
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vasiliki Gkretsi | -- | 2722 | 2024-01-24 14:19:36 | | | |
| 2 | Lindsay Dong | -1 word(s) | 2721 | 2024-01-25 01:38:15 | | |
Video Upload Options
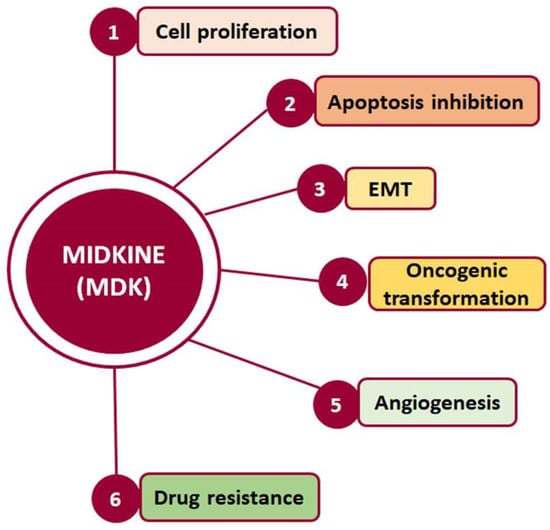
Midkine (MDK) is a multifunctional secreted protein that can act as a cytokine or growth factor regulating multiple signaling pathways and being implicated in fundamental cellular processes, such as survival, proliferation, and migration. Although its expression in normal adult tissues is barely detectable, MDK serum levels are found to be elevated in several types of cancer, including hepatocellular carcinoma (HCC).
1. Hepatocellular Carcinoma
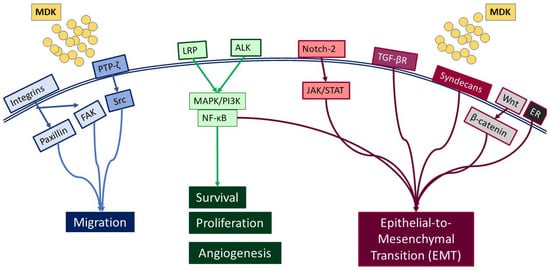
2. MDK-Mediated Signaling and Cellular Function
3. MDK Localization, Expression, and Detection
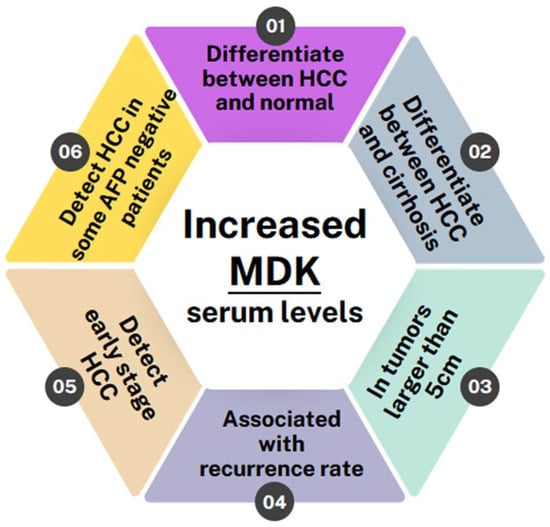
4. MDK’s Potential as a Biomarker for HCC Diagnosis and Progression
5. MDK in Comparison with AFP
Few studies showed no correlation with AFP levels. Specifically, Saad et al. [30] determined MDK mRNA expression using real-time PCR in 29 patients with HCC and compared it with that of 7 healthy individuals, 27 patients who had chronic HCV, and 18 patients who had liver cirrhosis. They found that MDK mRNA expression was higher in HCC patients compared with the other groups. However, no significant correlation was observed between MDK and tumor characteristics (number of nodules, lesion size, and extrahepatic metastases) or AFP levels. In agreement with the above, Shaheen et al. [7], evaluated MDK protein levels as a biomarker in patients with newly diagnosed HCC. Among 100 participants, including 40 HCC patients, 30 liver cirrhosis patients, and 30 controls, MDK was found to be significantly upregulated in HCC patients compared with both liver cirrhosis patients and controls but showed no association with tumor diameter, number of nodules, AFP levels, or Barcelona Clinic liver cancer (BCLC) staging, which is widely used for staging primary liver cancer. However, it is worth noting that MDK showed greater sensitivity compared with AFP in HCC diagnosis, especially in the early stages, highlighting its potential as a novel marker, especially in differentiating HCC from liver cirrhosis.
The majority of studies, however, showed that MDK provides some additional advantages as a biomarker compared with AFP. Specifically, in a recent study [31], serum MDK levels were assessed in 238 individuals, including 78 HCC patients with HCV-related HCC, 40 with HCV-related liver cirrhosis, 40 with chronic HCV without liver cirrhosis, and 80 healthy controls. The findings consistently confirmed elevated MDK levels in HCC patients compared with other groups. However, although no association with tumor size was found, and while there was no significant difference in MDK protein expression between the other groups, the authors highlighted that MDK diagnostic accuracy for HCC diagnosis was high.
Interestingly, in a recent phase II validation study, Zhu et al. [32] evaluated MDK as a diagnostic biomarker in early-stage HCC for those with negative AFP. Their study also included in vitro work in nine HCC cell lines (Bel-7402, Huh-7, HCCLM3, MHCC97H, PLC, HepG2, and Hep3B, with L-O2 and Chang liver cells serving as controls), as well as evaluation of 88 HCC samples, their corresponding adjacent tissues, and serum samples from 388 HCC cases and 545 controls. MDK expression was evaluated using IHC in tissue microarrays (TMA), ELISA in serum samples, and Western blotting in cell lines. They found elevated MDK protein expression in all HCC cell lines compared with controls, while IHC analysis in the TMA exhibited high MDK expression in the form of diffused cytoplasmic staining in 72% of HCCs compared with normal adjacent liver tissue or cancer-free cirrhotic samples. Although serum MDK levels did not exhibit correlations with tumor aggressiveness indicators, such as poor differentiation, microvascular invasion, larger tumor size, advanced tumor stage, survival, and tumor recurrence, they did correlate with MDK expression in tumor tissues. No association was found between MDK and BCLC staging. Compared with AFP, in AFP-positive patients, MDK and AFP had similar specificities; however, MDK showed superior sensitivity to AFP (86.9% and 51.9%, respectively). On the other hand, in patients with early-stage HCC and negative AFP, MDK had better performance compared with AFP for distinguishing early-stage HCC and small-sized tumors from non-HCC cases, including cirrhosis.
In another study, El-Shayeb et al. [33] tested MDK serum levels in 89 patients with liver cirrhosis without HCC, 86 patients with cirrhotic HCV-induced HCC, and 69 healthy controls. They found serum MDK levels to be increased in HCC patients compared with the other two control groups. Interestingly, however, MDK exhibited higher levels in patients with multiple focal lesions, lesions exceeding 5 cm, and those with portal vein thrombosis, compared with those with single focal lesions, lesions smaller than 5 cm, and those without portal vein thrombosis. Finally, MDK was proven to have superior performance compared with AFP in differentiating HCC patients from individuals with liver cirrhosis.
6. Conclusions
References
- Dehghani, S.H.H.a.M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129.
- Calderon-Martinez, E.; Landazuri-Navas, S.; Vilchez, E.; Cantu-Hernandez, R.; Mosquera-Moscoso, J.; Encalada, S.; Al Lami, Z.; Zevallos-Delgado, C.; Cinicola, J. Prognostic Scores and Survival Rates by Etiology of Hepatocellular Carcinoma: A Review. J. Clin. Med. Res. 2023, 15, 200–207.
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606.
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48.
- Tsuchiya, N.; Sawada, Y.; Endo, I.; Saito, K.; Uemura, Y.; Nakatsura, T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 10573–10583.
- Saikia, M.; Cheung, N.; Singh, A.K.; Kapoor, V. Role of Midkine in Cancer Drug Resistance: Regulators of Its Expression and Its Molecular Targeting. Int. J. Mol. Sci. 2023, 24, 8739.
- Shaheen, K.Y.; Abdel-Mageed, A.I.; Safwat, E.; AlBreedy, A.M. The value of serum midkine level in diagnosis of hepatocellular carcinoma. Int. J. Hepatol. 2015, 2015, 146389.
- Filippou, P.S.; Karagiannis, G.S.; Constantinidou, A. Midkine (MDK) growth factor: A key player in cancer progression and a promising therapeutic target. Oncogene 2020, 39, 2040–2054.
- Muramatsu, T. Structure and function of midkine as the basis of its pharmacological effects. Br. J. Pharmacol. 2014, 171, 814–826.
- Muramatsu, T. Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 410–425.
- Majaj, M.; Weckbach, L.T. Midkine-A novel player in cardiovascular diseases. Front. Cardiovasc. Med. 2022, 9, 1003104.
- Gowhari Shabgah, A.; Ezzatifar, F.; Aravindhan, S.; Olegovna Zekiy, A.; Ahmadi, M.; Gheibihayat, S.M.; Gholizadeh Navashenaq, J. Shedding more light on the role of Midkine in hepatocellular carcinoma: New perspectives on diagnosis and therapy. IUBMB Life 2021, 73, 659–669.
- Polykratis, A.; Katsoris, P.; Courty, J.; Papadimitriou, E. Characterization of heparin affin regulatory peptide signaling in human endothelial cells. J. Biol. Chem. 2005, 280, 22454–22461.
- Bie, C.; Chen, Y.; Tang, H.; Li, Q.; Zhong, L.; Peng, X.; Shi, Y.; Lin, J.; Lai, J.; Wu, S.; et al. Insulin-Like Growth Factor 1 Receptor Drives Hepatocellular Carcinoma Growth and Invasion by Activating Stat3-Midkine-Stat3 Loop. Dig. Dis. Sci. 2022, 67, 569–584.
- Sun, B.; Hu, C.; Yang, Z.; Zhang, X.; Zhao, L.; Xiong, J.; Ma, J.; Chen, L.; Qian, H.; Luo, X.; et al. Midkine promotes hepatocellular carcinoma metastasis by elevating anoikis resistance of circulating tumor cells. Oncotarget 2017, 8, 32523–32535.
- Dai, L.C.; Shao, J.Z.; Min, L.S.; Xiao, Y.T.; Xiang, L.X.; Ma, Z.H. Midkine accumulated in nucleolus of HepG2 cells involved in rRNA transcription. World J. Gastroenterol. 2008, 14, 6249–6253.
- Wu, L.; Chen, H.; Fu, C.; Xing, M.; Fang, H.; Yang, F.; Yang, Q.; Zhang, Y.; Li, W.; Chen, Z. Midkine mediates dysfunction of liver sinusoidal endothelial cells through integrin alpha4 and alpha6. Vasc. Pharmacol. 2022, 147, 107113.
- Li, J.; Li, J.; Hao, H.; Lu, F.; Wang, J.; Ma, M.; Jia, B.; Zhuo, M.; Wang, J.; Chi, Y.; et al. Secreted proteins MDK, WFDC2, and CXCL14 as candidate biomarkers for early diagnosis of lung adenocarcinoma. BMC Cancer 2023, 23, 110.
- Yuan, K.; Chen, Z.; Li, W.; Gao, C.E.; Li, G.; Guo, G.; Yang, Y.; Ai, Y.; Wu, L.; Zhang, M. MDK Protein Overexpression Correlates with the Malignant Status and Prognosis of Non-small Cell Lung Cancer. Arch. Med. Res. 2015, 46, 635–641.
- Hu, B.; Qin, C.; Li, L.; Wei, L.; Mo, X.; Fan, H.; Lei, Y.; Wei, F.; Zou, D. Midkine promotes glioblastoma progression via PI3K-Akt signaling. Cancer Cell Int. 2021, 21, 509.
- Jia, M.; Zhao, H.Z.; Cheng, Y.P.; Luo, Z.B.; Zhang, J.Y.; Li, S.S.; Xu, X.J.; Tang, Y.M. High expression of Midkine (MK) indicates poor prognosis in childhood acute lymphoblastic leukemia. Hematology 2016, 21, 69–77.
- Hu, X.F.; Yao, J.; Gao, S.G.; Yang, Y.T.; Peng, X.Q.; Feng, X.S. Midkine and syndecan-1 levels correlate with the progression of malignant gastric cardiac adenocarcinoma. Mol. Med. Rep. 2014, 10, 1409–1415.
- Zhou, Q.; Yang, C.; Mou, Z.; Wu, S.; Dai, X.; Chen, X.; Ou, Y.; Zhang, L.; Sha, J.; Jiang, H. Identification and validation of a poor clinical outcome subtype of primary prostate cancer with Midkine abundance. Cancer Sci. 2022, 113, 3698–3709.
- Chiu, T.J.; Chen, C.H.; Chen, Y.J.; Wee, Y.; Wang, C.S.; Luo, S.D. Prognosis of Midkine and AT1R expression in resectable head and neck squamous cell carcinoma. Cancer Cell Int. 2023, 23, 212.
- Yao, J.; Li, W.Y.; Li, S.G.; Feng, X.S.; Gao, S.G. Midkine promotes perineural invasion in human pancreatic cancer. World J. Gastroenterol. 2014, 20, 3018–3024.
- Tanabe, K.; Matsumoto, M.; Ikematsu, S.; Nagase, S.; Hatakeyama, A.; Takano, T.; Niikura, H.; Ito, K.; Kadomatsu, K.; Hayashi, S.; et al. Midkine and its clinical significance in endometrial carcinoma. Cancer Sci. 2008, 99, 1125–1130.
- Lin, H.; Zhou, Q.; Wu, W.; Ma, Y. Midkine Is a Potential Urinary Biomarker for Non-Invasive Detection of Bladder Cancer with Microscopic Hematuria. Onco. Targets Ther. 2019, 12, 11765–11775.
- Omran, M.M.; Farid, K.; Omar, M.A.; Emran, T.M.; El-Taweel, F.M.; Tabll, A.A. A combination of alpha-fetoprotein, midkine, thioredoxin and a metabolite for predicting hepatocellular carcinoma. Ann. Hepatol. 2020, 19, 179–185.
- Shang, B.; Wang, R.; Qiao, H.; Zhao, X.; Wang, L.; Sui, S. Multi-omics analysis of pyroptosis regulation patterns and characterization of tumor microenvironment in patients with hepatocellular carcinoma. PeerJ 2023, 11, e15340.
- Saad, Y.; El-Serafy, M.; Eldin, M.S.; Abdellatif, Z.; Khatab, H.; Elbaz, T.; Elgarem, H. New genetic markers for diagnosis of hepatitis C related hepatocellular carcinoma in Egyptian patients. J. Gastrointest. Liver Dis. 2013, 22, 419–425.
- Zekri, A.-R.N.; El Kassas, M.; Salam, E.S.E.; Hassan, R.M.; Mohanad, M.; Gabr, R.M.; Lotfy, M.M.; Abdel-Zaher, R.A.T.; Bahnassy, A.A.; Ahmed, O.S. The possible role of Dickkopf-1, Golgi protein- 73 and Midkine as predictors of hepatocarcinogenesis: A review and an Egyptian study. Sci. Rep. 2020, 10, 5156.
- Zhu, W.W.; Guo, J.J.; Guo, L.; Jia, H.L.; Zhu, M.; Zhang, J.B.; Loffredo, C.A.; Forgues, M.; Huang, H.; Xing, X.J.; et al. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin. Cancer Res. 2013, 19, 3944–3954.
- El-Shayeb, A.F.; El-Habachi, N.M.; Mansour, A.R.; Zaghloul, M.S. Serum midkine is a more sensitive predictor for hepatocellular carcinoma than Dickkopf-1 and alpha-L-fucosidase in cirrhotic HCV patients. Medicine 2021, 100, e25112.
- Hodeib, H.; ELshora, O.; Selim, A.; Sabry, N.M.; El-Ashry, H.M. Serum Midkine and Osteopontin Levels as Diagnostic Biomarkers of Hepatocellular Carcinoma. Electron. Physician 2017, 9, 3492–3498.
- Mashaly, A.H.; Anwar, R.; Ebrahim, M.A.; Eissa, L.A.; El Shishtawy, M.M. Diagnostic and Prognostic Value of Talin-1 and Midkine as Tumor Markers in Hepatocellular Carcinoma in Egyptian Patients. Asian Pac. J. Cancer Prev. 2018, 19, 1503–1508.
- Hung, Y.J.; Lin, Z.H.; Cheng, T.I.; Liang, C.T.; Kuo, T.M.; Kao, K.J. Serum midkine as a prognostic biomarker for patients with hepatocellular carcinoma. Am. J. Clin. Pathol. 2011, 136, 594–603.
- Daif, A.; Al-Azzawi, M.A.; Sakr, M.A.; Ismail, H.A.; Gadallah, M. Noninvasive identifcation of molecular biomarkers of hepatocellular carcinoma in HCV-Egyptian patients. J. Egypt. Nat. Cancer Instit. 2023, 35, 11.
- Vongsuvanh, R.; van der Poorten, D.; Iseli, T.; Strasser, S.I.; McCaughan, G.W.; George, J. Midkine Increases Diagnostic Yield in AFP Negative and NASH-Related Hepatocellular Carcinoma. PLoS ONE 2016, 11, e0155800.
- Piratvisuth, T.; Hou, J.; Tanwandee, T.; Berg, T.; Vogel, A.; Trojan, J.; De Toni, E.N.; Kudo, M.; Eiblmaier, A.; Klein, H.G.; et al. Development and clinical validation of a novel algorithmic score (GAAD) for detecting HCC in prospective cohort studies. Hepatol. Commun. 2023, 7, e0317.
- Eletreby, R.; Elsharkawy, M.; Taha, A.A.; Hassany, M.; Abdelazeem, A.; El-Kassas, M.; Soliman, A. Evaluation of GALAD Score in Diagnosis and Follow-up of Hepatocellular Carcinoma after Local Ablative Therapy. J. Clin. Transl. Hepatol. 2023, 11, 334–340.
- Guan, M.C.; Zhang, S.Y.; Ding, Q.; Li, N.; Fu, T.T.; Zhang, G.X.; He, Q.Q.; Shen, F.; Yang, T.; Zhu, H. The Performance of GALAD Score for Diagnosing Hepatocellular Carcinoma in Patients with Chronic Liver Diseases: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 949.
- Yang, J.D.; Addissie, B.D.; Mara, K.C.; Harmsen, W.S.; Dai, J.; Zhang, N.; Wongjarupong, N.; Ali, H.M.; Ali, H.A.; Hassan, F.A.; et al. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol. Biomark. Prev. 2019, 28, 531–538.
- Parikh, N.D.; Mehta, A.S.; Singal, A.G.; Block, T.; Marrero, J.A.; Lok, A.S. Biomarkers for the Early Detection of Hepatocellular Carcinoma. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2495–2503.
- Pinero, F.; Dirchwolf, M.; Pessoa, M.G. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells 2020, 9, 1370.
- Zheng, L.; Li, H.; Huang, J.; Shin, J.; Luo, S.; Guo, C.; Zhao, Y.; Li, F. Serum midkine levels for the diagnosis and assessment of response to interventional therapy in patients with hepatocellular carcinoma. J. Interv. Med. 2021, 4, 39–45.





