, and improve oxygenation without adverse effects, and is therefore a possible treatment strategy [
128].
9. Treatment
Treatments targeting NETs have been validated by several studies and are considered to be a feasible treatment for ARDS, capable of alleviating pulmonary symptoms. Therapies targeting NETs include degrading already-formed NETs and inhibiting the formation of NETs. The most common treatment for NET degradation is DNase Ⅰ, which degrades DNA components in NETs, thereby reducing the deposition of NETs, and is currently approved for clinical use without toxicity [
131].
Since PAD4 is the key to the formation of NETs, the inhibition of PAD4 can reduce histone citrullination and block NETosis [
130,
134]. PAD4 inhibitors Cl-amidine and GSK484, and selective inhibitors such as streptomycin, can reduce the levels of citrulline histone H3 (CitH3) and inflammatory factors, and reduce the formation of NETs, thereby reducing lung injury [
9,
135]. Treatment with the PAD2/PAD4 inhibitor YW356 has also been shown to reduce PAD activation and alleviate LPS-induced acute lung injury [
79].
As mentioned above, NE plays an important role in the formation of NETs, so inhibiting NE is another way to reduce the production of NETs. Using NE inhibitors can reduce the severity of lung injury [
26,
31]. The selective NE inhibitor GW311616A and the nanoparticle-mediated small molecule NE inhibitor Sivelestat have been shown to effectively inhibit the formation of NETs [
9,
62].
The inhibitors of some signaling pathways can also affect the formation of NETs; for example, inhibitors of p38 MAPK kinase can reduce NETs in ALI [
95], and high-mobility histone B1 (HMGB1) can further drive NETs, so blocking it can prevent NET formation [
36]. The hypoglycemic agent metformin can also reduce NETosis and lung inflammation by specifically inhibiting HMGB1, activating AMPK, and inhibiting the mTOR pathway [
9,
16]. Anti-PD-L1 antibodies affect the release levels of NETs by regulating neutrophil autophagy via inhibiting the PI3K/Akt/mTOR pathway [
94].
Other therapies that target NETs, including the use of Protectin D1 (PD1) [
139], iron-chelating agent Deferasirox [
140], mesenchymal stem cells (MSCs) [
141], osteopontin (OPN) [
142], colchicine [
143], disulfiram [
144], Methoxyeugenol [
145], anti-CLEC5A [
146], Selinexor [
147], etc., can inhibit or reduce the production of NETs. At the same time, the stimulation of the ear vagus nerve [
148] and intravenous vitamin C [
129] also block NETosis.
11. Conclusions
In the process of ARDS, neutrophils accumulate in and infiltrate the lungs to generate NETosis, and the NETs produced destroy the lung endothelial cell barrier, causing tissue damage, forming immune thrombosis, and aggravating the condition of ARDS. The production of NETs and the release of contents that affect the surrounding tissues constantly stimulate each other, forming a vicious circle, leading to the continuous formation of NETs. At the same time, NETs can also cause cytokine storms and affect the body’s immune balance, further promoting the development of the disease. However, although there has been much research on the formation of NETs in ARDS, the mechanism affecting disease progression, and the corresponding treatment methods, specifically exploration and interpretation, still need further study.