Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Adnan Iqbal | -- | 3558 | 2024-01-25 15:35:46 | | | |
| 2 | Sirius Huang | Meta information modification | 3558 | 2024-01-26 02:09:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zafar, A.; Takeda, C.; Manzoor, A.; Tanaka, D.; Kobayashi, M.; Wadayama, Y.; Nakane, D.; Majeed, A.; Iqbal, M.A.; Akitsu, T. Industrial Applications of Microfluidic Systems. Encyclopedia. Available online: https://encyclopedia.pub/entry/54356 (accessed on 08 February 2026).
Zafar A, Takeda C, Manzoor A, Tanaka D, Kobayashi M, Wadayama Y, et al. Industrial Applications of Microfluidic Systems. Encyclopedia. Available at: https://encyclopedia.pub/entry/54356. Accessed February 08, 2026.
Zafar, Ayesha, China Takeda, Asif Manzoor, Daiki Tanaka, Masashi Kobayashi, Yoshitora Wadayama, Daisuke Nakane, Adnan Majeed, Muhammad Adnan Iqbal, Takashiro Akitsu. "Industrial Applications of Microfluidic Systems" Encyclopedia, https://encyclopedia.pub/entry/54356 (accessed February 08, 2026).
Zafar, A., Takeda, C., Manzoor, A., Tanaka, D., Kobayashi, M., Wadayama, Y., Nakane, D., Majeed, A., Iqbal, M.A., & Akitsu, T. (2024, January 25). Industrial Applications of Microfluidic Systems. In Encyclopedia. https://encyclopedia.pub/entry/54356
Zafar, Ayesha, et al. "Industrial Applications of Microfluidic Systems." Encyclopedia. Web. 25 January, 2024.
Copy Citation
With the proliferation of microelectromechanical systems (MEMS) technology, the syntheses of microfluidic devices or microdroplets have become increasingly important as tools for chemical analysis and synthesis. Microfluidic devices (microreactors, microchemical chips, etc.) that integrate the functions of chemical synthesis, analysis equipment, and chemical plants in a compact form are being investigated.
microfluidic system
droplet synthesis
microfluidic organic synthesis
organic compounds
1. Pharmaceutical Applications
Photochemical reactions can be enhanced through the implementation of continuous flow technology. Scientists have developed a High Throughput Experimentation (HTE) droplet microfluidic platform that enables high-throughput drug discovery in flow to generate pharmaceutically relevant compound libraries. It is possible to handle several samples simultaneously over a long period by segmenting them with an immiscible phase [1][2]. Greater alkene compatibility and enhanced reactivity were observed with pharmacologically relevant sulfonyl acetamides containing heteroarenes, as shown in Scheme 1A. The in-droplet reaction discovery screen enabled researchers to expand upon their previously reported alkene amino arylation methodology to include substrates with increased structural complexity. It also allowed them to identify reactivity trends and [3] structure–activity relationships to inform ongoing mechanistic investigations [4]. The present study introduces a microfluidic redox-neutral electrochemistry platform (µRN-eChem), which is widely applicable to single-electron transfer chemistry (SET), such as radical–radical cross-coupling, Minisci reactions, and nickel-catalyzed C(sp2)-O cross-couplings (Scheme 1B). A microfluidic channel facilitates selective transformation by accelerating molecular diffusion across a cathode and anode simultaneously while outpacing the decomposition of intermediates. Due to the excellent conductivity of the microfluidic channel, no additional electrolyte was required. An electrosynthesis of a nematic liquid crystal compound was demonstrated using µRN-eChem in two steps [5].
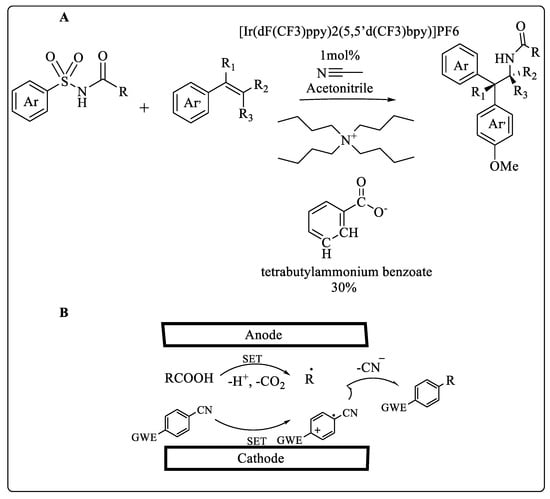
Scheme 1. (A) Microfluidic droplet system enabled HTE reaction discovery (B) μRN-eChem-based decarboxylative arylation.
An isoporous nanostructured membrane is incorporated into microfluidics-integrated microscale organic electrochemical transistors (OECTs). These label-free devices were used to cure Alzheimer’s disease (AD) by detecting Aβ protein aggregates in human serum with a performance exceeding those of several other systems [6][7]. To capture protein aggregates, Congo Red (CR) molecules were functionalized on the membrane. During the accumulation process, the membrane surface’s capacitance changed, modulating the gate voltage felt by the transistor channel. Microfluidic channels served as immunoreaction chambers that reduced analytical time compared to microwells while using minimal sample/reagents. There was a drastic difference between transistor characteristics for all devices as a result of the binding event. Both buffer and human serum samples were detected with Microfluidic-based OECTs in a broad range of concentrations. Since accumulation-mode devices require less power and have higher current changes during binding, they outperform depletion-mode devices. Unlike electronic immunosensors that rely on reference electrodes or electroactive labels, the simple detection method does not require reference electrodes [8].
In order to synthesize organic TPE nanoparticles (NPs), a new supercritical antisolvent (SAS) process was developed with a microreactor. A model organic molecule, tetraphenyl ethylene (TPE), was solubilized in tetrahydrofuran (THF) [9], and supercritical carbon dioxide (sc-CO2) was used as an antisolvent. It is possible to obtain sizes below 15 nm using µSAS. Narrow dispersion size (±3 nm) was obtained. This technique is very useful for producing very small organic nanoparticles, paving the way for a wide range of practical applications [10].
Metal–organic framework microcrystals with HKUST-1 topology [11] of different sizes, shapes, and chemistry of metal clusters have been synthesized using the microfluidic system consisting of the microfluidic chip (MFC) and syringe pumps. The MFC contains two channels, the upper channel contained metallic salt (CuSO4, Cu(NO3)2 or Ni(NO3)2 preliminarily dissolved in N,N-Dimethylformamide), whereas the bottom channel was filled with the ligand (benzene-1,3,5-tricarboxylic acid or trimesic acid preliminarily dissolved in N,N-Dimethylformamide) shown in Scheme 2. The reaction rapidly started at the junction of two channels. For the temperature regulation, the MFC was placed on a refrigerant and a heating plate. Variations of the reaction temperature in the case of all three metallic salts used resulted in a change of the morphology of the obtained MOF microcrystals [12].
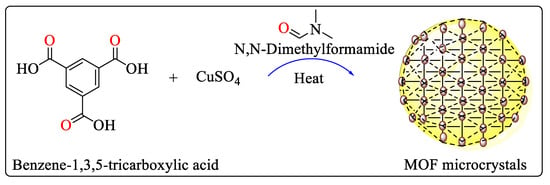
Scheme 2. Microfluidic reaction to formulate MOF microcrystals.
Previous studies have shown that a multifunctional nanoparticle drug delivery system (NDDS) is capable of delivering hydrophilic doxorubicin (DOX) and near-infrared photosensitizer dye IR780 simultaneously using a nanoscale zeolitic imidazolate framework-90 (ZIF-90) core and a SAD shell using a microfluidics-based approach. ZIF-DH obtained by IR780 has a 10-fold loading capacity over ZIF-90 obtained by conventional means. A spermine-modified acetalated dextran (SAD) shell could further enhance the pH-responsive release and prevent drug leakage in physiological solutions by improving the pH-responsive release performance of nanoscale ZIF-90. ZIF-90 is also capable of targeting tumors due to its conjugation of hyaluronic acid (HA) via amine groups on its SAD shell. A synergistic dual-mode chemo-photodynamic therapy using IR780/DOX@ZIF-DH was shown to be effective against cancer both in vitro and in vivo. As a result of this study, multiple problems encountered in nanoscale ZIF are simultaneously solved by combining intelligent polymer design and controllable microfluidics-based production. It is expected that this technology has great potential for cancer therapy clinical applications [13].
There are a number of foodborne pathogens, including Salmonella, that are found throughout the world. It can cause diarrhea, gastroenteritis, typhoid, and other symptoms when found in a wide range of foods. To prevent and control foodborne diseases, rapid and sensitive detection of Salmonella is essential [14]. In conventional methods, bacteria are mainly detected by culture, enzyme-linked immunosorbent assays (ELISAs) and polymerase chain reactions (PCRs) [15]. An automatic, rapid, sensitive and automated detection system for foodborne pathogens has been successfully developed using a microfluidic biosensor [16][17]. An MNB–Salmonella–MOF complex was formed using the immune magnetic nanobeads (MNBs) and the immune magnetic nanofoams (MOFs) to detect Salmonella typhimurium. As a result of the catalysis by the complexes, yellow 2,3-diaminophenazine (DAP) was produced by combining o-phenylenediamine with H2O2 as shown in Figure 1. The catalyst was then photographed using a narrow-band blue light. With greater sensitivity, the improved image processing was demonstrated to be effective for rapidly mixing the solutions in the vibrating mixer. Detection of Salmonella typhimurium at a concentration of 14 CFU/mL was achieved using the microfluidic biosensor, which integrated mixing, incubation, separation, catalysis, and detection. Despite its microfluidic design, this microfluidic biosensor does not cost more than USD 200. For ensuring food safety, it can be extended for in-field screening of other foodborne bacteria [18].
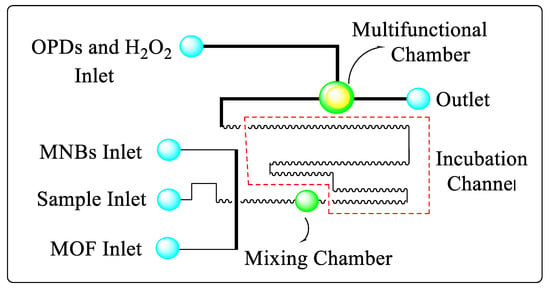
Figure 1. Diagram of the microfluidic chip to detect Salmonella typhimurium bacteria.
Approximately 30 thousand tons of cephalexin, which is a kind of β-lactam, are produced each year and the antibiotic sells for USD 15 billion annually [19]. An integrated microfluidic platform was developed to manufacture cephalexin continuously through an enzymatic reaction. Microspace is used on this platform as an excellent environment in which to perform continuous reaction–transport processes. This platform offers simultaneous synthesis and separation of reaction products in combination with an aqueous two-phase system (ATPS). It is screened for the composition of ATPS that allows the separation of the enzyme from the reaction product. In order to synthesize cephalexin, the bottom (salt) phase of ATPS is used as a reaction medium. During the kinetic regime, reaction conditions are set so that maximum cephalexin yields can be achieved. Afterward, slug-flow microfluidic platforms are used to test the enzyme recycle of cephalexin synthesis. A modular microfluidic system with a gravity settler and a microdialysis unit is utilized to continuously synthesize cephalexin [20].
Metal–organic nanoparticles (MONs) were prepared from diethyldithiocarbamate copper Cu(DDC)2 using a Stabilized Metal Ion Ligand Nanocomplex (SMILE) technique [21]. In addition to being a superior yielding and high-concentration method, the SMILE method also requires a simplified process for formulation and preparation, and it has excellent formulation properties. The 3D-printed microfluidic device was used to further develop the SMILE technology for continuous production of bovine serum albumin Cu(DDC)2 MONs (Scheme 3). The microfluidic device was used to achieve precise mixing control, to easily scale up preparation for mass production, and to demonstrate great potential for treating breast cancer [22].
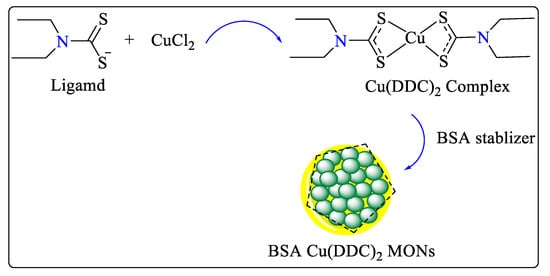
Scheme 3. Synthetic reaction performed in microfluidic system to synthesize BSA Cu(DDC)2 MONs.
A microfluidic chip based on glass capillaries was used to produce solid–lipid nanoparticles (SLNs) for the first time. The current synthesis method showed several advantages over conventional bulk methods, which usually suffer from multiple preparation steps, low production rates and poor reproducibility, as well as being able to produce SLNs continuously with high yields, to be highly reproducible, and to be precisely controlled over their physical properties. For the purpose of testing the efficacy of SLN-based nano formulations in cancer therapy, sorafenib (SFN) and paclitaxel (PTX) were used as model drugs. As a result of the microfluidic production of SLNs, the drugs were encapsulated efficiently and loaded sufficiently to sustain a sustained release. In addition, fluorescence assisted imaging was used to confirm tumor penetration and cellular uptake as well as the anti-cancer efficacy of the drug-loaded SLN formulations [23].
A microreactor device was used for the successful preparation of lignin/chitosan pH-responsive polymer nanoparticles (Lig/Chi NPs) used for drug delivery. The electrostatic assembly of amino groups of chitosan and carboxyl groups of lignin formed Lig/Chi NPs during the mixing of positively charged chitosan and negatively charged lignin solutions in a microreactor. There is a positive charge on the surface of the nanoparticles, and they are very dispersible as shown in Figure 2. With the microreactor, lignin-based nanoparticles (LNP) suspensions with high stability and controlled size distributions can be produced with more uniform mixing and better control of the solvent/antisolvent ratio throughout the process. Lig/Chi NPs have been demonstrated as pH-responsive drug delivery carriers in these studies, and their production and application have reached a large-scale and commercial level [24].
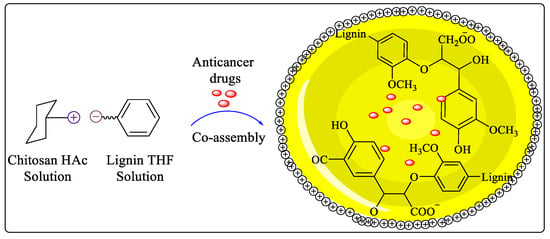
Figure 2. Microfluidic synthesis of Lig/Chi NPs.
Poly(ε-caprolactone) (PCL) is a polymer made from synthetic materials and has significant drug-loading capacity (LC), biodegradability, non-immunogenicity, and permeability, all of which improve the controlled release of drugs [25]. PCL nanoparticles (NPs) containing clarithromycin (CLR) have been synthesized using the microfluidic technique. To ensure the most homogeneous solution, 0.04 g of PCL granules were added to 20 mL of acetone, and the mixture was agitated for 1 h at 45 °C and 1 h at room temperature. Subsequently, CLR powder was dissolved in a cooled polymer solution in a tightly closed container. To produce NPs, distilled water was injected into the adjacent channels at a flow rate of 50 mL/h, whereas the PCL/CLR solution was injected into the middle channel at a rate of 2.5 mL/h. Subsequently, the two fluid flows were joined by a hydrodynamic flow-focusing event in the mixing region, where two liquid continuous phase streams surrounded the scattered phase stream and split it into droplets. To conduct additional analyses, the solution was lyophilized after it was removed from the outlet channel, after which the PCL NPs were formed (Figure 3). The resulting NPs were smaller, more uniform, and more stable than the NPs prepared using conventional methods [26].
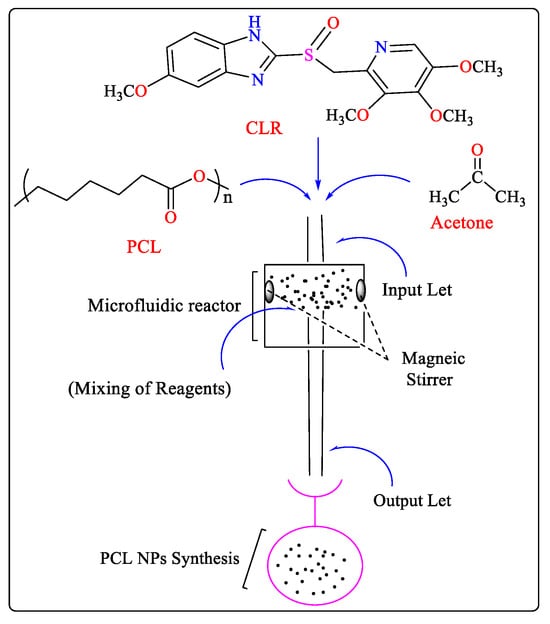
Figure 3. Square-shaped microfluidic reactor-based synthesis of PCL NPs.
2. Photocatalytic Applications
Synthetic organic photocatalytic chemistry is an effective method for easily producing both naturally occurring chemicals and compounds with highly complex structures under favorable circumstances. However, implementing a photochemical mechanism in an industrial process has proven challenging owing to scaling-up difficulties. With flow chemistry, it is possible to have more control over the reaction parameters, as well as higher reaction selectivity and reproducibility [27][28][29]. In contrast to conventional continuous-flow systems, droplet microfluidic technology uses multiple immiscible fluids to create a series of droplets that serve as analysis units. This avoids the issues of sample diffusion and cross-contamination, while further reducing the amount of reagent used. This method modifies fluids in microscale channels and offers a framework for several chemical synthesis approaches, leading to materials and molecules [30].
Metal catalysts and large quantities of hazardous organic solvents are required for the practical production of polyconjugate polymeric materials. Aqueous solutions containing micelle-forming agents can easily substitute for non-aqueous solvents, even when used for the manufacture of water-insoluble organics. Recently, the researchers described a selective metal-free photoinduced microfluidic system-based direct arylation procedure conducted in a laboratory setting with water, ambient temperature, and minimal competitive dehalogenation. The surfactant Kolliphor EL (K-EL) and a specially formulated photo redox mediator (S-PTh), which also functioned as a cosurfactant, partitioned numerous reactive species into different compartments of the association colloid formed in water, resulting in unexpected selectivity. The synthesized arylated compounds prepared by batch process had several drawbacks in terms of reagent amount, selectivity, reproducibility, and preparation time. To overcome these problems, the reaction was performed in a microfluidic reactor system and the results were surprising [31].
Compared to conventional batch synthesis, microfluidic reactors have demonstrated considerable advantages in photocatalytic organic synthesis because they transfer heat and mass rapidly, have short molecular diffusion distances, are easy to control, and are light transplant [32][33][34]. Researchers reported a cationic poly(p-phenylene ethynylene terthiophene) (PPET3-N2) as a sensitizer for effective photocatalytic oxidation of a series of organic sulfides in microfluidic device. In the present study, photocatalytic oxidation of 4-methoxythioanisole was performed. The channel in the microfluidic reactor was filled with mixed solution of PPET3-N2 and 4-methoxythioanisole in methanol. In order to keep the microfluidic reactor in an oxygen rich environment, oxygen was delivered into another channel using a syringe pump [35].
3. Biochemical Applications
Several new methods have been demonstrated recently for the construction of multifunctional wound healing materials. As the substrate, a polyvinylpyrrolidone (PVP)-oriented membrane was spun by microfluidics to create an oriented microfiber membrane. A zeolitic metal–organic framework-8 at ascorbic acid (ZIF-8@AA) was used as a framework to load ascorbic acid in drug-delivery nanomaterials. Following microfluidic spinning, the PVP-oriented membrane showed robust antibacterial and drug release performance. Biocompatibility and hemocompatibility of the fabricated PVP membrane were excellent in vitro. The present work offers a method of constructing regular microfiber arrangements and allowing active materials to be loaded into substrates in the biomedical field for disease treatment [36].
An insulin delivery system based on a metal–organic framework (MOF) has been developed using microfluidics. Through a continuous-flow, microfluidic mixing system, ZIF-8 was synthesized with insulin and gold nanoparticles (AuNPs). To oxidize glucose molecules absorbed by porous ZIF-8, glucose oxidase mimicking the function of AuNPs was used. As glucose was oxidized inside the MOF, gluconic acid and hydrogen peroxide were produced. It is possible to use these synthetic bioactive MOFs to develop stimulus-responsive drug delivery systems and to exploit them for biosensing applications [37].
In Figure 4, enzyme–MOF composites were synthesized using a double-Y-shaped microfluidic channel. First, zinc ions (Zn2+) were mixed with 2-methylimidazole (2-MeIM), followed by protein. Through centrifugation and washing steps, the product was continuously collected from the outlet. As the ratio of reactants in the microchannel continuously changed, mesopores appeared in the resultant products. The multimodal distribution of pore sizes enabled enzymes to be immobilized while reducing resistance to mass transfer. As a result, enzyme–MOF composites prepared from conventional bulk solution synthesis are believed to have a nearly twofold higher enzymatic activity than enzyme–MOF composites. As a result of this defect-assisted synthesis, a new approach to enhancing enzyme–MOF composite activity has been proposed [38].
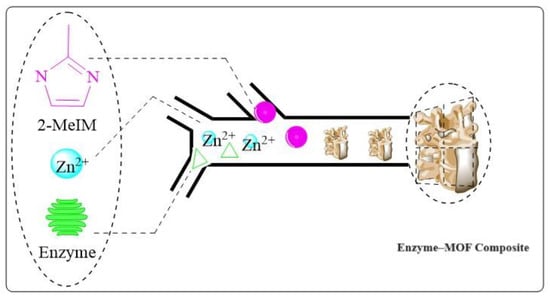
Figure 4. Microfluidic system-based synthesis of enzyme–MOF composites.
Through a microfluidic approach, researchers have developed BioZIF-8 MOFs that are aptamer-functionalized in a single step and using a single chip. Microfluidics enabled the encapsulation of nucleic acids, proteins, and small drug molecules while simultaneously functionalizing BioZIF-8 MOFs with an aptamer (RNA and DNA aptamers). It was found that neat BioZIF-8 MOFs had a much lower toxicity profile and were more effective at targeting lymph nodes and tumor masses than the aptamer-BioZIF-8 MOFs [39][40][41]. In this study, BioZIF-8 MOFs are prepared using a simple one-step and one-chip microfluidic method [42].
4. Fine Chemical Production
Azo compounds are adaptable substances used in a wide range of products including batteries and anticancer medications and are typically produced via challenging solution-based processes [43][44]. To facilitate the chemical reaction, diazotization is typically performed in a highly concentrated aqueous HCl solution. The pH is brought back to neutral because excessive amounts of either alkaline or acidic reagents can cause product deterioration. However, these conventional solution-phase synthesis methods require challenging procedures involving metal catalysts, precise temperature, and environmental control. Consequently, a unique method for producing azo compounds known as microfluidics-based pH control, in which all of the difficult steps are completed in tiny droplets, has been developed. Using the microdroplet technique, the required azo molecule could be produced quickly, allowing the reaction to be conducted at ambient temperature (23 °C) as opposed to 0 °C and reducing the reaction time from 1 h to 3 s or less. Furthermore, pH control can be easily attained, and the reagent concentration can be lowered to one-tenth of what is typically required. In addition, the presented microfluidic device makes it simple to alter the reagent concentration by changing the rate at which it flows, and synthetic experiments may be conducted under various conditions (Figure 5).
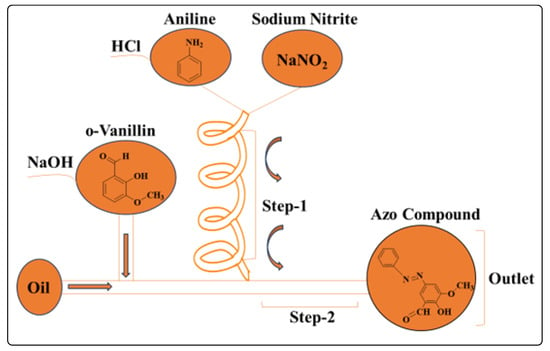
Figure 5. Microfluidic system for the synthesis of azo compounds.
Aniline, sodium nitrite, and o-vanillin were used to prepare azo compounds according to Scheme 5. Aqueous HCl and sodium hydroxide solutions were used to dissolve aniline and o-vanillin, respectively. To investigate their impact on the chemical reaction, the flow rate varied with pH and reagent concentration. After filtration, the azo compound was isolated [45].
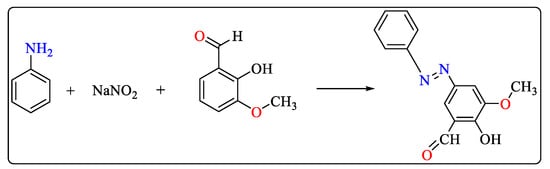
Scheme 5. Reaction scheme for the synthesis of azo compounds.
The two-step microfluidic system-based synthesis of a Cu(II) complex with a Schiff base is shown in Figure 6. Isoleucine and salicylaldehyde are injected from inlets A and B, respectively, at a rate of flow 5 L/min. The Schiff base ligand is produced in Step 1. Inlet C is used to induce the formation of a Cu(II) acetate dihydrate solution (20 mmol/L) at a flow rate of 10 L/min. The Cu(II) complex ligand is produced and removed as a solution from outlet D. Syringes and syringe pumps are used to inject the reactants dissolved in methanol into the inlets. This facilitates the precise control of the flow rate of the microfluidic device and the amount of reactants added [46].
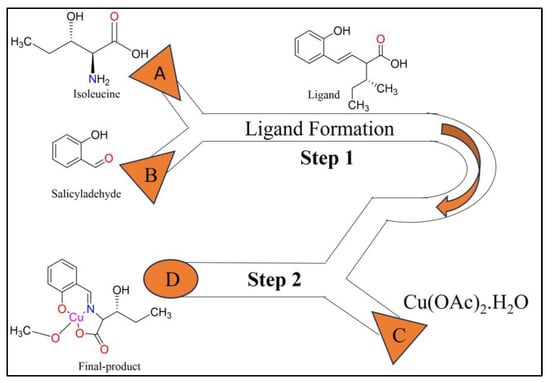
Figure 6. Microfluidic synthesis of Schiff base Cu(II) complex.
Schiff bases are produced via the combination of amino and carbonyl groups containing multidentate ligands to synthesize highly significant complexes with metal ions. These compounds are used as polymer stabilizing substances [47], anti-corrosion [48], dyes, pigments, catalysts, antioxidants [49], carcinogens, and antimicrobial agents [50]. For the first time, microfluidic technology allowed for temperature-free synthesis of the Schiff base Cu(II) complex in 20 s, and the reaction performance was approximately 700 times greater than that of the synthesis utilizing the beaker [51] (Scheme 6).
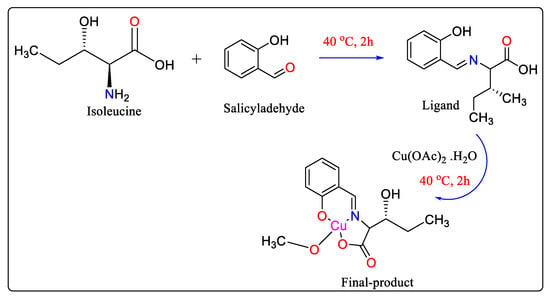
Scheme 6. Conventional beaker-based synthesis of Schiff base Cu(II) complex.
Another Cu(II) complex was produced like the one described above. As shown in Scheme 7, as a starting point, a ligand is prepared using 3,5-dichlorosalicylaldehyde and (1R,2R)-(+)-1,2-diphenyl-ethylenediamine in a beaker with methanol as the solvent. Next, a droplet-merging device is used to synthesize a copper complex using the ligand and Cu(II) acetate monohydrate. The ligand and Cu(II) acetate monohydrate are effectively combined during the merging stage in a methanol solution. Immediately upon their merging, the droplet interiors were transparent; however, a complex crystal started to emerge after 0.2 s. The synthesis is almost finished in one second at an ambient temperature below 25 °C, as opposed to two hours at 40 °C for the beaker-level work [52].
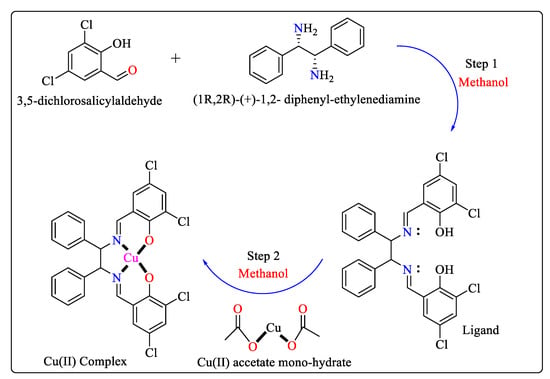
Scheme 7. Schematic pathway for the synthesis of Cu(II) complex. Step 1 for the synthesis of ligands and Step 2 for the synthesis of metal complexes.
Covalent organic frameworks (COFs) are a new class of molecular materials that rely on the precise chemical fusion of organic subunits to form 2D or 3D permeable crystalline frameworks coupled by covalent bonds with deterministic control over porosity, composition, and topology [53][54][55][56]. The majority of recently developed COFs are based on self-condensation reactions with other boronic acids or boronic acids condensed with catechol. Solvothermal reaction conditions are required to produce porous crystalline materials. However, the limited chemical stability of these COFs, despite their thermal durability, severely restricts their use in various applications [57][58]. Researchers reported the rapid synthesis of a crystalline COF at room temperature as part of our efforts to overcome these limitations.
Using femtosecond laser micromachining, microfluidic chips (MFCs) consisting of centimeter-level 3D channels with large volume and high density were developed for timesaving, economically viable, and hazard-free flow synthesis. Its advantages have been demonstrated by forming aryldiazonium salts in situ and borylating them with bis(pinacolato)diboron. The 3D MFC-based flow synthesis technology offers several important advantages, such as (1) altering the reaction temperature from an ice bath to room temperature; (2) reducing the residence time by 10 times; and (3) significantly increasing yields, as several aryl-boronates were produced with higher yields than traditional batch processes. It is therefore anticipated that a novel, simplified flow synthetic protocol will be developed for green organic synthesis using MFCs [59].
A microfluidic system was discovered here that automates chemical reaction screening and optimization inside microliter liquid droplets. Droplet “micro-reactors” are generated, merged, and flowed, and reaction conditions, including reagent volumes, temperature, and time, are precisely controlled by the system. Input parameters can be thoroughly monitored by allowing multiple reaction conditions to be screened simultaneously due to the high level of control coupled. Furthermore, there is a remarkable reduction in reagent consumption. The researcher demonstrated the use of a microfluidic platform to screen a model imine formation-(E)-1-(2-nitrophenyl)-N-phenethylmethanimine using ethanol as a solvent through a condensation of o-nitrobenzaldehyde and phenylethylamine. Tests were conducted using microfluidics to determine (i) the ratio of reagents (aldehyde and amine), (ii) the temperature, (iii) the reaction time, as well as (iv) the effect of p-toluenesulfonic acid as a catalyst [60].
References
- Hwang, Y.-J.; Coley, C.W.; Abolhasani, M.; Marzinzik, A.L.; Koch, G.; Spanka, C.; Lehmann, H.; Jensen, K.F. A segmented flow platform for on-demand medicinal chemistry and compound synthesis in oscillating droplets. Chem. Commun. 2017, 53, 6649–6652.
- Beulig, R.; Warias, R.; Heiland, J.; Ohla, S.; Zeitler, K.; Belder, D. A droplet-chip/mass spectrometry approach to study organic synthesis at nanoliter scale. Lab Chip 2017, 17, 1996–2002.
- Liu, D.; Wang, K.; Wang, Y.; Wang, Y.; Luo, G. A simple online phase separator for the microfluidic mass transfer studies. Chem. Eng. J. 2017, 325, 342–349.
- Sun, A.C.; Steyer, D.J.; Allen, A.R.; Payne, E.M.; Kennedy, R.T.; Stephenson, C.R. A droplet microfluidic platform for high-throughput photochemical reaction discovery. Nat. Commun. 2020, 11, 6202.
- Mo, Y.; Lu, Z.; Rughoobur, G.; Patil, P.; Gershenfeld, N.; Akinwande, A.I.; Buchwald, S.L.; Jensen, K.F. Microfluidic electrochemistry for single-electron transfer redox-neutral reactions. Science 2020, 368, 1352–1357.
- Zhang, Y.; Ren, B.; Zhang, D.; Liu, Y.; Zhang, M.; Zhao, C.; Zheng, J. Design principles and fundamental understanding of biosensors for amyloid-β detection. J. Mater. Chem. B 2020, 8, 6179–6196.
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356.
- Koklu, A.; Wustoni, S.; Musteata, V.-E.; Ohayon, D.; Moser, M.; McCulloch, I.; Nunes, S.P.; Inal, S. Microfluidic integrated organic electrochemical transistor with a nanoporous membrane for amyloid-β detection. ACS Nano 2021, 15, 8130–8141.
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940.
- Jaouhari, T.; Zhang, F.; Tassaing, T.; Fery-Forgues, S.; Aymonier, C.; Marre, S.; Erriguible, A. Process intensification for the synthesis of ultra-small organic nanoparticles with supercritical CO2 in a microfluidic system. Chem. Eng. J. 2020, 397, 125333.
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material n. Science 1999, 283, 1148–1150.
- Koryakina, I.G.; Bachinin, S.V.; Gerasimova, E.N.; Timofeeva, M.V.; Shipilovskikh, S.A.; Bukatin, A.S.; Sakhatskii, A.; Timin, A.S.; Milichko, V.A.; Zyuzin, M.V. Microfluidic synthesis of metal-organic framework crystals with surface defects for enhanced molecular loading. Chem. Eng. J. 2023, 452, 139450.
- Shen, J.; Ma, M.; Zhang, H.; Yu, H.; Xue, F.; Hao, N.; Chen, H. Microfluidics-assisted surface trifunctionalization of a zeolitic imidazolate framework nanocarrier for targeted and controllable multitherapies of tumors. ACS Appl. Mater. Interfaces 2020, 12, 45838–45849.
- Silva, N.F.; Magalhães, J.M.; Freire, C.; Delerue-Matos, C. Electrochemical biosensors for Salmonella: State of the art and challenges in food safety assessment. Biosens. Bioelectron. 2018, 99, 667–682.
- Kong, R.; Lee, S.; Law, T.; Law, S.; Wu, R. Rapid detection of six types of bacterial pathogens in marine waters by multiplex PCR. Water Res. 2002, 36, 2802–2812.
- Sayad, A.; Ibrahim, F.; Uddin, S.M.; Cho, J.; Madou, M.; Thong, K.L. A microdevice for rapid, monoplex and colorimetric detection of foodborne pathogens using a centrifugal microfluidic platform. Biosens. Bioelectron. 2018, 100, 96–104.
- Tian, F.; Lyu, J.; Shi, J.; Tan, F.; Yang, M. A polymeric microfluidic device integrated with nanoporous alumina membranes for simultaneous detection of multiple foodborne pathogens. Sens. Actuators B Chem. 2016, 225, 312–318.
- Qi, W.; Zheng, L.; Wang, S.; Huang, F.; Liu, Y.; Jiang, H.; Lin, J. A microfluidic biosensor for rapid and automatic detection of Salmonella using metal-organic framework and Raspberry Pi. Biosens. Bioelectron. 2021, 178, 113020.
- Chandel, A.K.; Rao, L.V.; Narasu, M.L.; Singh, O.V. The realm of penicillin G acylase in β-lactam antibiotics. Enzym. Microb. Technol. 2008, 42, 199–207.
- Vobecká, L.; Tichá, L.; Atanasova, A.; Slouka, Z.; Hasal, P.; Přibyl, M. Enzyme synthesis of cephalexin in continuous-flow microfluidic device in ATPS environment. Chem. Eng. J. 2020, 396, 125236.
- Chen, W.; Yang, W.; Chen, P.; Huang, Y.; Li, F. Disulfiram copper nanoparticles prepared with a stabilized metal ion ligand complex method for treating drug-resistant prostate cancers. ACS Appl. Mater. Interfaces 2018, 10, 41118–41128.
- Chang, Y.; Jiang, J.; Chen, W.; Yang, W.; Chen, L.; Chen, P.; Shen, J.; Qian, S.; Zhou, T.; Wu, L. Biomimetic metal-organic nanoparticles prepared with a 3D-printed microfluidic device as a novel formulation for disulfiram-based therapy against breast cancer. Appl. Mater. Today 2020, 18, 100492.
- Arduino, I.; Liu, Z.; Rahikkala, A.; Figueiredo, P.; Correia, A.; Cutrignelli, A.; Denora, N.; Santos, H.A. Preparation of cetyl palmitate-based PEGylated solid lipid nanoparticles by microfluidic technique. Acta Biomater. 2021, 121, 566–578.
- Chai, Y.; Wang, Y.; Li, B.; Qi, W.; Su, R.; He, Z. Microfluidic synthesis of lignin/chitosan nanoparticles for the pH-responsive delivery of anticancer drugs. Langmuir 2021, 37, 7219–7226.
- Sudhakar, K.; Rao, K.M.; Sudhakar, P.; ChandraBabu, A.; Kumara Babu, P.; Subha, M.; Chowdoji Rao, K. Development of pH-sensitive polycaprolactone-based microspheres for in vitro release studies of Triprolidine Hydrochloride. Des. Monomers Polym. 2014, 17, 617–623.
- Tavana, B.; Khatibi, A.; Jafarkhani, S.; Zahedi, P.; Zamani, M.H.; Jafari, S.H.; Najafi, M. Simulation and in vitro evaluations of microfluidically-fabricated clarithromycin-poly (ε-caprolactone) nanoparticles. J. Ind. Eng. Chem. 2023, 124, 211–223.
- Buglioni, L.; Raymenants, F.; Slattery, A.; Zondag, S.D.; Noël, T. Technological innovations in photochemistry for organic synthesis: Flow chemistry, high-throughput experimentation, scale-up, and photoelectrochemistry. Chem. Rev. 2021, 122, 2752–2906.
- Hone, C.A.; Kappe, C.O. Towards the standardization of flow chemistry protocols for organic reactions. Chem.-Methods 2021, 1, 454–467.
- Dinter, R.; Willems, S.; Nissalk, T.; Hastürk, O.; Brunschweiger, A.; Kockmann, N. Development of a microfluidic photochemical flow reactor concept by rapid prototyping. Front. Chem. 2023, 11, 1244043.
- Chu, W.-Y.; Chiou, Y.-R.; Luo, R.-H.; Chen, T.-H.; Yu, C.-J.; Chou, Y.-J.; Chang, H.-T.; Chen, C.-F. Partially miscible droplet microfluidics to enhance interfacial adsorption of hydrophilic nanoparticles for colloidosome synthesis. Chem. Eng. J. 2023, 471, 144223.
- Pallini, F.; Sangalli, E.; Sassi, M.; Roth, P.M.; Mattiello, S.; Beverina, L. Selective photoredox direct arylations of aryl bromides in water in a microfluidic reactor. Org. Biomol. Chem. 2021, 19, 3016–3023.
- Baek, J.; Shen, Y.; Lignos, I.; Bawendi, M.G.; Jensen, K.F. Multistage microfluidic platform for the continuous synthesis of III–V core/shell quantum dots. Angew. Chem. Int. Ed. 2018, 57, 10915–10918.
- Chowdhury, M.S.; Zheng, W.; Kumari, S.; Heyman, J.; Zhang, X.; Dey, P.; Weitz, D.A.; Haag, R. Dendronized fluorosurfactant for highly stable water-in-fluorinated oil emulsions with minimal inter-droplet transfer of small molecules. Nat. Commun. 2019, 10, 4546.
- Jayamohan, H.; Smith, Y.R.; Gale, B.K.; Mohanty, S.K.; Misra, M. Photocatalytic microfluidic reactors utilizing titania nanotubes on titanium mesh for degradation of organic and biological contaminants. J. Environ. Chem. Eng. 2016, 4, 657–663.
- Li, J.; An, Z.; Sun, J.; Tan, C.; Gao, D.; Tan, Y.; Jiang, Y. Highly selective oxidation of organic sulfides by a conjugated polymer as the photosensitizer for singlet oxygen generation. ACS Appl. Mater. Interfaces 2020, 12, 35475–35481.
- Zhang, Y.; Li, T.-T.; Sun, L.; Shiu, B.-C.; Zhang, L.; Lin, J.-H.; Lou, C.-W. Oriented ascorbic acid onto zeolitic metal-organic framework-8 membrane via microfluidic spinning for biomedical care. Colloids Surf. B Biointerfaces 2023, 229, 113442.
- Rohra, N.; Gaikwad, G.; Dandekar, P.; Jain, R. Microfluidic Synthesis of a Bioactive Metal–Organic Framework for Glucose-Responsive Insulin Delivery. ACS Appl. Mater. Interfaces 2022, 14, 8251–8265.
- Hu, C.; Bai, Y.; Hou, M.; Wang, Y.; Wang, L.; Cao, X.; Chan, C.-W.; Sun, H.; Li, W.; Ge, J. Defect-induced activity enhancement of enzyme-encapsulated metal-organic frameworks revealed in microfluidic gradient mixing synthesis. Sci. Adv. 2020, 6, eaax5785.
- Lian, J.; Ozga, A.J.; Sokol, C.L.; Luster, A.D. Targeting lymph node niches enhances type 1 immune responses to immunization. Cell Rep. 2020, 31, 107679.
- Tian, Y.; Wang, H.; Liu, Y.; Mao, L.; Chen, W.; Zhu, Z.; Liu, W.; Zheng, W.; Zhao, Y.; Kong, D. A peptide-based nanofibrous hydrogel as a promising DNA nanovector for optimizing the efficacy of HIV vaccine. Nano Lett. 2014, 14, 1439–1445.
- Xu, C.; Hong, H.; Lee, Y.; Park, K.S.; Sun, M.; Wang, T.; Aikins, M.E.; Xu, Y.; Moon, J.J. Efficient lymph node-targeted delivery of personalized cancer vaccines with reactive oxygen species-inducing reduced graphene oxide nanosheets. ACS Nano 2020, 14, 13268–13278.
- Balachandran, Y.L.; Li, X.; Jiang, X. Integrated microfluidic synthesis of aptamer functionalized biozeolitic imidazolate framework (BioZIF-8) targeting lymph node and tumor. Nano Lett. 2021, 21, 1335–1344.
- Zhao, R.; Tan, C.; Xie, Y.; Gao, C.; Liu, H.; Jiang, Y. One step synthesis of azo compounds from nitroaromatics and anilines. Tetrahedron Lett. 2011, 52, 3805–3809.
- Aritake, Y.; Takanashi, T.; Yamazaki, A.; Akitsu, T. Polarized spectroscopy and hybrid materials of chiral Schiff base Ni (II), Cu (II), Zn (II) complexes with included or separated azo-groups. Polyhedron 2011, 30, 886–894.
- Tanaka, D.; Sawai, S.; Hattori, S.; Nozaki, Y.; Yoon, D.H.; Fujita, H.; Sekiguchi, T.; Akitsu, T.; Shoji, S. Microdroplet synthesis of azo compounds with simple microfluidics-based pH control. RSC Adv. 2020, 10, 38900–38905.
- Kobayashi, M.; Akitsu, T.; Furuya, M.; Sekiguchi, T.; Shoji, S.; Tanii, T.; Tanaka, D. Efficient Synthesis of a Schiff Base Copper (II) Complex Using a Microfluidic Device. Micromachines 2023, 14, 890.
- Dhar, D.N.; Taploo, C. Schiff bases and their applications. J. Sci. Ind. Res. 1982, 41, 501–506.
- Abd El-Lateef, H.M.; Abu-Dief, A.M.; Mohamed, M.A. Corrosion inhibition of carbon steel pipelines by some novel Schiff base compounds during acidizing treatment of oil wells studied by electrochemical and quantum chemical methods. J. Mol. Struct. 2017, 1130, 522–542.
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Innovation of imine metal chelates as corrosion inhibitors at different media: A collective study. Int. J. Mol. Sci. 2022, 23, 9360.
- Ghanghas, P.; Choudhary, A.; Kumar, D.; Poonia, K. Coordination metal complexes with Schiff bases: Useful pharmacophores with comprehensive biological applications. Inorg. Chem. Commun. 2021, 130, 108710.
- Khanam, W.; Dubey, N. Recent advances in immobilized ω-transaminase for chiral amine synthesis. Mater. Today Chem. 2022, 24, 100922.
- Yoon, D.H.; Jamshaid, A.; Ito, J.; Nakahara, A.; Tanaka, D.; Akitsu, T.; Sekiguchi, T.; Shoji, S. Active microdroplet merging by hydrodynamic flow control using a pneumatic actuator-assisted pillar structure. Lab Chip 2014, 14, 3050–3055.
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. science 2005, 310, 1166–1170.
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. 2008, 120, 8958–8962.
- Spitler, E.L.; Dichtel, W.R. Lewis acid-catalysed formation of two-dimensional phthalocyanine covalent organic frameworks. Nat. Chem. 2010, 2, 672–677.
- Jiang, J.-X.; Cooper, A.I. Microporous organic polymers: Design, synthesis, and function. In Functional Metal-Organic Frameworks: Gas Storage, Separation and Catalysis; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–33.
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568.
- Colson, J.W.; Dichtel, W.R. Rationally synthesized two-dimensional polymers. Nat. Chem. 2013, 5, 453–465.
- Ren, J.; Wu, M.; Dong, K.; Zhang, M.; Cheng, Y.; Shi, G. Highly efficient synthesis and application of aryl diazonium salts via femtosecond laser-tailored 3D flow microfluidic chips. Chin. Chem. Lett. 2023, 34, 107694.
- Jankowski, P.; Kutaszewicz, R.; Ogończyk, D.; Garstecki, P. A microfluidic platform for screening and optimization of organic reactions in droplets. J. Flow Chem. 2020, 10, 397–408.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
26 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




