1. Introduction
As the global incidence of cancer continues to increase, there is a rising need for innovative and efficacious treatment approaches
[1]. This demand extends to alternative therapies that can address the shortcomings of traditional treatments and improve patient outcomes. In this context, there has been a notable focus on treatments that harness the properties of molecular oxygen, leveraging its reactive states, which parallel those naturally employed by the body. This emphasis is especially prominent in the realm of photodynamic therapy
[2].
It is widely recognized that cells naturally produce reactive oxygen species (ROS) to perform essential functions, such as cell signaling and defending against pathogens
[3]. At physiological levels, ROS also play beneficial roles, including the regulation of gene expression, cell differentiation, and the maintenance of stem cells
[4]. These endogenous ROS mainly originate from natural sources like mitochondrial respiration and various enzymes, including NADPH oxidases (NOXs), among other contributors. However, they can also be generated by external factors such as ultraviolet light (UV) and ionizing radiation (IR), drugs, and other substances like tobacco and alcohol
[3]. When reactive oxygen species (ROS) levels exceed the physiological range, they can indeed become detrimental to cellular components, including nucleic acids, lipids, and proteins, causing cellular disruption and cell death through mechanisms like apoptosis, necrosis, and autophagy
[3][4][5]. These adverse effects of ROS are also associated with processes related to aging, cancer, and neurodegenerative diseases
[6]. Nonetheless, despite their potential for causing cellular damage, ROS are gaining recognition as valuable assets in cancer therapy.
One approach to exploit the potential of ROS for therapeutic purposes is through photodynamic therapy (PDT). PDT studies have shown promise in directing the harmful effects of ROS toward cancer cells by utilizing the body’s endogenous oxygen in combination with a photosensitizer (PS) and light at a specific wavelength
[5][6][7]. Photodynamic therapy (PDT) is a minimally invasive method that has captured significant attention as an emerging tool for the treatment of cancer. Some studies have also reported its potential to offer several advantages when applied in combination with conventional therapies, such as chemotherapy and radiation
[8][9].
The dynamics of PDT are mainly dependent on the electronic interactions between an excited photosensitizer compound (PS) and molecular oxygen. These further lead to energy transfer reactions, culminating in the generation of ROS, primarily singlet oxygen (
1O
2)
[5]. PDT is gaining considerable recognition for its selectivity, relative ease of therapeutic application, high tolerability by patients, and efficacy in treating certain cancers, including inoperable tumors
[10][11]. A significant advantage of this therapeutic approach is its ability to focus disruptive oxygenated reactions on a specific target site within the body, like tumors, thereby minimizing damage to the surrounding healthy tissue
[11].
2. Photodynamic Therapy: Principles and Reaction Mechanisms
Photodynamic therapy (PDT) is a therapeutic approach that hinges on the combined effects of light, a photosensitizer (PS), and molecular oxygen. Initially, the patient is administered a PS, either through intravenous injection or topical application. Following this, there is a crucial period termed the ‘drug-light interval’. During this phase, the PS selectively accumulates in the target tissue, ensuring that the desired concentration is reached. Once the optimal distribution is achieved, light of a specific wavelength is applied to the target area, which subsequently results in the activation of the PS
[5][12][13].
The selection of the wavelength is determined by the absorption spectrum unique to the chosen PS. For PDT applications, the chosen wavelengths typically fall within the range of 600 to 850 nanometers (nm) in the absorption spectrum
[12][14]. This spectrum defines the range of light wavelengths that a PS can effectively absorb. Each wavelength within this spectrum contains the exact energy needed to activate the PS, thus enabling energy transitions. Activation requires that the absorbed light’s energy precisely matches the energy difference required for electrons to shift between their respective energy levels
[13][15]. This concept is visually represented by the peaks in the absorption spectrum, which correspond to the energy differences between the electron energy states of the molecule
[16].
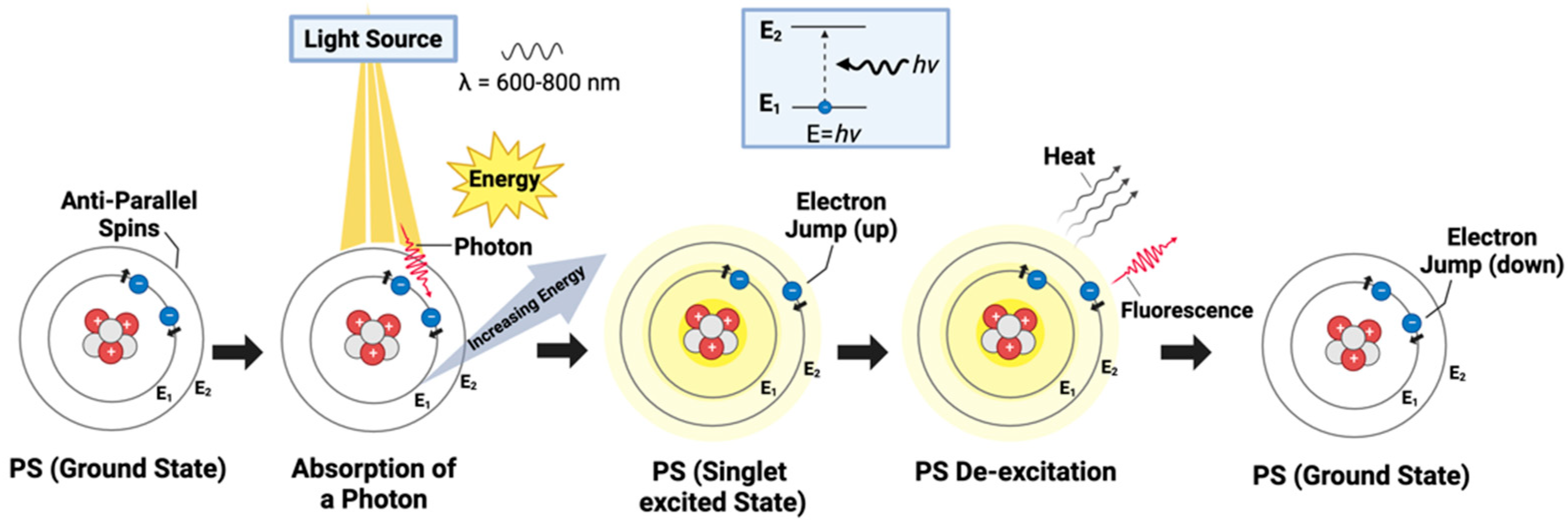
Upon irradiation, and the subsequent absorption of the requisite energy, electrons within the PS are propelled to higher energy states. This facilitates the transition of the PS molecule from its stable ground state to an excited state (
Figure 1)
[13][17]. A ground state is characterized by electrons residing in the lowest energy level of an atom or molecule (n = 1), thereby maintaining minimal energy and ensuring stability due to their proximity to the nucleus and minimized interactions
[18]. This shift from the ground to the excited state is critical, as it initiates photodynamic reactions and results in the transformation of the PS to a transient excited singlet state (
1O
2). In this state, the PS becomes electronically unstable and highly reactive, characterized by one electron occupying a higher energy level than its counterpart in the pair, thereby possessing surplus energy
[13][19].
Figure 1. Basic mechanism of photosensitizer excitation in PDT: The photosensitizer (PS) is represented as a single atom for conceptual ease, highlighting the electron transitions between energy levels. The diagram illustrates the initial stage of PS activation, where the PS, in its ground state, absorbs a photon within the therapeutic window (600–800 m), elevating an electron to the singlet excited state. The inset clarifies the electron’s energy jump and subsequent fluorescence emission as it returns to the ground state, a precursor event before intersystem crossing and reactive oxygen species generation in PDT.
To clarify this concept further, typically, during this fleeting phase, the electrons within the PS, which ordinarily exist in pairs with opposite spins, undergo a process of unpairing (
Figure 1). The unpairing specifically results from one electron in the pair absorbing energy and moving to a higher level, while the other remains at a lower energy level
[13][18]. This altered electronic configuration, where electrons with opposite spins are temporarily unpaired and reside in distinct energy levels, contributes to the heightened reactivity of the singlet excited state
[13][18]. The singlet excited state is transitory as electrons seek to return swiftly to a stable, paired configuration
[13]. The energy imbalance and unpaired state of the electrons make the molecule more susceptible to engaging in chemical reactions
[13][18]. Moreover, as the PS seeks to dissipate its excess energy and return to its ground state, it can engage in other mechanisms. Such mechanisms may include fluorescence, where the excess energy is emitted as light, or internal conversion, which involves the transformation of excess energy into heat
[13][20].
Simultaneously, the singlet PS in its excited state can undergo intersystem crossing, where it transitions to a triplet state, the most efficient state for PDT applications
[21]. The triplet state is characterized by having two unpaired electrons, like the singlet state. However, in the triplet state, these electrons have parallel spins, which makes it more stable compared to the singlet excited state
[21]. In this brief yet reactive phase, the PS can efficiently transfer its excess energy to nearby molecules, like oxygen (O
2) found in the blood, leading to the production of reactive oxygen species (ROS), notably singlet oxygen (
1O
2)
[22]. This shift in the spin direction of an electron or intersystem crossing is a key process in PDT and typically occurs in a non-radiative manner since it takes place without the emission of a photon. The shift may be influenced by external factors, such as the presence of heavy atoms in the PS molecule, external magnetic fields, or the specific molecular environment. Additionally, intersystem crossing can also be significantly affected by a phenomenon known as spin–orbit coupling. This coupling is a natural quantum mechanical process involving the interaction between an electron’s spin and its orbital motion around the nucleus
[21][23]. Furthermore, the longer-lived triplet state of the PS enables the direct transfer of energy to molecular oxygen (O
2), resulting in the formation of singlet oxygen (
1O
2), the preferential byproduct resulting from the photochemical reaction
[24][25].
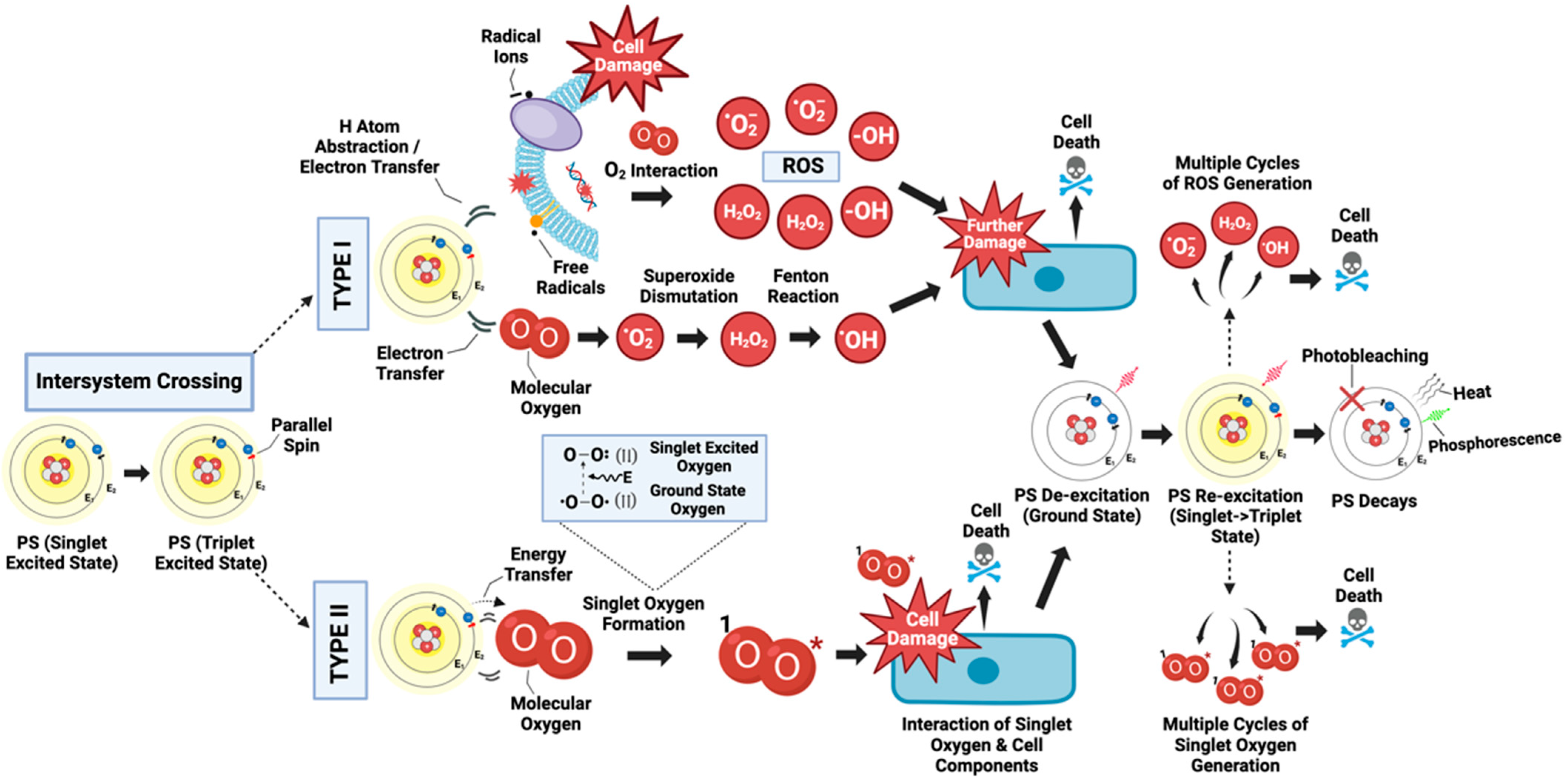
PDT involves two main types of reactions: type I and type II. Both contribute to the therapeutic effects of PDT by inducing cell death through different processes, and they can occur simultaneously
[5][26]. The balance between these reactions is influenced by several factors, including the nature of the PS, the substrate or target tissue, the local oxygen concentration, and the binding affinity of the sensitizer to the substrate
[5][27]. Both Type I and Type II are characterized by the PS initially being in a singlet excited state due to photon energy transfer from light irradiation. In this state, the PS can then undergo intersystem crossing to progress to a more stable, long-lived, electronically excited triplet state. Each reaction has different product types and is primarily classified according to the mechanism involved
[5][27].
In a Type I reaction, the triplet excited state of the PS directly interacts with a substrate, which could be a cell membrane or another molecule (
Figure 2). This biochemical interaction can directly cause damage through two different processes: hydrogen atom abstraction or electron transfer reactions. Following this, highly reactive free radicals and radical ions can be produced from reactions with cellular components such as lipids from the lipid membrane and proteins to induce immediate cellular damage. Additionally, these free radicals can further engage with molecular oxygen (O
2) to generate various reactive oxygen species (ROS), such as superoxide anions (O
2−•), hydroxyl radicals (HO
•), and hydrogen peroxide (H
2O
2). These ROS can cause subsequent oxidative damage to biological structures, ultimately leading to cell death
[5][24][28][29]. However, the literature on hydrogen atom abstraction and energy transfer in the context of PDT is limited, as there is a scarcity of information available on these topics.
Figure 2. Triplet excited state dynamics in PDT: The diagram captures the photodynamic action of a PS which, upon light absorption, transitions from a ground state to a singlet excited state. It then undergoes intersystem crossing to achieve a triplet excited state. In Type I reactions, the triplet PS engages with cellular substrates to simultaneously generate radical ions or free radicals. These entities can inflict damage on cellular substrates and, through further reactions with molecular oxygen, give rise to diverse ROS that cause enhanced damage. Concurrently, the PS can directly interact with oxygen to initiate a reaction cascade, leading to potent damage from the hydroxyl radical, resulting in cell death. In Type II reactions, the triplet PS directly transfers energy to molecular oxygen, forming singlet oxygen (1O2) that damages cellular components. Both Type I and Type II pathways can proceed in tandem, with repeated cycles of ROS generation culminating in extensive cell damage, cell death, and the degradation of the PS.
Castaño et al. are among the few to describe a parallel mechanism in Type I photodynamic therapy, where the triplet excited PS directly engages with molecular oxygen (
Figure 2). This interaction prompts an electron transfer, resulting in superoxide anion production (O
2−•). Superoxide itself, although reactive, is generally less damaging compared to other oxygen reactive species like the hydroxyl radical (HO
•). Subsequently, superoxide is further processed into hydrogen peroxide (H
2O
2) and oxygen through the process of dismutation, which can occur spontaneously or enzymatically through the Superoxide Dismutase enzyme (SOD). The critical damage occurs when superoxide participates in the Fenton reaction react with metal ions like iron (Fe
3+), causing their reduction (Fe
2+). The reduced metals can then engage with H
2O
2 to generate highly reactive hydroxyl radicals. These hydroxyl radicals, capable of penetrating cells, initiate a series of reactions that severely harm cellular components by bonding with or extracting electrons from intracellular molecules, ultimately resulting in cell death
[17].
The Type II reaction, on the other hand, predominantly occurs due to the direct transfer of energy from the triplet-excited PS to molecular oxygen (O
2), which concludes in the formation of singlet oxygen (
1O
2) and the return of the PS to its ground state (
Figure 2)
[30]. The energy transfer specifically occurs because of collisions, or proximity interactions, between the excited-triplet-state PS and oxygen (O
2). A PS molecule can produce a significant amount of singlet oxygen, typically ranging from 10
3 to 10
5 molecules, before it undergoes decay
[30]. Singlet oxygen is highly reactive and can interact with numerous biological substrates, inducing oxidative damage, and subsequently, cell death. Notably, the Type II reaction is predominant during PDT, and singlet oxygen is identified as the primary cytotoxic agent responsible for the biological effects observed
[30]. The molecules that are affected are determined by the location of the accumulated PS, and they can be either proteins, lipids, or DNA
[31]. Moreover, the PS can undergo multiple cycles of energy transfer, repeatedly transitioning to and from its ground state without degradation
[30]. Nevertheless, over time, it may experience a loss of activity, typically due to photobleaching
[32].
Additionally, the literature describes another mechanism known as the Type III reaction in PDT. Unlike the Type II reaction, this pathway operates independently of oxygen, allowing a PS to directly interact with specific target biomolecules
[33][34][35]. For the Type III mechanism to take effect, the PS itself should possess a specific targeting property toward proteins, nucleic acids, and other cellular molecules. Upon combination with a Type III PS, the biological target molecule can be directly and efficiently destroyed by the PS in its excited state following exposure to light. This mechanism offers a distinctive advantage by completely bypassing the ‘bottleneck’ of oxygen concentration that is often encountered in traditional PDT
[33][34][35][36]. However, it is worth noting that only a limited number of PSs possessing this intrinsic selectivity feature have been identified thus far, as detailed in the following section. Further research is strongly encouraged to identify and develop more promising Type III reaction molecules. These molecules have the potential to overcome the limitations associated with oxygen availability in current PSs, thus expanding the effectiveness of PDT
[35].
3. Evolution of Photosensitizer Design
As ongoing clinical investigations continue to generate evidence in the field of PDT, a wide variety of PSs have emerged over the years
[36]. In cancer therapy, PSs have been categorized according to their usage and effectivity for specific cancer types
[36][37]. Most of the PSs approved for cancer treatment are derived from the tetrapyrrole molecule, resembling the structure of a component of hemoglobin known as the protoporphyrin group
[37]. These compounds belong to the first generation of PSs and include the hematoporphyrin derivative (HpD) and photofrin II, a purified form of HpD
[38]. They have found applications in treating lung, colorectal, brain, and breast cancers. However, the first-generation PSs had several limitations due to their complex molecular structures, synthesis challenges, low quantum yields, and hydrophobic nature. These factors, combined with limited selectivity, significantly impeded their ability to penetrate tissues
[39]. To overcome these constraints, efforts were made to modify existing molecules by introducing chemical groups into their tetrapyrrole ring. While these modifications improved the solubility to some extent, not all issues were resolved
[40].
The significant breakthrough in the field of PSs came with the introduction of the second generation. Phthalocyanines and chlorins, which are part of this generation, proved to be more efficient, offering increased selectivity and improved tissue penetration, especially in the near-infrared spectrum, making them valuable for cancer treatment
[38][39][40]. An example of a widely recognized second-generation PS is 5-aminolevulinic acid (5-ALA). When metabolized, it transforms into protoporphyrin IX (PPIX), which functions as a PS
[39]. PpIX naturally occurs in all living cells and is used for the synthesis of heme groups, essential iron-containing compounds within hemoglobin
[41].
Administering 5-ALA to oncology patients results in a higher PpIX aggregation in cancer cells compared to normal cells
[39]. The selective accumulation of PpIX in tumor cells has been attributed to the altered metabolism of these cells, which is distinct from that of normal cells. Tumor cells exhibit a higher reliance on glycolysis for energy production, a phenomenon known as the Warburg effect, rather than oxidative phosphorylation. Consequently, tumor cells do not efficiently produce heme, which in turn results in the accumulation of PpIX within the tumor cells
[42].
An advantage of PpIX over other porphyrin-based photosensitizers is its relatively short elimination time (typically within 28 to 48 h), reducing the risk of long-term photosensitivity
[39]. However, individuals with certain porphyrias, a genetic disorder, can experience PpIX accumulation, which potentially leads to liver failure
[43]. Boronated porphyrins (BOPPs) are another class of second-generation PSs. When administered to a patient, BOPPs enable a dual treatment approach, combining both PDT and Boron Neutron Capture Therapy (BNCT) to target tumors, especially in the brain
[44]. This combination therapy takes advantage of boron’s natural affinity for cancerous tissue, providing an additional layer of treatment. However, BNCT’s clinical applications are evolving, and its availability varies across healthcare settings due to ongoing research and development initiatives
[44].
Recent efforts have led to significant advances in the development of a third generation of PSs. These novel compounds have been built upon well-known PS molecules like porphyrins, chlorins, and phthalocyanines. They are ingeniously combined with specific proteins, amino acids, antibodies, or carbohydrates, conferring them the ability to precisely target cellular components and disrupt vital cellular functions
[45]. This new class of PSs often employs advanced delivery systems, including attachment or encapsulation with nanoparticles
[45]. Such innovations hold the potential to enhance PS specificity, which can result in a higher accumulation at the tumor site and reduced systemic toxicity
[45].
In parallel with the development of third-generation PSs, the incorporation of nanozymes has also emerged as a noteworthy advancement in the field
[45][46]. Nanozymes are nanomaterials with enzyme-like properties capable of catalyzing various chemical reactions
[45]. In the context of PDT, they have been shown to regulate tumor oxygen deficiency as well as intensify the generation of ROS at the localized target site, ultimately leading to the more effective and rapid destruction of cancer cells
[45][46]. Furthermore, ongoing research in nanotechnology has highlighted its significant potential in PDT applications, as it enhances drug delivery precision, targetability, safety, and overall treatment efficacy in cancer therapy, some of which are discussed in the following section
[47].
As we can observe, most of the advances have been oriented toward enhancing the strength of PDT as a therapeutic approach in cancer, which relies on both light and PS accumulation. Thanks to this bifunctionality, PDT has been oriented to be more target-specific, causing less damage to healthy tissues within the body
[37].
Accumulating the knowledge from the different generations of PSs, an optimal PS molecule should be selected based on specific criteria, with the first parameter being its absorption spectrum, ideally falling within the range of 600 and 800 mm
[34]. Longer wavelengths, such as red and infrared, are preferred because they can penetrate deeper into the target tissue, as they are less likely to be absorbed and scattered by tissue components
[34][37]. Precise control of the wavelength is crucial, as exceeding 800 nm can result in insufficient energy for exciting oxygen to a singlet state, rendering the photosensitivity reaction nonviable
[34][37].
The selected PS should also be able to attain an appropriate quantum yield of its triplet excited form. In other words, an optimal number of PS molecules must reach a triplet excited state, following irradiation, to enable maximal interactions with oxygen, leading to the subsequent generation of a substantial quantity of ROS
[34]. In terms of toxicity, the PS should possess the ability to clear non-target tissues relatively quickly and must remain non-reactive in the absence of light
[34][37]. While it is advisable to have a long therapeutic window, allowing the PS to disperse from normal tissue and accumulate in the tumor, some studies now suggest that applying light shortly after PS administration might enhance effectiveness. This is because, during this early stage, a significant amount of the PSs may remain in the tumor blood vessels, potentially resulting in significant vascular damage and better treatment outcomes
[37][48].
In further considering the selection of an ideal PS, it is essential to factor in the potential benefits of photobleaching that refers to the process in which a PS becomes damaged or destroyed when exposed to light
[37]. This process has also been found to be advantageous in PDT treatment by contributing to the control of light dosage. Photobleaching can help to prevent over-treatment by limiting the continued activation of the PS, ensuring that the therapeutic effect is precisely targeted
[37][49].