Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tao Wang | -- | 4364 | 2024-01-29 08:15:38 | | | |
| 2 | Tao Wang | -704 word(s) | 3660 | 2024-01-29 09:13:47 | | | | |
| 3 | Hideki Kanda | Meta information modification | 3660 | 2024-01-29 12:31:32 | | | | |
| 4 | Hideki Kanda | Meta information modification | 3660 | 2024-01-29 12:35:14 | | | | |
| 5 | Hideki Kanda | Meta information modification | 3660 | 2024-01-29 12:35:49 | | | | |
| 6 | Hideki Kanda | Meta information modification | 3660 | 2024-01-29 12:36:56 | | | | |
| 7 | Lindsay Dong | + 12 word(s) | 3672 | 2024-01-30 02:19:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, T.; Zhu, L.; Mei, L.; Kanda, H. Liquefied Dimethyl Ether Extraction Technology for Microalgae. Encyclopedia. Available online: https://encyclopedia.pub/entry/54463 (accessed on 07 February 2026).
Wang T, Zhu L, Mei L, Kanda H. Liquefied Dimethyl Ether Extraction Technology for Microalgae. Encyclopedia. Available at: https://encyclopedia.pub/entry/54463. Accessed February 07, 2026.
Wang, Tao, Li Zhu, Li Mei, Hideki Kanda. "Liquefied Dimethyl Ether Extraction Technology for Microalgae" Encyclopedia, https://encyclopedia.pub/entry/54463 (accessed February 07, 2026).
Wang, T., Zhu, L., Mei, L., & Kanda, H. (2024, January 29). Liquefied Dimethyl Ether Extraction Technology for Microalgae. In Encyclopedia. https://encyclopedia.pub/entry/54463
Wang, Tao, et al. "Liquefied Dimethyl Ether Extraction Technology for Microalgae." Encyclopedia. Web. 29 January, 2024.
Copy Citation
Microalgae are a sustainable source for the production of biofuels and bioactive compounds. Dimethyl ether (DME), which is characterized by its low boiling point and safety as an organic solvent, exhibits remarkable properties that enable high extraction rates of various active compounds, including lipids and bioactive compounds, from high-water-content microalgae without the need for drying.
microalgae
green solvent
dimethyl ether
extraction
natural products
1. Introduction
Plants contain a wide variety of naturally occurring organic compounds that are produced and metabolized in their bodies. These compounds include waxes, terpenoids, lipids, phenolic compounds, polar glucosides, alkaloids, sugars, peptides, and various other substances [1]. The most important physiologically active plant compounds include phenolic compounds (including flavonoids), saponins, and cyclins, which play key roles as dietary supplements [2]. Natural phytonutrients are widely distributed and contain a diverse range of compounds with low to high molecular weights [3][4][5]. Compounds with strong physiological activities against living organisms have attracted considerable attention from researchers, leading to the exploration of new natural products and structural modifications, particularly in fields such as medicine, pharmaceuticals, and nutraceuticals [3][4][5][6][7][8].
Organic compounds obtained from nature serve as dietary supplements that help improve health, delay aging, prevent chronic disease, prolong life, and support the structure and function of the body [9][10][11]. The market for dietary supplements was estimated to be approximately USD 353 billion in 2019 and it is growing steadily [12].
Substances such as antibiotics, chemical preservatives, and alkaloids have been used in the formulation and extraction of bioactive compounds in various food industries, including sugarcane [13], tea [14], coffee [15], and plant extracts [16][17]. The preparation of natural material samples involves several critical steps: The initial phase includes preliminary washing of plant materials, drying or lyophilization, and grinding for homogenization. The next steps include extraction and qualitative/quantitative analyses [18]. The production of natural materials is expensive and has the drawback of reduced nutrient concentrations in the raw material itself; this poses a significant obstacle for the natural materials industry [19][20]. Consequently, several natural ingredients require solvent-based extraction and purification to produce dietary supplements [21][22].
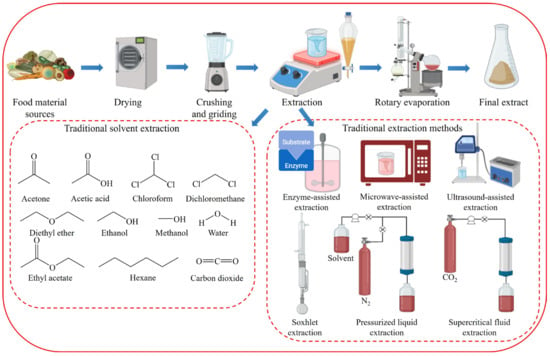
2. Disadvantages of Conventional Extraction Techniques
2.1. Disadvantages of Traditional Solvent Extraction
Conventional extraction methods for natural organic compounds have historically relied on various solid–liquid extraction techniques using organic solvents, such as the Soxhlet extraction method, immersion method, and steam distillation [23]. Commonly used solvents in these methods include acetone, acetic acid, chloroform, dichloromethane, diethyl ether, ethanol, and hexane. The quantitative and qualitative performance of the extraction depends heavily on the polarity of the solvent used. This necessitates the selection of a solvent appropriate for the polarity of the target component, without a predefined method or solvent [24]. Despite their simplicity, conventional extraction methods suffer from low selectivity, low recovery and extraction rates, labor intensiveness, time consumption, and the use of large amounts of often toxic organic solvents, leading to potential trace residues in the extracts [25].
Traditionally, organic solvents such as chloroform, hexane, methanol, and dichloromethane have been used to extract lipids and other functional compounds [26][27] (Figure 1). However, owing to significant waste generation and risks to the environment and human health, the demand for sustainable, nontoxic extraction methods has increased. Conventional extraction processes require dried algal starting materials and consume considerable time and energy [28]. For lipid extraction from microalgae, 90% of process energy consumption is attributed to lipid extraction [29]. Wet extraction, which accounts for 70% of total energy consumption, appears to be promising.

Figure 1. Conventional organic solvent extraction of phytochemicals.
Ethanol is a low molecular weight organic compound that exhibits both hydrophilic and lipophilic properties, making it suitable for the extraction of bioactive compounds such as phenolic acids, flavonoids, and phenolic acid esters [30]. However, the use of ethanol in food processing is prohibited in certain regions and cultures, necessitating the use of alternative solvents. When ethanol is used to extract highly aqueous samples, the addition of benzene to the ethanol–water mixture is required to increase water volatility and prevent water contamination via co-boiling, thereby preserving the non-polar components [31]. Considering the effect of ethanol on human health, dietary supplements should either avoid its use or maintain its concentration as low as possible [32][33].
Supercritical carbon dioxide (scCO2) exhibits an intermediate state between gas and liquid when maintained at high temperatures and pressures above the critical point (31.3 °C, 7.38 MPa) [34]. The use of scCO2 remarkably improves the extraction efficiency of functional components, enabling selective extraction via temperature or pressure control [35][36][37]. The extraction of functional components using scCO2 has been applied to various natural food sources, such as essential oils [38], γ-oryzanol [39], chamomile seed oil [40], and hops [41], as well as for caffeine removal [42] and oil extraction from microalgae [43][44][45]. However, the extraction of functional components from highly aqueous samples using supercritical methods is challenging [46]. This is because of the non-polar nature of scCO2, which often requires the addition of entrainer solvents such as methanol [47][48], ethanol [49], and acetone [50] to facilitate extraction [51].
2.2. Disadvantages of Traditional Extraction Methods
Pre-drying is essential for moisture-containing natural products because moisture content inhibits solvent extraction [52]. For example, coffee hulls have a high moisture content, ranging from 18% to 80% w/w, which includes bound water trapped in the fine structure of solid particles. Therefore, most solvent extraction techniques require dried samples [53]. Historically, drying natural materials under natural conditions has been the primary method of preservation. However, this method is now being abandoned owing to its lengthy process and the inability to adjust the drying parameters.
The production of instant coffee requires high thermal energy (21.10 and 8.50 MJ/kg product) for spray drying and extraction, accounting for three-quarters of the total process [54]. Spray drying is the most commonly used method for this purpose and requires 10–20 times more energy per kilogram of evaporated water than drying using an evaporator. To reduce energy consumption, researchers use evaporators to preconcentrate coffee samples before drying [55]. Heat-sensitive substances require extraction at room temperature or cold solvent removal, such as via lyophilization. High extraction temperatures result in solvent loss and component degradation. Anthocyanins, which are valuable, colored bioactive compounds, are increasingly extracted worldwide, but their functionality is limited by their decomposition at 50–60 °C, indicating limited temperature stability [56].
The extraction of natural compounds requires a series of complex operations, such as sample drying, pulverization, extraction, and solvent removal [57] (Figure 1). The extraction process begins with solvent selection and involves the use of extraction techniques with higher extraction rates [58]. Traditional extraction techniques such as maceration, Soxhlet extraction, and decoction have significant drawbacks, including long extraction times, poor selectivity, expensive solvents, and the need for significant solvent evaporation [59].
Maceration involves coarsely grinding the raw material, placing it in a container, pouring the solvent to completely cover the material, and extracting it while stirring until the soluble substances dissolve [60]. However, this method typically takes several days to weeks due to its long soaking time [61].
Soxhlet extraction, a model extraction technique traditionally used to extract compounds, particularly lipids, from solid or semi-solid matrices, has several drawbacks, such as long extraction times (12–24 h), high solvent volumes, high energy consumption, and issues regarding selectivity and efficiency [62][63][64].
Decoction is used to extract thermally stable bioactive compounds by boiling the raw materials in water [65]. However, this limits the extraction of water-soluble components, increases the solvent-to-solid ratio, and introduces numerous water-soluble impurities into the extract [66].
3. Advantages of Liquefied DME as an Extractant
3.1. Physical Properties of DME
DME is a simple ether with the chemical formula CH3–O–CH3 and lacks a direct C–C bond. DME has a high oxygen content (34.8%) and low carbon-to-hydrogen ratio (C:H) [67]. The two methyl groups in DME form two polarized bonds oriented at an angle of 111.8 ± 0.2°, resulting in a bent V-shaped molecular geometry around the central oxygen atom [68]. DME contains two types of bonds (C–O and C–H). Although there is a 0.4-unit electronegativity difference between C–H bonds, which results in weak polarity, C–O bonds have a 1-unit electronegativity difference, indicating a higher polarity [69]. Because of the uneven distribution of charged electron clouds throughout the molecule, DME exhibits a dipole moment of 1.3 D, making it a polar substance [70].
DME is a gas under standard conditions and has a boiling point of −24.8 °C [71]. This gaseous state results in minimal residue in the extracted materials [72]. Although denser than dry air, DME exists as a vapor at 0.1 MPa and 25 °C. Moreover, it transitions from the vapor to the liquid phase above a saturated vapor pressure of 0.59 MPa at 25 °C [73][74]. The density of liquid DME at 25 °C is 668 kg/m3 [75].
The dielectric constant (ε) of liquid DME at 30.5 °C and 6.3 MPa is 5.34 [76]. In comparison, the dielectric constant (ε) of water (30 °C, 25 MPa) is 80 [77]. This suggests that the polarity of DME is suitable for dissolving non-polar to moderately polar substances [78]. DME can bind to both polar and non-polar compounds via the oxygen atom at its molecular center. It forms hydrogen bonds with the hydrogen atoms of other molecules, thereby increasing extractability [79][80].
3.2. Cell Destruction and Drying-Free Extraction Techniques
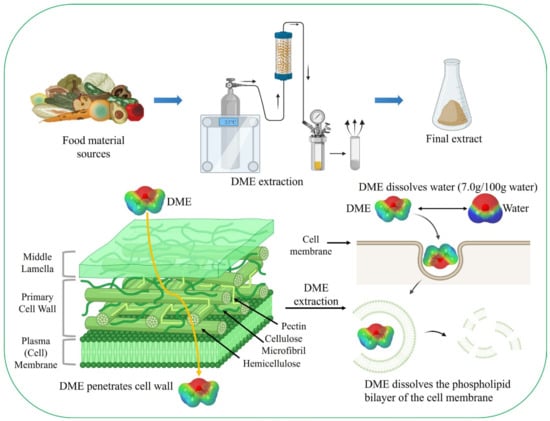
Plant cells are surrounded by a cell wall, which is mainly composed of cellulose. For example, microalgae such as Chlorella species possess a robust cell wall approximately 88 nm in thickness [81]. Consequently, the extraction of active components, such as lipids, from cells requires long processing times, hydrophobic extraction solvents, and energy-intensive mechanical disruption methods [82]. Because phospholipids in cell membranes are amphiphilic molecules, they require a mixture of polar and non-polar organic solvents for extraction. Liquid DME is well-known for extracting neutral and complex lipids from dairy products [83]. Liquid DME is used to extract compounds from various wet and dry biomasses containing lipid-rich compounds without cell disruption [84][85][86][87][88] (Figure 2).

Figure 2. Extraction process using liquefied DME; liquefied DME dissolves the phospholipid bilayer of the cell membrane and water.
3.3. Safety of Liquefied DME as an Extraction Solvent
DME, a recognized organic solvent with a low boiling point that is safe for human use, has significant potential for the extraction of various active ingredients. It has been approved for use as an extraction solvent in the food industries of the United States, Europe, and other regions. The European Food Safety Authority (EFSA), in its assessment of the safety of DME as an extraction solvent for the removal of fats from animal protein sources, stated that residual levels of up to 9 μg/kg in extracted animal protein do not pose a significant safety concern [89]. Food Standards Australia New Zealand (FSANZ) approved the use of DME as an extraction solvent processing aid for all dairy ingredients and products [90].
In experiments with rats exposed to DME, DME residues in the bodies of the rats were in the range of 14–19 ppm when the airborne concentration of DME reached 1000 ppm. These concentrations were equivalent to 1/61 of the airborne concentrations accumulated in the body. After inhalation for 60 min, the rats’ various organs showed DME concentrations that decreased to less than 4 ppm within 90 min [91][92].
3.4. Environmental Issues Caused by Liquefied DME Extraction
The synthesis of DME from renewable sources, utilizing biomass-derived CO2 and hydrogen generated via water electrolysis (powered by solar or wind energy), enables the production of DME from renewable feedstocks [93]. The lack of explosive peroxide formation in DME allows safe storage [94]. As it does not form peroxide aerosols, DME has attracted considerable attention as a propellant for household hairsprays [95]. Generally considered biodegradable, nontoxic, non-carcinogenic, and non-corrosive, DME has proven to be ideal for various everyday applications such as personal care products (hairsprays, shaving creams, foams, and antiperspirants), household products, paints, coatings, food, insect repellents, and animal products [96][97]. DME then undergoes photochemical reactions with OH radicals to produce CO2 and H2O [98]. Experimental modeling under ultraviolet radiation indicates that DME has a degradation half-life of 3–30 h, reaching approximately 100–150 h in the upper atmospheric regions up to an altitude of approximately 10 km. Although freon compounds may take several years or decades to degrade, DME degradation occurs in approximately 0.014 years (5.1 days) [98].
DME has similar physical properties to LPG and has been developed as a synthetic fuel. In China, it is used commercially as a substitute for LPG in city gas, often blended with 20% propane for consumer use [99]. It is also used as a fuel in automotive and industrial applications. However, pure DME has an explosive range of 3.427 vol% in air, which is a significant safety concern when used as an extraction solvent [99]. To address this issue, the blending of DME with CO2 has been investigated. When the mole fraction of CO2 exceeds 0.882, the mixture falls out of the explosive range and becomes non-flammable [99].
3.5. Liquefied DME Extraction
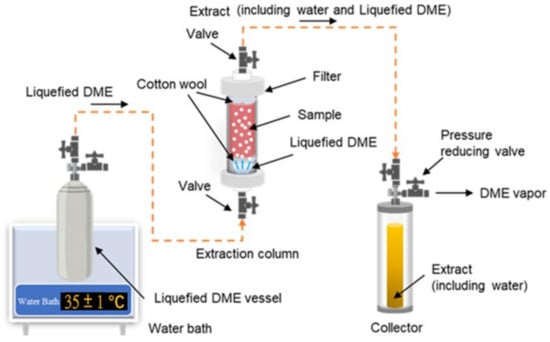
Figure 3 shows a schematic of a laboratory-scale liquefied DME extraction system [100]. This DME extraction system consists of a series of connections, including a metal tank containing liquefied DME (500 mL capacity), an extraction column (10–100 mL capacity), and an extraction solution collection tank (96 mL capacity).

Figure 3. Schematic of a laboratory-scale extraction system using liquefied DME.
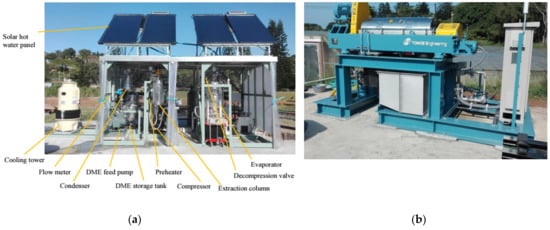
Kanda et al. pioneered the design and development of the first DME ambient-temperature drying and purification process prototype [101]. Using this prototype, ambient temperature dewatering and deodorization of high-moisture coal and sewage sludge were achieved [101]. Kanda et al. (2019) developed the largest microalgal oil extraction apparatus in the world (Figure 4a). This apparatus successfully extracts oil from high-moisture microalgae without drying [102]. In addition, they were able to limit CO2 emissions during the extraction process to a level determined by the CO2 captured from the oil. A centrifugal separator was used to recover microalgae from a 300-ton raceway cultivation tank at 1500–2100 G and at a processing rate of 3–7 tons per hour (Figure 4b).

Figure 4. (a) Experimental setup of a microalgae oil extraction system using liquefied DME; (b) A microalgae recovery system [102].
4. Applications of Liquefied DME Extraction
4.1. Lipid Extraction from Microalgae
Microalgal biomass is a rich source of various nutrients, including fatty acids, carotenoids, proteins, minerals, and other essential nutrients that can be used as functional food ingredients [102][103][104][105][106][107][108][109][110]. Many species of microalgae grow well in saline water, such as seawater, thus avoiding the need for limited freshwater resources [111]. Some oleaginous species of microalgae overproduce lipids and fatty acids by modifying the physical and chemical properties of the culture medium [112]. The lipid content of some microalgae may reach 77%, exceeding the index of higher plants (such as soybeans) [113]. The protein content of Arthrospira maxima has been found to reach 71% [114].
Microalgal oils have recently been used as alternatives to fish and vegetable oils with low nutritional values [115]. Microalgal oils contain highly utilizable and nontoxic fatty acids, such as polyunsaturated fatty acids (PUFA), arachidonic acid (ARA), c-linolenic acid (GLA), eicosapentaenoic acid, and docosahexaenoic acid (DHA) [116]. Long-chain polyunsaturated fatty acids such as eicosapentaenoic acid (ω-3 C20:5) and DHA (ω-3 C22:6) obtained from microalgae are essential for humans due to their beneficial effects on health, including neurodevelopment and the prevention of chronic diseases [117].
4.2. Extraction of Functional Components from Natural Resources
In addition to lipids, liquefied DME has been used to extract bioactive compounds from various sources, including spices, green tea, algae, fruits, vegetables, grains, natural plants, and fish. In 2003, Catchpole et al. used liquefied DME to extract specific pungent compounds from ginger, black pepper, and chili powder [118]. Despite the significant extraction of water, liquefied DME showed similar efficacy as scCO2 in isolating pungent compounds from spices. Complete extraction was achieved with minimal solvent consumption. At temperatures of 35, 40, 50, and 60 °C, liquefied DME showed nearly equivalent extraction rates. Subsequently, subcritical propane was suggested as a cost-effective alternative to CO2 because of its lower operating pressure and reduced energy consumption during spice extraction, similar to liquefied DME. However, subcritical propane is the least effective at dissolving pungent components and is unsuitable for carotenoid extraction [118][119].
Liquefied DME has been previously used to decaffeinate green tea [120]. The main functional components of green tea are caffeine and catechins [121]. Excessive caffeine consumption can lead to health problems, such as dizziness, increased heart rate, tremors, and insomnia, owing to overstimulation of the central nervous system [122]. Liquefied DME enables catechin extraction while completely removing caffeine. Ciulla et al. also demonstrated higher extraction rates of caffeine from coffee beans and powder using liquefied DME rather than using scCO2-based extraction methods [123].
Natural carotenoids exhibit several beneficial effects, including antioxidant, anti-inflammatory, antiproliferative, and antiapoptotic properties [124]. As antioxidants, carotenoids detoxify intracellular free radicals, thereby reducing the incidence of oxidative damage and associated diseases [125]. Carotenoids, which are widely distributed in nature, are biosynthesized by various organisms, including photosynthetic organisms such as algae, plants, fungi, and bacteria [125].
Using an enzyme-assisted DME and ethanol co-solvent extraction method, Billakanti et al. were able to extract almost all lipids, including polyunsaturated fatty acids and fucoxanthin, from the wet, brown seaweed Undaria pinnatifida [126]. Undaria pinnatifida contains a mixture of sulfated and branched chain polysaccharides that are tightly bound to the cell wall [127]. Therefore, extracting bioactive compounds from brown seaweed biomass is difficult because the cell wall is a major obstacle [128].
Microalgae have attracted widespread attention as natural sources of carotenoids because they grow faster than other higher plants. The Liquefied DME extraction method successfully extracted 7.70 mg/g of astaxanthin, a carotenoid, and 30.0% of its dry weight of lipids from microalgae (Haematococcus pluvialis) [129]. The extraction rate of astaxanthin was 1.82% lower than that achieved through acetone extraction using drying and cell disruption. Liquefied DME extraction removed 92% of the water from the microalgae and increased the carbon and hydrogen contents. Babadi et al. reported the extraction of total carotenoids (4.14 mg/g algal dry weight) and total chlorophyll (8.45 mg/g algal dry weight) from the microalgae Chlorococcum humicola using liquefied DME [130].
5. Theoretical Study of Liquefied DME
The use of Hansen solubility parameters (HSP) to evaluate the solubility of various analytes of natural origin has increased [131]. HSP is used to quantify molecular interactions and solubility [132][133].
HSP is based on three interaction forces: dispersion, dipole, and hydrogen bonding forces. The dispersion force (δd) indicates random interactions between molecules and represents the non-polar nature of the molecules. The dipole force (δp) indicates polar interactions between molecules and represents the polar nature of the molecule. The hydrogen bonding force (δh) represents hydrogen bonding interactions between molecules and their hydrogen bonding abilities [134]. These interaction forces can be summed to obtain the HSP. The solubility of a substance in a solvent is higher when its HSP is similar to that of the solvent.
HSPs are typically estimated using experimental data or molecular modeling techniques [135][136]. The HSP distance between two substances is expressed by the following equation [137]:
The difference in the HSP Ra [MPa1/2] can be obtained by taking the sum of the squares of the differences between the three parameters and determining their square roots [135]. The smaller the difference, the more similar the interactions between the substances and the higher the solubility and compatibility.
Based on the experimental data on solute–solvent interactions, plotting the solubility parameters of good and poor solvents for a solute in a three-dimensional diagram produces a Hansen solubility sphere, with regions of good solvents clustered together [131]. The spherical region indicates the extent to which the substance interacts with the solvent. The radius of the sphere represents the interaction radius R0 [MPa1/2]. The ratio of Ra to R0 is the relative energy difference (RED), which can be calculated using Equation (2). Here, RED ≤ 1 indicates a good solvent and RED > 1 indicates a poor solvent. RED can be used as an indicator of solubility [131].
6. Bioactive Extraction to Biomedical Advances
Tuna are one of the most important marine fish species worldwide [138]. Tuna giblets are rich in bioactive compounds such as unsaturated fatty acids, vitamins, and proteins. These compounds have antioxidant properties and can be converted into value-added products [139]. However, the internal organs, particularly the livers, of tuna are difficult to process and are often discarded [140].
Fang et al. reported that liquefied DME can be used to extract lipids and vitamins from tuna liver [141]. Compared to the conventional scCO2 method, liquefied DME extraction can prevent lipid oxidation and effectively reduce damage to omega-3 polyunsaturated fatty acids (n-3 PUFAs) and vitamins, thereby obtaining high-quality liver oil with excellent yield. The pressure used in liquefied DME extraction is much lower (0.8 MPa) than that used in scCO2 extraction (35 MPa), and no freeze-drying pretreatment is required.
Lipids, water, and vitamins can be extracted from tuna liver using liquefied DME to precipitate high-quality proteins. Currently, pH shifts, including alkaline or acidic extraction, isoelectric precipitation, centrifugation, and lyophilization, are the best processing methods for obtaining proteins from tuna liver [142]. However, this method is complex and time-consuming, and the lyophilization process is time- and energy-intensive [143].
Kanda et al. crystallized glycine from an aqueous solution using liquefied DME as an antisolvent [144]. Liquefied DME can be operated at 20–25 °C, potentially reducing the energy consumption of drying or crystallization with ethanol. Kanda et al. also prepared liposomes by dissolving soy lecithin and cholesterol in liquefied DME and infusing them into warm water [145]. Transmission electron microscopy, dynamic light scattering for particle size distribution measurements, and zeta potential measurements revealed that the resulting liposomes ranged in size from approximately 60 to 300 nm, with a zeta potential of approximately −57 mV. This indicates that the liquefied DME injection method successfully produces liposomes similar to those produced using conventional diethyl ether at temperatures above 45 °C. The liquefied DME method does not require the residue of conventional diethyl ether in the final product of liposomes or the high-temperature and high-pressure conditions of scCO2.
Organ transplantation is a treatment option for patients with severe organ failure. During organ transplantation, cells derived from the patient are grown on a three-dimensional scaffold to create an organ that will not be rejected. When porcine tissue is decellularized to create a scaffold, the porcine aorta is similar in structure to the human aorta, making it suitable for transplantation into humans [146]. The decellularization of tissues from different species involves three steps: extraction of lipids using sodium dodecyl sulfate (SDS), DNA fragmentation using DNase, and the removal of DNA fragments via washing with water and ethanol [147]. However, long processing times, inflammation caused by SDS at the contact site, and difficulty in completely removing the toxic surfactant from the tissue may cause certain problems. Liquefied DME was used to extract lipids, DNA, and cell nuclei from ostrich carotid tissue and porcine aorta [147][148][149][150]. Demonstrating that ostrich carotid tissue can be used as an alternative to porcine scaffolds, researchers can decellularize the porcine aorta after lipid extraction using DME, followed by DNase treatment and washing for at least five days. Furthermore, the introduction of liquefied DME into conventional decellularization eliminates the need for surfactants.
References
- Manuel F. Balandrin; James A. Klocke; Eve Syrkin Wurtele; Wm. Hugh Bollinger; Natural Plant Chemicals: Sources of Industrial and Medicinal Materials. Sci.. 1985, 228, 1154-1160.
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health-a comprehensive review. Vet. Q. 2020, 41, 1–29.
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Innovative Natural Functional Ingredients from Microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170.
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463.
- Vieira da Silva, B.; Barreira, J.C.M.; Oliveira, M.B.P.P. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends Food Sci. Technol. 2016, 50, 144–158.
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614.
- Uhrin, P.; Wang, D.; Mocan, A.; Waltenberger, B.; Breuss, J.M.; Tewari, D.; Mihaly-Bison, J.; Huminiecki, Ł.; Starzyński, R.R.; Tzvetkov, N.T.; et al. Vascular smooth muscle cell proliferation as a therapeutic target. Part 2: Natural products inhibiting proliferation. Biotechnol. Adv. 2018, 36, 1608–1621.
- Waltenberger, B.; Mocan, A.; Šmejkal, K.; Heiss, E.H.; Atanasov, A.G. Natural Products to Counteract the Epidemic of Cardiovascular and Metabolic Disorders. Molecules 2016, 21, 807.
- Vijaykrishnaraj, M.; Wang, K. Dietary natural products as a potential inhibitor towards advanced glycation end products and hyperglycemic complications: A phytotherapy approaches. Biomed. Pharmacother. 2021, 144, 112336.
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216.
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901.
- Lordan, R. Dietary supplements and nutraceuticals market growth during the coronavirus pandemic—Implications for consumers and regulatory oversight. PharmaNutrition 2021, 18, 100282.
- Dias, M.O.d.S.; Maciel Filho, R.; Mantelatto, P.E.; Cavalett, O.; Rossell, C.E.V.; Bonomi, A.; Leal, M.R.L.V. Sugarcane processing for ethanol and sugar in Brazil. Environ. Dev. 2015, 15, 35–51.
- Rajapaksha, D.S.W.; Shimizu, N. Valorization of spent black tea by recovery of antioxidant polyphenolic compounds: Subcritical solvent extraction and microencapsulation. Food Sci. Nutr. 2020, 8, 4297–4307.
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60.
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180.
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436.
- Buszewski, B.; Szultka, M. Past, Present, and Future of Solid Phase Extraction: A Review. Crit. Rev. Anal. Chem. 2012, 42, 198–213.
- Adeyi, O.; Oke, E.O.; Okolo, B.I.; Adeyi, A.J.; Otolorin, J.A.; Nwosu-Obieogu, K.; Adeyanju, J.A.; Dzarma, G.W.; Okhale, S.; Ogu, D.; et al. Process optimization, scale-up studies, economic analysis and risk assessment of phenolic rich bioactive extracts production from Carica papaya L. leaves via heat-assisted extraction technology. Heliyon 2022, 8, e09216.
- Martinez-Fernandez, J.S.; Gu, X.; Chen, S. Techno-economic assessment of bioactive compound recovery from potato peels with sequential hydrothermal extraction. J. Clean. Prod. 2021, 282, 124356.
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latham, L.Y. Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10.
- Lemes, A.C.; Egea, M.B.; de Oliveira Filho, J.G.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds from Agro-industrial By-products: A Review. Front. Bioeng. Biotechnol. 2021, 9, 802543.
- Patrice Didion, Y.; Gijsbert Tjalsma, T.; Su, Z.; Malankowska, M.; Pinelo, M. What is next? the greener future of solid liquid extraction of biobased compounds: Novel techniques and solvents overpower traditional ones. Sep. Purif. Technol. 2023, 320, 124147.
- Medina-Medrano, J.R.; Torres-Contreras, J.E.; Valiente-Banuet, J.I.; Mares-Quiñones, M.D.; Vázquez-Sánchez, M.; Álvarez-Bernal, D. Effect of the solid–liquid extraction solvent on the phenolic content and antioxidant activity of three species of Stevia leaves. Sep. Sci. Technol. 2019, 54, 2283–2293.
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81.
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328.
- Cravotto, C.; Fabiano-Tixier, A.S.; Claux, O.; Abert-Vian, M.; Tabasso, S.; Cravotto, G.; Chemat, F. Towards Substitution of Hexane as Extraction Solvent of Food Products and Ingredients with No Regrets. Foods 2022, 11, 3412.
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263.
- Lardon, L.; Hélias, A.; Sialve, B.; Steyer, J.-P.; Bernard, O. Life-Cycle Assessment of Biodiesel Production from Microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481.
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid.-Based Complement. Altern. Med. 2015, 2015, 595393.
- Shen, W.-C.; Chien, I.L. Design and Control of Ethanol/Benzene Separation by Energy-Saving Extraction–Distillation Process Using Glycerol as an Effective Heavy Solvent. Ind. Eng. Chem. Res. 2019, 58, 14295–14311.
- Ethanol Content in Herbal Medicinal Products and Traditional Herbal Medicinal Products Used in Children-Scientific Guideline. Available online: https://www.ema.europa.eu/en/ethanol-content-herbal-medicinal-products-traditional-herbal-medicinal-products-used-children (accessed on 4 December 2023).
- Over-the-Counter Drug Products Intended for Oral Ingestion That Contain Alcohol. Available online: https://www.federalregister.gov/documents/1995/03/13/95-6128/over-the-counter-drug-products-intended-for-oral-ingestion-that-contain-alcohol (accessed on 4 December 2023).
- Goto, M. Chemical recycling of plastics using sub- and supercritical fluids. J. Supercrit. Fluids 2009, 47, 500–507.
- Gopalan, B.; Goto, M.; Kodama, A.; Hirose, T. Supercritical Carbon Dioxide Extraction of Turmeric (Curcuma longa). J. Agric. Food Chem. 2000, 48, 2189–2192.
- Goto, M.; Sato, M.; Hirose, T. Extraction of Peppermint Oil by Supercritical Carbon Dioxide. J. Chem. Eng. Jpn. 1993, 26, 401–407.
- Hoshino, Y.; Wahyudiono; Machmudah, S.; Hirayama, S.; Kanda, H.; Hoshino, M.; Goto, M. Extraction of Functional Components from Freeze-Dried Angelica furcijuga Leaves Using Supercritical Carbon Dioxide. ACS Omega 2022, 7, 5104–5111.
- Triques, C.C.; da Silva, E.A.; Santos, K.A.; Klein, E.J.; Slusarski-Santana, V.; Fagundes-Klen, M.R.; Fiorese, M.L. Supercritical Fluid Extraction of Essential Oils from Natural Sources. In Essential Oils: Extraction Methods and Applications; Inamuddin, Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 707–739.
- Kamchonemenukool, S.; Wongwaiwech, D.; Thongsook, T.; Weerawatanakorn, M. Subcritical liquified dimethyl ether and supercritical fluid carbon dioxide extraction of gamma oryzanol from rice bran acid oil. J. Agric. Food Res. 2023, 14, 100672.
- Milovanovic, S.; Grzegorczyk, A.; Świątek, Ł.; Grzęda, A.; Dębczak, A.; Tyskiewicz, K.; Konkol, M. A Novel Strategy for the Separation of Functional Oils from Chamomile Seeds. Food Bioprocess Technol. 2023, 16, 1806–1821.
- Fischer, B.; Gevinski, E.V.; da Silva, D.M.; Júnior, P.A.L.; Bandiera, V.J.; Lohmann, A.M.; Rigo, D.; Duarte, P.F.; Franceschi, E.; Zandoná, G.P.; et al. Extraction of hops pelletized (Humulus lupulus) with subcritical CO2 and hydrodistillation: Chemical composition identification, kinetic model, and evaluation of antioxidant and antimicrobial activity. Food Res. Int. 2023, 167, 112712.
- De Marco, I.; Riemma, S.; Iannone, R. Life cycle assessment of supercritical CO2 extraction of caffeine from coffee beans. J. Supercrit. Fluids 2018, 133, 393–400.
- Crampon, C.; Boutin, O.; Badens, E. Supercritical Carbon Dioxide Extraction of Molecules of Interest from Microalgae and Seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953.
- Santana, A.; Jesus, S.; Larrayoz, M.A.; Filho, R.M. Supercritical Carbon Dioxide Extraction of Algal Lipids for the Biodiesel Production. Procedia Eng. 2012, 42, 1755–1761.
- Lorenzen, J.; Igl, N.; Tippelt, M.; Stege, A.; Qoura, F.; Sohling, U.; Brück, T. Extraction of microalgae derived lipids with supercritical carbon dioxide in an industrial relevant pilot plant. Bioprocess Biosyst. Eng. 2017, 40, 911–918.
- Soh, L.; Zimmerman, J. Biodiesel production: The potential of algal lipids extracted with supercritical carbon dioxide. Green Chem. 2011, 13, 1422–1429.
- Hedayati, A.; Ghoreishi, S.M. Supercritical carbon dioxide extraction of glycyrrhizic acid from licorice plant root using binary entrainer: Experimental optimization via response surface methodology. J. Supercrit. Fluids 2015, 100, 209–217.
- Anderson, K.E.; Siepmann, J.I. Solubility in Supercritical Carbon Dioxide: Importance of the Poynting Correction and Entrainer Effects. J. Phys. Chem. B 2008, 112, 11374–11380.
- Ghoreishi, S.M.; Hedayati, A.; Mohammadi, S. Optimization of periodic static-dynamic supercritical CO2 extraction of taxifolin from Pinus nigra bark with ethanol as entrainer. J. Supercrit. Fluids 2016, 113, 53–60.
- Shimizu, S.; Abbott, S. How Entrainers Enhance Solubility in Supercritical Carbon Dioxide. J. Phys. Chem. B 2016, 120, 3713–3723.
- Pilařová, V.; Al Hamimi, S.; Cunico, L.P.; Nováková, L.; Turner, C. Extending the design space in solvent extraction—From supercritical fluids to pressurized liquids using carbon dioxide, ethanol, ethyl lactate, and water in a wide range of proportions. Green Chem. 2019, 21, 5427–5436.
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Integrated strategies for water removal and lipid extraction from coffee industry residues. Sustain. Energy Technol. Assess. 2018, 29, 26–35.
- Gómez-de la Cruz, F.J.; Cruz-Peragón, F.; Casanova-Peláez, P.J.; Palomar-Carnicero, J.M. A vital stage in the large-scale production of biofuels from spent coffee grounds: The drying kinetics. Fuel Process. Technol. 2015, 130, 188–196.
- Okada, M.; Rao, M.A.; Lima, J.E.; Torloni, M. Energy consumption and the potential for conservation in a spray-dried coffee plant. J. Food Sci. 1980, 45, 685–688.
- Ramírez, C.A.; Patel, M.; Blok, K. From fluid milk to milk powder: Energy use and energy efficiency in the European dairy industry. Energy 2006, 31, 1984–2004.
- Oancea, S. A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat. Antioxidants 2021, 10, 1337.
- Khonchaisri, R.; Sumonsiri, N.; Prommajak, T.; Rachtanapun, P.; Leksawasdi, N.; Techapun, C.; Taesuwan, S.; Halee, A.; Nunta, R.; Khemacheewakul, J. Optimization of Ultrasonic-Assisted Bioactive Compound Extraction from Green Soybean (Glycine max L.) and the Effect of Drying Methods and Storage Conditions on Procyanidin Extract. Foods 2022, 11, 17755.
- Li, J.; Sobańtka, A. A Systematic Analysis of the Effect of Extraction Solvents on the Chemical Composition of Extraction Solutions and the Analytical Implications in Extractables and Leachables Studies. J. Pharm. Biomed. Anal. 2023, 222, 115081.
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10.
- Gori, A.; Boucherle, B.; Rey, A.; Rome, M.; Fuzzati, N.; Peuchmaur, M. Development of an innovative maceration technique to optimize extraction and phase partition of natural products. Fitoterapia 2021, 148, 104798.
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585.
- Talekar, S.; Patti, A.F.; Singh, R.; Vijayraghavan, R.; Arora, A. From waste to wealth: High recovery of nutraceuticals from pomegranate seed waste using a green extraction process. Ind. Crops Prod. 2018, 112, 790–802.
- Weggler, B.A.; Gruber, B.; Teehan, P.; Jaramillo, R.; Dorman, F.L. Chapter 5—Inlets and sampling. In Separation Science and Technology; Snow, N.H., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 12, pp. 141–203.
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20.
- Mahmudati, N.; Wahyono, P.; Djunaedi, D. Antioxidant activity and total phenolic content of three varieties of Ginger (Zingiber officinale) in decoction and infusion extraction method. J. Phys. Conf. Ser. 2020, 1567, 022028.
- Perera, P.R.D.; Ekanayake, S.; Ranaweera, K.K.D.S. Antidiabetic Compounds in Syzygium cumini Decoction and Ready to Serve Herbal Drink. Evid. Based Complement. Altern. Med. 2017, 2017, 1083589.
- Makoś, P.; Słupek, E.; Sobczak, J.; Zabrocki, D.; Hupka, J.; Rogala, A. Dimethyl ether (DME) as potential environmental friendly fuel. In Proceedings of the International Conference on Advances in Energy Systems and Environmental Engineering (ASEE19), Wroclaw, Poland, 9–12 June 2019.
- Tamagawa, K.; Takemura, M.; Konaka, S.; Kimura, M. Molecular structure of dimethylether as determined by a joint analysis of gas electron diffraction and microwave spectroscopic data. J. Mol. Struct. 1984, 125, 131–142.
- Che, C.; Li, Y.; Zhang, G.; Deng, D. Doped Amorphous Carbon Films Prepared by Liquid Phase Electrodeposition. Open J. Appl. Sci. 2014, 3, 5–13.
- Ascenzi, D.; Cernuto, A.; Balucani, N.; Tosi, P.; Ceccarelli, C.; Martini, L.M.; Pirani, F. Destruction of dimethyl ether and methyl formate by collisions with He+. Astron. Astrophys. 2019, 625, A72.
- Wu, J.; Zhou, Y.; Lemmon, E.W. An Equation of State for the Thermodynamic Properties of Dimethyl Ether. J. Phys. Chem. Ref. Data 2011, 40, 023104.
- Zheng, Q.; Watanabe, M. Advances in low-temperature extraction of natural resources using liquefied dimethyl ether. Resour. Chem. Mater. 2022, 1, 16–26.
- Park, S.H.; Lee, C.S. Combustion performance and emission reduction characteristics of automotive DME engine system. Prog. Energy Combust. Sci. 2013, 39, 147–168.
- Thomas, G.; Feng, B.; Veeraragavan, A.; Cleary, M.J.; Drinnan, N. Emissions from DME combustion in diesel engines and their implications on meeting future emission norms: A review. Fuel Process. Technol. 2014, 119, 286–304.
- Sezer, İ. Thermodynamic, performance and emission investigation of a diesel engine running on dimethyl ether and diethyl ether. Int. J. Therm. Sci. 2011, 50, 1594–1603.
- Eltringham, W.; Catchpole, O.J. Relative Permittivity Measurements of Gaseous, Liquid, and Supercritical Dimethyl Ether. J. Chem. Eng. Data 2007, 52, 363–367.
- Kruse, A.; Dahmen, N. Water—A magic solvent for biomass conversion. J. Supercrit. Fluids 2015, 96, 36–45.
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and Efficient Extraction Methods for Marine-Derived Compounds. Mar. Drugs 2015, 13, 3182–3230.
- Tallon, S.J.; Catchpole, O.J.; Eltringham, W. Development and scale up of new compressed gas extraction technologies for food and natural product processing. In Proceedings of the AIChE Centennial: Chemical Engineering Education: Past and Future, AIChE Annual Meeting, Conference Proceedings, AIChE 100, Philadelphia, PA, USA, 16–21 November 2008; ISBN 978–081691050–2.
- Kanda, H.; Kamo, Y.; Machmudah, S.; Wahyudiono; Goto, M. Extraction of Fucoxanthin from Raw Macroalgae excluding Drying and Cell Wall Disruption by Liquefied Dimethyl Ether. Mar. Drugs 2014, 12, 2383–2396.
- He, X.; Liu, J.; Van Vleck, E. Preface-SI: SIAM-2015. J. Comput. Appl. Math. 2016, 307, 1.
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process. Technol. 2019, 191, 232–247.
- Catchpole, O.J.; Tallon, S.J.; Grey, J.B.; Fletcher, K.; Fletcher, A.J. Extraction of lipids from a specialist dairy stream. J. Supercrit. Fluids 2008, 45, 314–321.
- Kanda, H.; Hoshino, R.; Murakami, K.; Wahyudiono; Zheng, Q.; Goto, M. Lipid extraction from microalgae covered with biomineralized cell walls using liquefied dimethyl ether. Fuel 2020, 262, 116590.
- Catchpole, O.; Ryan, J.; Zhu, Y.; Fenton, K.; Grey, J.; Vyssotski, M.; MacKenzie, A.; Nekrasov, E.; Mitchell, K. Extraction of lipids from fermentation biomass using near-critical dimethylether. J. Supercrit. Fluids 2010, 53, 34–41.
- Liu, S.; Hu, W.; Fang, Y.; Cai, Y.; Zhang, J.; Liu, J.; Ding, Y. Extraction of oil from wet Antarctic krill (Euphausia superba) using a subcritical dimethyl ether method. RSC Adv. 2019, 9, 34274–34282.
- Kerdsiri, J.; Wisuitiprot, W.; Boonnoun, P.; Chantakul, R.; Netsopa, S.; Nuengchamnong, N.; Waranuch, N. Effect of extraction methods on biological activities of Thai rice bran extracts. Songklanakarin J. Sci. Technol. 2020, 42, 1007–1015.
- Kanda, H.; Shimakata, M.; Wang, T.; Zhu, L.; Wahyudiono; Goto, M. Supercritical methanol-induced esterification of microalgal lipids employing biomineralized cell walls as the catalyst. Fuel 2022, 330, 125707.
- Commission Directive (EU) 2016/1855 of 19 October 2016 Amending Directive 2009/32/EC of the European Parliament and of the Council on the Approximation of the Laws of the Member States on Extraction Solvents Used in the Production of Foodstuffs and Food Ingredients (Text with EEA Relevance). Available online: http://data.europa.eu/eli/dir/2016/1855/oj (accessed on 4 December 2023).
- Food Standards Australia New Zealand, Application A1056, Dimethyl Ether as a Processing Aid for Dairy Ingredients & Products Approval Report. 2012. Available online: https://www.foodstandards.gov.au/sites/default/files/food-standards-code/applications/Documents/A1056%20DME%20as%20a%20PA%20(dairy)%20AR%20FINAL1.pdf (accessed on 18 December 2023).
- Bohnenn, L.J.M. SPC. Soap, Perfumery, and Cosmetics; United Trade Press: London, UK, 1979; p. 300.
- Bohnenn, L.J.M. DME-A Promising Alternative. Propellant in the Fluorocarbon Crisis. Aerosol Rep. 1979, 18, 70–79.
- Catizzone, E.; Freda, C.; Braccio, G.; Frusteri, F.; Bonura, G. Dimethyl ether as circular hydrogen carrier: Catalytic aspects of hydrogenation/dehydrogenation steps. J. Energy Chem. 2021, 58, 55–77.
- Putrasari, Y.; Lim, O. Dimethyl Ether as the Next Generation Fuel to Control Nitrogen Oxides and Particulate Matter Emissions from Internal Combustion Engines: A Review. ACS Omega 2022, 7, 32–37.
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511.
- Fleisch, T.H.; Basu, A.; Sills, R.A. Introduction and advancement of a new clean global fuel: The status of DME developments in China and beyond. J. Nat. Gas Sci. Eng. 2012, 9, 94–107.
- Saravanan, K.; Ham, H.; Tsubaki, N.; Bae, J.W. Recent progress for direct synthesis of dimethyl ether from syngas on the heterogeneous bifunctional hybrid catalysts. Appl. Catal. B 2017, 217, 494–522.
- Good, D.A.; Francisco, J.S.; Jain, A.K.; Wuebbles, D.J. Lifetimes and global warming potentials for dimethyl ether and for fluorinated ethers: CH3OCF3 (E143a), CHF2OCHF2 (E134), CHF2OCF3 (E125). J. Geophys. Res. Atmos. 1998, 103, 28181–28186.
- Kanda, H.; Fukuta, Y.; Wahyudiono; Goto, M. Enhancement of Lipid Extraction from Soya Bean by Addition of Dimethyl Ether as Entrainer into Supercritical Carbon Dioxide. Foods 2021, 10, 1223.
- Kanda, H.; Zhu, L.; Zhu, W.; Wang, T. Ethanol-free extraction of curcumin and antioxidant activity of components from wet Curcuma longa L. by liquefied dimethyl ether. Arab. J. Chem. 2023, 16, 104585.
- Kanda, H. Super-Energy-Saving Dewatering Method for High-Specific-Surface-Area Fuels by Using Dimethyl Ether. Adsorpt. Sci. Technol. 2008, 26, 345–349.
- Kanda, H. Final Report 2022, Production of Biofuels Using Algal Biomass, Science and Technology Research Partnership for Sustainable Development (SATREPS), Japan Science and Technology Agency. Available online: https://www.jst.go.jp/global/kadai/pdf/h2705_final.pdf (accessed on 18 December 2023).
- Dexso Butanex 345/600 mm Extractor. Available online: https://verdampftnochmal.de/products/en/Dexso-Butanex-345-/-600mm-Extractor_2 (accessed on 12 December 2023).
- Liquid Gas Extraction Technology Uses Dimethyl Ether to Extract Organic Material. Available online: https://www.pcne.eu/article/liquid-gas-extraction-technology/ (accessed on 12 December 2023).
- High Quality Butane Hexane Ethanol Solvent Extraction Herb Equipment/Oil Extraction Machine/Subcritical Extraction Equipment. Available online: https://www.alibaba.com/product-detail/High-quality-Butane-hexane-ethanol-solvent_62547269161.html?spm=a2700.details.0.0.106e350agQzf21 (accessed on 12 December 2023).
- Mini Solvent Extraction Unit for Lab. Available online: https://www.bestextractionmachine.com/products/pilot-solvent-extraction-unit.html (accessed on 12 December 2023).
- Tanaka, K.; Higashi, Y. Measurements of the Isobaric Specific Heat Capacity and Density for Dimethyl Ether in the Liquid State. J. Chem. Eng. Data 2010, 55, 2658–2661.
- Park, S.H.; Lee, C.S. Applicability of dimethyl ether (DME) in a compression ignition engine as an alternative fuel. Energy Convers. Manag. 2014, 86, 848–863.
- Zhen, X.; Wang, Y. An overview of methanol as an internal combustion engine fuel. Renew. Sustain. Energy Rev. 2015, 52, 477–493.
- Machmudah, S.; Diono, W.; Kanda, H.; Goto, M. Supercritical fluids extraction of valuable compounds from algae: Future perspectives and challenges. Eng. J. 2018, 22, 13–30.
- Draaisma, R.B.; Wijffels, R.H.; Slegers, P.M.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177.
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal lipids: A review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenergy 2021, 150, 106108.
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306.
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210.
- Santigosa, E.; Brambilla, F.; Milanese, L. Microalgae Oil as an Effective Alternative Source of EPA and DHA for Gilthead Seabream (Sparus aurata) Aquaculture. Animals 2021, 11, 971.
- Volkman, J.K.; Jeffrey, S.W.; Nichols, P.D.; Rogers, G.I.; Garland, C.D. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1989, 128, 219–240.
- Huang, Y.; Zhang, D.; Xue, S.; Wang, M.; Cong, W. The Potential of Microalgae Lipids for Edible Oil Production. Appl. Biochem. Biotechnol. 2016, 180, 438–451.
- Catchpole, O.J.; Grey, J.B.; Perry, N.B.; Burgess, E.J.; Redmond, W.A.; Porter, N.G. Extraction of Chili, Black Pepper, and Ginger with Near-Critical CO2, Propane, and Dimethyl Ether: Analysis of the Extracts by Quantitative Nuclear Magnetic Resonance. J. Agric. Food Chem. 2003, 51, 4853–4860.
- Daood, H.G.; Illés, V.; Gnayfeed, M.H.; Mészáros, B.; Horváth, G.; Biacs, P.A. Extraction of pungent spice paprika by supercritical carbon dioxide and subcritical propane. J. Supercrit. Fluids 2002, 23, 143–152.
- Kanda, H.; Li, P.; Makino, H. Production of decaffeinated green tea leaves using liquefied dimethyl ether. Food Bioprod. Process. 2013, 91, 376–380.
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and Caffeine Content of Green Tea Dietary Supplements and Correlation with Antioxidant Capacity. J. Agric. Food Chem. 2006, 54, 1599–1603.
- Ito, M.; Ando, T.; Yamamoto, K.; Ishido, A. Caffeine intoxication as a result of excessive consumption of bottled coffee products: A case report. J. Rural Med. 2023, 18, 200–204.
- Ciulla, M.; Canale, V.; Wolicki, R.D.; Ferrone, V.; Carlucci, G.; Fontana, A.; Siani, G.; D’Alessandro, N.; Di Profio, P. Comparison of extraction methods for active biomolecules using sub-critical dimethyl ether and n-butane. Eur. Food Res. Technol. 2023, 249, 367–374.
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed. Pharmacother. 2022, 145, 112179.
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049.
- Billakanti, J.M.; Catchpole, O.J.; Fenton, T.A.; Mitchell, K.A.; MacKenzie, A.D. Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process Biochem. 2013, 48, 1999–2008.
- Avila-Peltroche, J.; Won, B.Y.; Cho, T.O. An improved protocol for protoplast production, culture, and whole plant regeneration of the commercial brown seaweed Undaria pinnatifida. Algal Res. 2022, 67, 102851.
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Robert Waaland, J.; Rabiei, R. Fatty Acids, Amino Acids, Mineral Contents, and Proximate Composition of Some Brown Seaweeds. J. Phycol. 2012, 48, 285–292.
- Boonnoun, P.; Kurita, Y.; Kamo, Y.; Wahyudiono; Machmudah, S.; Okita, Y.; Ohashi, E.; Kanda, H.; Goto, M. Wet Extraction of Lipids and Astaxanthin from Haematococcus pluvialis by Liquefied Dimethyl Ether. J. Nutr. Food Sci. 2014, 4, 1000305.
- Eghbali Babadi, F.; Boonnoun, P.; Nootong, K.; Powtongsook, S.; Goto, M.; Shotipruk, A. Identification of carotenoids and chlorophylls from green algae Chlorococcum humicola and extraction by liquefied dimethyl ether. Food Bioprod. Process. 2020, 123, 296–303.
- Sánchez-Camargo, A.d.P.; Bueno, M.; Parada-Alfonso, F.; Cifuentes, A.; Ibáñez, E. Hansen solubility parameters for selection of green extraction solvents. TrAC Trends Anal. Chem. 2019, 118, 227–237.
- Zhou, K.-G.; Mao, N.-N.; Wang, H.-X.; Peng, Y.; Zhang, H.-L. A Mixed-Solvent Strategy for Efficient Exfoliation of Inorganic Graphene Analogues. Angew. Chem. Int. 2011, 50, 10839–10842.
- Terrell, E. Estimation of Hansen solubility parameters with regularized regression for biomass conversion products: An application of adaptable group contribution. Chem. Eng. Sci. 2022, 248, 117184.
- Srinivas, K.; King, J.W.; Monrad, J.K.; Howard, L.R.; Hansen, C.M. Optimization of Subcritical Fluid Extraction of Bioactive Compounds Using Hansen Solubility Parameters. J. Food Sci. 2009, 74, E342–E354.
- Louwerse, M.J.; Maldonado, A.; Rousseau, S.; Moreau-Masselon, C.; Roux, B.; Rothenberg, G. Revisiting Hansen Solubility Parameters by Including Thermodynamics. ChemPhysChem 2017, 18, 2999–3006.
- Venkatram, S.; Kim, C.; Chandrasekaran, A.; Ramprasad, R. Critical Assessment of the Hildebrand and Hansen Solubility Parameters for Polymers. J. Chem. Inf. Model. 2019, 59, 4188–4194.
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007.
- Hiraoka, Y.; Okochi, Y.; Ohshimo, S.; Shimose, T.; Ashida, H.; Sato, T.; Ando, Y. Lipid and fatty acid dynamics by maternal Pacific bluefin tuna. PLoS ONE 2019, 14, e0222824.
- Fraterrigo Garofalo, S.; Cavallini, N.; Demichelis, F.; Savorani, F.; Mancini, G.; Fino, D.; Tommasi, T. From tuna viscera to added-value products: A circular approach for fish-waste recovery by green enzymatic hydrolysis. Food Bioprod. Process. 2023, 137, 155–167.
- Hilborn, R.; Costello, C. The potential for blue growth in marine fish yield, profit and abundance of fish in the ocean. Mar. Policy 2018, 87, 350–355.
- Fang, Y.; Liu, S.; Hu, W.; Zhang, J.; Ding, Y.; Liu, J. Extraction of Oil from High-Moisture Tuna Livers by Subcritical Dimethyl Ether: A Comparison with Different Extraction Methods. Eur. J. Lipid Sci. Technol. 2019, 121, 1800087.
- Lynch, S.A.; Álvarez, C.; O’Neill, E.E.; Keenan, D.F.; Mullen, A.M. Optimization of protein recovery from bovine lung by pH shift process using response surface methodology. J. Sci. Food Agric. 2018, 98, 1951–1960.
- Dang, T.T.; Vuong, Q.V.; Schreider, M.J.; Bowyer, M.C.; Altena, I.A.V.; Scarlett, C.J. The Effects of Drying on Physico-Chemical Properties and Antioxidant Capacity of the Brown Alga (Hormosira banksii (Turner) Decaisne). J. Food Process. Preserv. 2017, 41, e13025.
- Kanda, H.; Katsube, T.; Hoshino, R.; Kishino, M.; Wahyudiono; Goto, M. Ethanol-free antisolvent crystallization of glycine by liquefied dimethyl ether. Heliyon 2020, 6, e05258.
- Kanda, H.; Katsube, T.; Wahyudiono; Goto, M. Preparation of Liposomes from Soy Lecithin Using Liquefied Dimethyl Ether. Foods 2021, 10, 1789.
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965.
- Kanda, H.; Ando, D.; Hoshino, R.; Yamamoto, T.; Wahyudiono; Suzuki, S.; Shinohara, S.; Goto, M. Surfactant-Free Decellularization of Porcine Aortic Tissue by Subcritical Dimethyl Ether. ACS Omega 2021, 6, 13417–13425.
- Kanda, H.; Ando, D.; Oya, K.; Wahyudiono; Goto, M. Surfactant-free preparation of an ostrich carotid artery scaffold using liquefied dimethyl ether and DNase. Arab. J. Chem. 2021, 14, 103280.
- Kanda, H.; Oya, K.; Wahyudiono; Goto, M. Surfactant-Free Decellularization of Porcine Auricular Cartilage Using Liquefied Dimethyl Ether and DNase. Materials 2023, 16, 3172.
- Kanda, H.; Oya, K.; Irisawa, T.; Wahyudiono; Goto, M. Tensile strength of ostrich carotid artery decellularized with liquefied dimethyl ether and DNase: An effort in addressing religious and cultural concerns. Arab. J. Chem. 2023, 16, 104578.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
7 times
(View History)
Update Date:
30 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




