
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pritee Mangesh Patil (Chunarkar) | -- | 5386 | 2024-01-23 12:44:47 | | | |
| 2 | Peter Tang | Meta information modification | 5386 | 2024-01-24 03:07:34 | | |
Video Upload Options
Malignancies cause one out of six mortalities, which is a serious health problem. Cancer therapy has always been challenging, apart from major advances in immunotherapies, stem cell transplantation, targeted therapies, hormonal therapies, precision medicine, and palliative care, and traditional therapies such as surgery, radiation therapy, and chemotherapy. Natural products are integral to the development of innovative anticancer drugs in cancer research, offering the scientific community the possibility of exploring novel natural compounds against cancers. The role of natural products like Vincristine and Vinblastine has been thoroughly implicated in the management of leukemia and Hodgkin’s disease. The computational method is the initial key approach in drug discovery, among various approaches.
1. Introduction
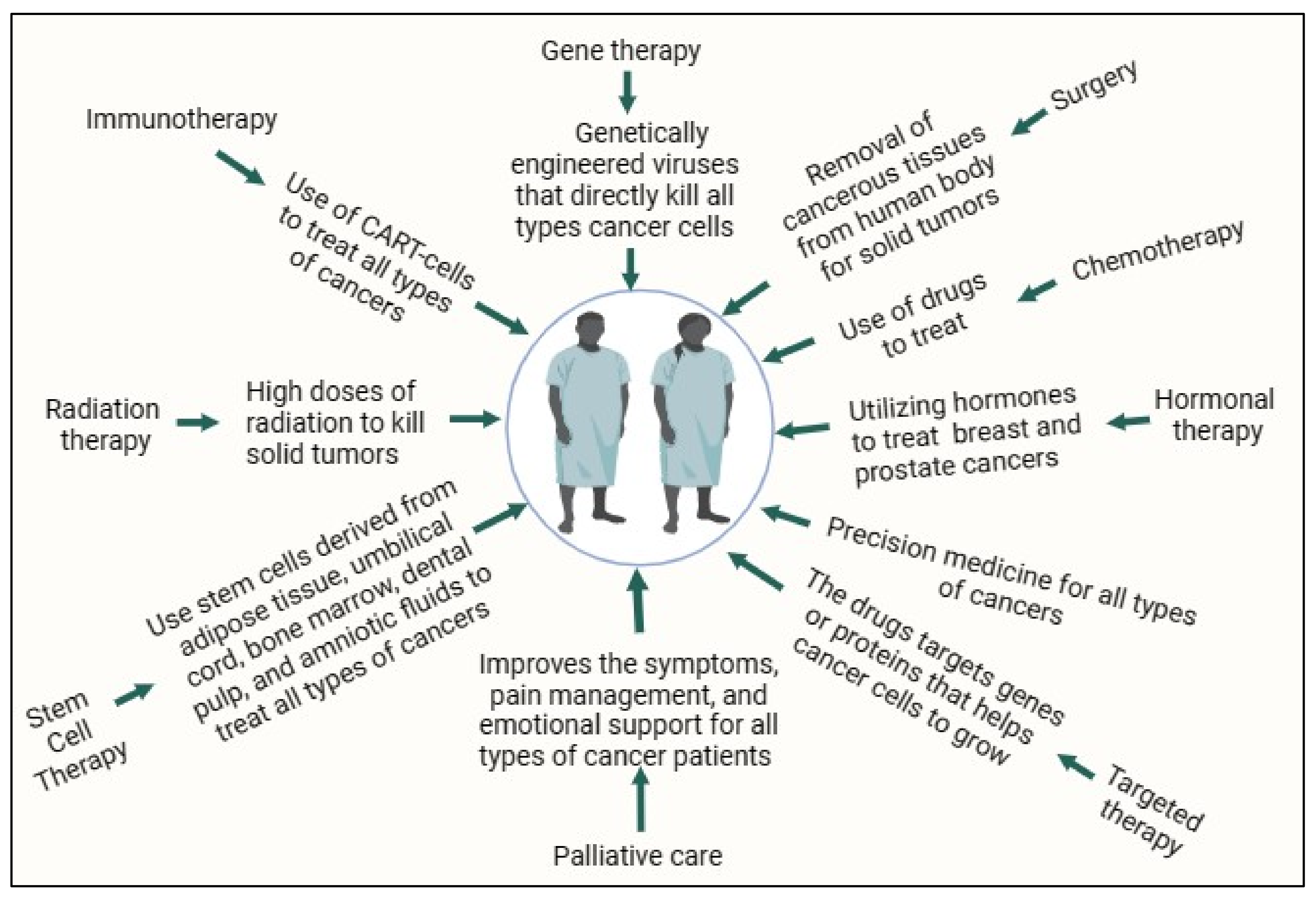
2. Historical Perspective
3. The Importance and Potential of Natural Products in Drug Discovery
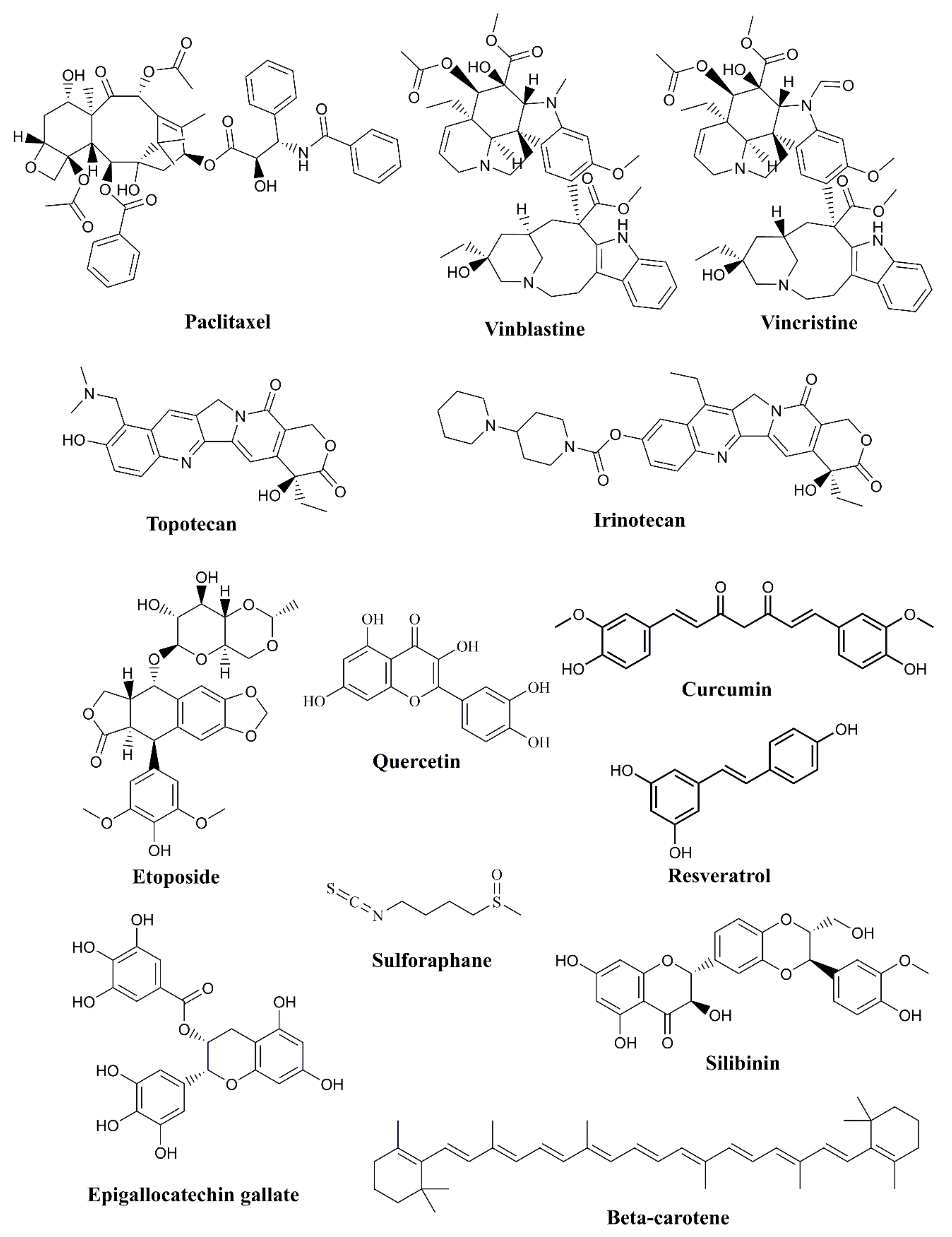
-
Paclitaxel: derived from the Pacific yew tree, and is used to treat various cancers, including breast, ovarian, and lung cancers [59].
-
Vinblastine and Vincristine: these alkaloids are derived from the Madagascar periwinkle plant and are used in the treatment of leukemia and lymphoma [60].
-
Etoposide: derived from the mayapple plant, etoposide is used to treat lung and testicular cancer [63].
|
Natural Compound |
Source |
Mechanism of Action |
Target Genes |
Cancer |
Reference |
|---|---|---|---|---|---|
|
Curcumin |
Turmeric (Curcuma longa) |
Inhibits cell proliferation, induces apoptosis. |
TNF, IL-1, VEGF, EGF, FGF, EGFR, HER-2, AR, NF-κB, AP-1, STAT |
Breast, lung, skin, gastrointestinal, colorectal, prostate, head and neck. |
[81] |
|
Resveratrol |
Grapes, berries, peanuts |
Antioxidant, affects cell cycle regulation. |
APE1/Ref-1, NF-κB, LSD1, MCP-1 |
Breast, cervical, uterine, blood, kidney, liver, eye, bladder, thyroid, esophageal, prostate, brain, lung, skin, gastric, colon, head and neck, bone, ovarian, and cervical. |
[82] |
|
Paclitaxel (Taxol) |
Pacific yew tree (Taxus brevifolia) |
Disrupts microtubule function. |
AP-1, JNK1, p38, ERK1, IL-1α, IL-1β, TNF-α |
Breast, ovarian, lung cancers. |
[83] |
|
Epigallocatechin gallate (EGCG) |
Green tea |
Antioxidant, induces apoptosis, inhibits proliferation. |
retinoic acid receptor β (RARβ), CDH1 (e-cadherine gene), DAPK1, DNMT1, DNMT3B, HDAC1 |
Breast, lung, bladder, head and neck, prostate, colorectal. |
[84] |
|
Sulforaphane |
Cruciferous vegetables |
Induces detoxification enzymes, pro-apoptotic. |
TIMP1, AURKA, CEP55, CRYAB, PLCE1, and MMP28, CRC |
Colorectal. |
[85] |
|
Genistein |
Soybeans |
Inhibits angiogenesis, modulates hormone activity. |
p21-WAF1, p16-INK4a, p21-WAF1 and p16-INK4a |
Breast, colorectal, lung, pancreatic. |
[86] |
|
Quercetin |
Apples, onions, tea, red wine |
Antioxidant, anti-inflammatory, inhibits proliferation. |
bcl-2-associated X protein (BAX), Cytochrome c release, Cysteine-aspartic proteases (caspase)-3, Caspase-9, Transforming growth factor β (TGF-β), Anti-apoptotic Bcl-2 |
Breast, prostate, colorectal, lung. |
[87] |
|
Capsaicin |
Chili peppers |
Induces apoptosis, inhibits cell growth. |
c-myc, c-Ha-ras, p53 |
Breast, lung, bladder, colon and pancreatic, colorectal. |
[88] |
|
Silymarin (Silibinin) |
Milk thistle |
Antioxidant, anti-inflammatory, cell regeneration. |
NF-кB, TGF-β, TNF-α, interferon-gamma, IL-2, IL-4, and COX-2 |
Breast, lung, colorectal, skin, pancreatic, prostate, gastrointestinal. |
[89] |
|
Berberine |
Berberis plants |
Inhibits cell progression, promotes apoptosis. |
IL-1, TNF, IL-6, cyclooxygenase 2 and prostaglandin E2 |
Colon. |
[90] |
|
Ellagic acid |
Pomegranates, berries, nuts |
Antioxidant, anti-proliferative. |
p53-dependent genes, NF-kB p50, p65, and the PPAR family |
Colorectal, prostate, lung, bladder, ovarian, breast. |
[91] |
|
Lycopene |
Tomatoes, watermelon, pink grapefruit |
Antioxidant, anti-proliferative. |
IGFBP-3, c-fos, and uPAR |
Breast, colorectal, lung, pancreatic, ovarian, cervical. |
[92] |
|
Indole-3-carbinol |
Cruciferous vegetables |
Modulates estrogen metabolism, apoptosis. |
CYP1A1, CYP1B1 and AhR |
Lung, head and neck, bladder, breast. |
[93] |
|
Beta-glucans |
Oats, barley, mushrooms |
Stimulates immune response. |
TLR-2/6, CR3 |
Breast, colorectal, prostate, ovarian. |
[94] |
|
Allicin |
Garlic |
Antioxidant, anti-proliferative, pro-apoptotic. |
E2F1, E2F2, and E2F3 |
Breast, bladder, lung, colorectal, prostate. |
[95] |
|
Catechins |
Tea, cocoa, fruits |
Antioxidant, anti-inflammatory, anti-proliferative. |
JNK, MAP kinase, JAKs, BCL-2, and Nrf2 |
Colorectal, pancreatic, lung, breast. |
[96] |
|
Ursolic acid |
Apples, basil, cranberries |
Inhibits metastasis, induces apoptosis. |
MMP-9, CT45A2, Bcl-2, Bcl-xL, and BAX |
Breast. |
[97] |
|
Limonene |
Citrus peels |
Induces detoxification enzymes, anti-proliferative. |
Bcl-2-associated X protein (BAX), Cytochrome c release, Cysteine-aspartic proteases (caspase)-3, Caspase-9, Transforming growth factor β (TGF-β), Anti-apoptotic Bcl-2 |
These are not directly associated with causing specific cancers, but rather are involved in cellular pathways related to apoptosis (programmed cell death) and regulation of cell survival. |
[98] |
|
Vinblastine |
Periwinkle plant (Catharanthus roseus) |
Inhibits microtubule assembly. |
CCNB1 and AURKA |
Breast, colorectal, lung, ovarian, prostate. |
[99] |
|
Vincristine |
Periwinkle plant (Catharanthus roseus) |
Binds to tubulin, inhibits microtubule formation. |
CYP3A4, CYP3A5 |
Liver. |
[60] |
|
Topotecan |
Happy tree (Camptotheca acuminata) |
Inhibits DNA topoisomerase I. |
ABCB1, ABCG2, ALDH1A1, IFIH1, SAMD4 and EPHA3 |
Breast, ovarian, colon. |
[100] |
|
Irinotecan |
Happy tree (Camptotheca acuminata) |
Inhibits DNA topoisomerase I. |
UGT1A1 |
It is not directly responsible for causing cance; variations in this gene can influence how the body processes certain chemotherapy drugs used in cancer treatment. |
[101] |
|
Etoposide |
Mayapple plant (Podophyllum peltatum) |
Inhibits DNA topoisomerase II. |
SEMA5A, SLC7A6 and PRMT7 |
For these genes, ongoing research might reveal their specific associations with certain cancers or their roles in cancer biology. The understanding of their involvement in cancer development and progression might evolve as more studies uncover their molecular mechanisms and connections to different cancer types. |
[102] |
|
Beta-carotene |
Carrots, sweet potatoes, spinach |
Antioxidant, modulates immune response. |
CD38, NCF1B, and ITGAL |
These genes are involved in various biological processes, including immune response and cell signalling. Their associations with specific cancers are not as prominent as some other genes, but they have been implicated in certain contexts. |
[103] |
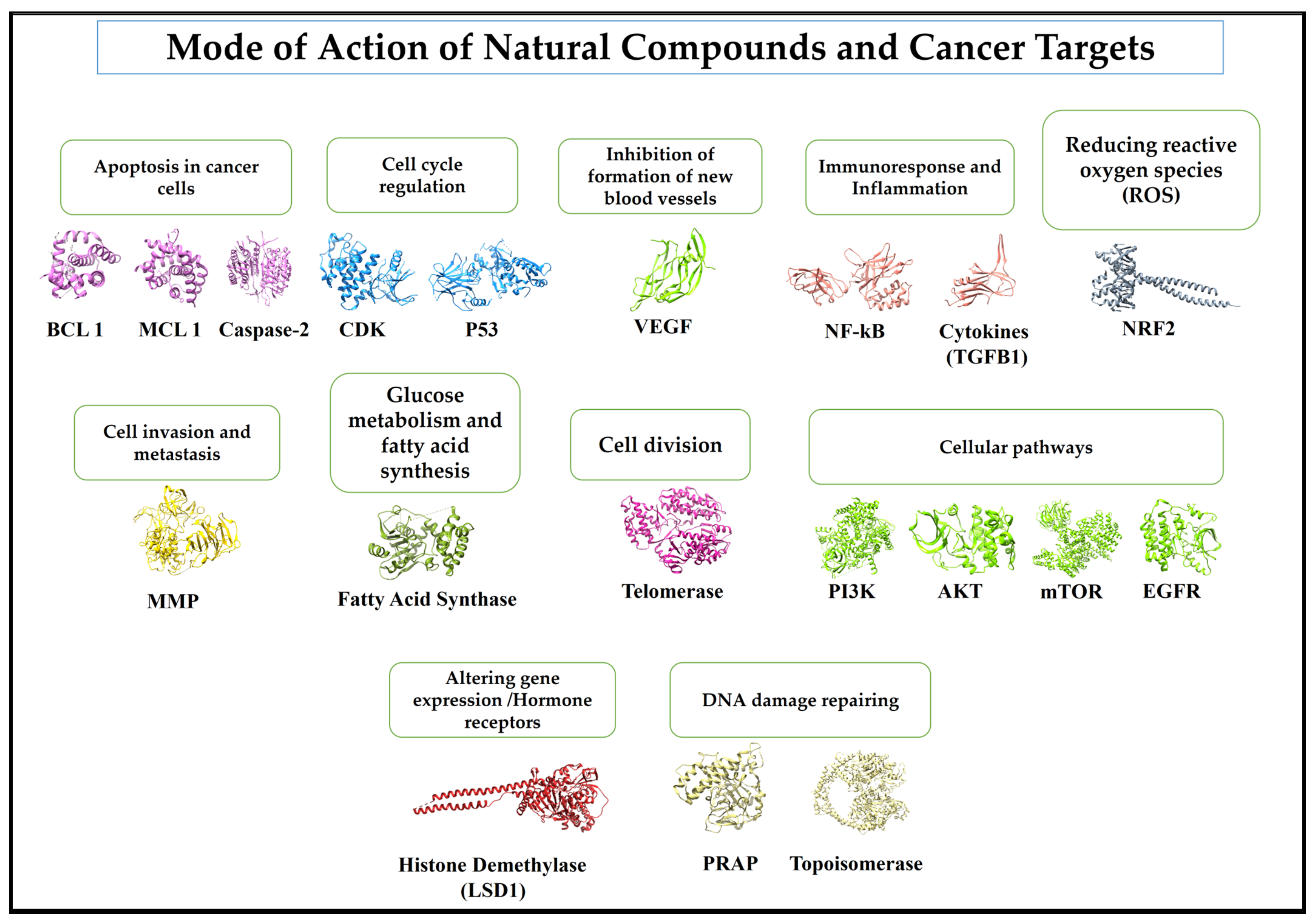
4. Present Status of Natural Compounds
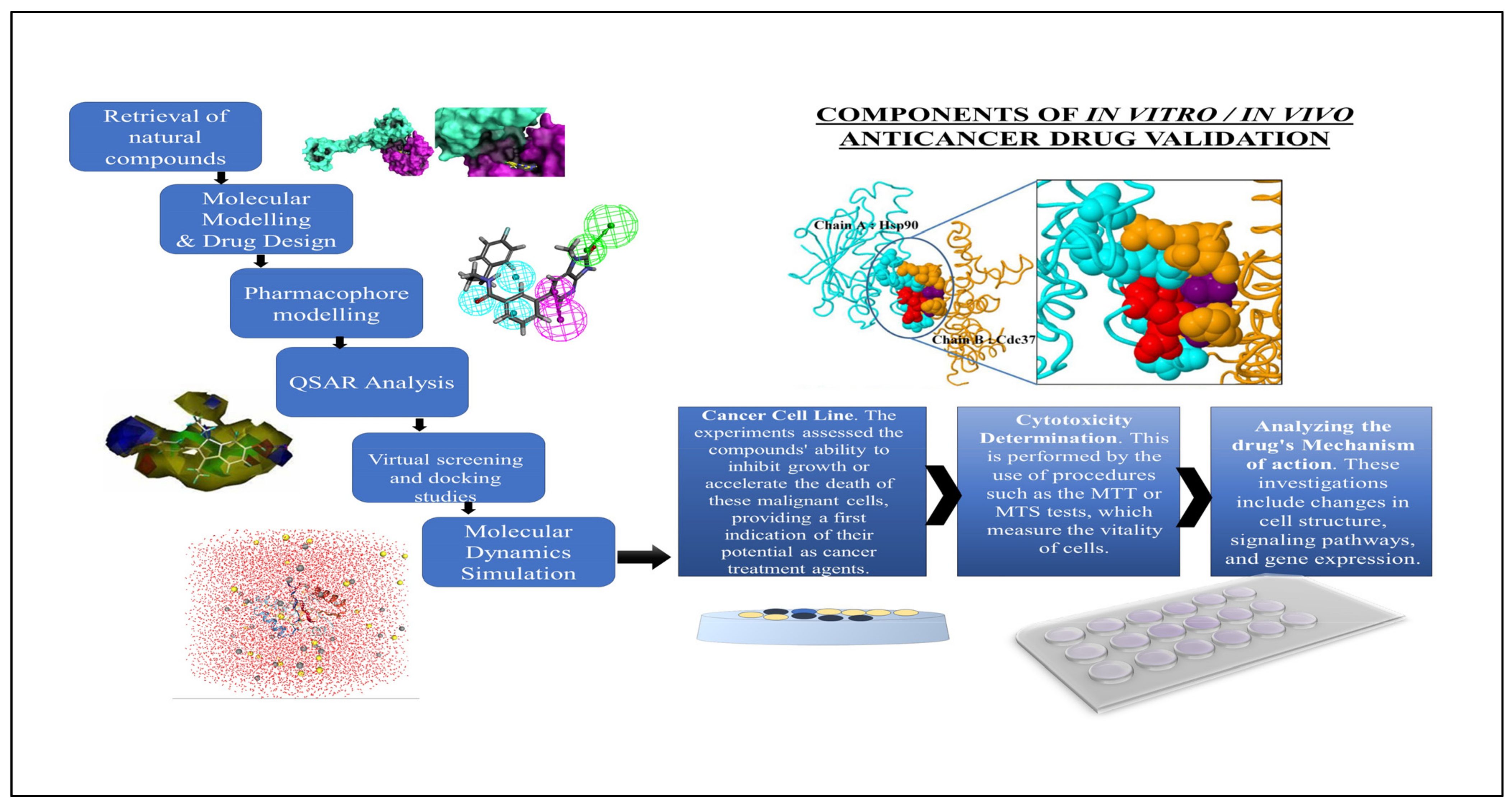
5. Computational Approaches in Drug Discovery
5.1. Molecular Modelling and Drug Design
References
- Magalhaes, L.G.; Ferreira, L.L.G.; Andricopulo, A.D. Recent Advances and Perspectives in Cancer Drug Design. An. Acad. Bras. Ciências 2018, 90, 1233–1250.
- Kaleem, M.; Perwaiz, M.; Nur, S.M.; Abdulrahman, A.O.; Ahmad, W.; Al-Abbasi, F.A.; Kumar, V.; Kamal, M.A.; Anwar, F. Epigenetics of Triple-Negative Breast Cancer via Natural Compounds. Curr. Med. Chem. 2021, 29, 1436–1458.
- Karn, V.; Sandhya, S.; Hsu, W.; Parashar, D.; Singh, H.N.; Jha, N.K.; Gupta, S.; Dubey, N.K.; Kumar, S. CRISPR/Cas9 System in Breast Cancer Therapy: Advancement, Limitations and Future Scope. Cancer Cell Int. 2022, 22, 234.
- Alhosin, M.; Sharif, T.; Mousli, M.; Etienne-Selloum, N.; Fuhrmann, G.; Schini-Kerth, V.B.; Bronner, C. Down-Regulation of UHRF1, Associated with Re-Expression of Tumor Suppressor Genes, Is a Common Feature of Natural Compounds Exhibiting Anti-Cancer Properties. J. Exp. Clin. Cancer Res. 2011, 30, 41.
- Parvez, A.; Choudhary, F.; Mudgal, P.; Khan, R.; Qureshi, K.A.; Aspatwar, A.; Farooqi, H. PD-1 and PD-L1: Architects of Immune Symphony and Immunotherapy Breakthroughs in Cancer Treatment. Front. Immunol. 2023, 14, 1296341.
- Chatterjee, A.; Mambo, E.; Sidransky, D. Mitochondrial DNA Mutations in Human Cancer. Oncogene 2006, 25, 4663–4674.
- Watson, I.R.; Takahashi, K.; Futreal, P.A.; Chin, L. Emerging Patterns of Somatic Mutations in Cancer. Nat. Rev. Genet. 2013, 14, 703–718.
- Piñeros, M.; Parkin, D.M.; Ward, K.; Chokunonga, E.; Ervik, M.; Farrugia, H.; Gospodarowicz, M.; O’Sullivan, B.; Soerjomataram, I.; Swaminathan, R.; et al. Essential TNM: A Registry Tool to Reduce Gaps in Cancer Staging Information. Lancet Oncol. 2019, 20, e103–e111.
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789.
- Loud, J.T.; Murphy, J. Cancer Screening and Early Detection in the 21 St Century. Semin. Oncol. Nurs. 2017, 33, 121–128.
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566.
- Wang, D.R.; Wu, X.L.; Sun, Y.L. Therapeutic Targets and Biomarkers of Tumor Immunotherapy: Response versus Non-Response. Signal Transduct. Target. Ther. 2022, 7, 331.
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366.
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552.
- Tiwari, P.K.; Ko, T.-H.; Dubey, R.; Chouhan, M.; Tsai, L.-W.; Singh, H.N.; Chaubey, K.K.; Dayal, D.; Chiang, C.-W.; Kumar, S. CRISPR/Cas9 as a Therapeutic Tool for Triple Negative Breast Cancer: From Bench to Clinics. Front. Mol. Biosci. 2023, 10, 1214489.
- Kumar, M.; Dubey, R.; Kumar Shukla, P.; Dayal, D.; Kumar Chaubey, K.; Tsai, L.-W.; Kumar, S. Identification of Small Molecule Inhibitors of RAD52 for Breast Cancer Therapy: In Silico Approach. J. Biomol. Struct. Dyn. 2023, 1–14.
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193.
- Salas-Benito, D.; Pérez-Gracia, J.L.; Ponz-Sarvisé, M.; Rodriguez-Ruiz, M.E.; Martínez-Forero, I.; Castañón, E.; López-Picazo, J.M.; Sanmamed, M.F.; Melero, I. Paradigms on Immunotherapy Combinations with Chemotherapy. Cancer Discov. 2021, 11, 1353–1367.
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27, 87–97.
- Choi, H.Y.; Chang, J.-E. Targeted Therapy for Cancers: From Ongoing Clinical Trials to FDA-Approved Drugs. Int. J. Mol. Sci. 2023, 24, 13618.
- Deli, T.; Orosz, M.; Jakab, A. Hormone Replacement Therapy in Cancer Survivors—Review of the Literature. Pathol. Oncol. Res. 2020, 26, 63–78.
- Chu, D.-T.; Nguyen, T.T.; Tien, N.L.B.; Tran, D.-K.; Jeong, J.-H.; Anh, P.G.; Van Thanh, V.; Truong, D.T.; Dinh, T.C. Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications. Cells 2020, 9, 563.
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of Precision Cancer Medicine: Evolution of the Treatment Paradigm. Cancer Treat. Rev. 2020, 86, 102019.
- Dumanovsky, T.; Augustin, R.; Rogers, M.; Lettang, K.; Meier, D.E.; Morrison, R.S. The Growth of Palliative Care in U.S. Hospitals: A Status Report. J. Palliat. Med. 2016, 19, 8–15.
- Ma, D.-L.; Chan, D.S.-H.; Leung, C.-H. Molecular Docking for Virtual Screening of Natural Product Databases. Chem. Sci. 2011, 2, 1656–1665.
- Schuster, D.; Wolber, G. Identification of Bioactive Natural Products by Pharmacophore-Based Virtual Screening. Curr. Pharm. Des. 2010, 16, 1666–1681.
- Cavalcanti, É.B.V.S.; Félix, M.B.; Scotti, L.; Scotti, M.T. Virtual Screening of Natural Products to Select Compounds with Potential Anticancer Activity. Anti-Cancer Agents Med. Chem. 2019, 19, 154–171.
- Anjum, F.; Ali, F.; Mohammad, T.; Shafie, A.; Akhtar, O.; Abdullaev, B.; Hassan, I. Discovery of Natural Compounds as Potential Inhibitors of Human Carbonic Anhydrase II: An Integrated Virtual Screening, Docking, and Molecular Dynamics Simulation Study. OMICS J. Integr. Biol. 2021, 25, 513–524.
- Yu, E.; Xu, Y.; Shi, Y.; Yu, Q.; Liu, J.; Xu, L. Discovery of Novel Natural Compound Inhibitors Targeting Estrogen Receptor α by an Integrated Virtual Screening Strategy. J. Mol. Model. 2019, 25, 278.
- Poornima, P.; Kumar, J.D.; Zhao, Q.; Blunder, M.; Efferth, T. Network Pharmacology of Cancer: From Understanding of Complex Interactomes to the Design of Multi-Target Specific Therapeutics from Nature. Pharmacol. Res. 2016, 111, 290–302.
- Iqbal, M.J.; Javed, Z.; Sadia, H.; Qureshi, I.A.; Irshad, A.; Ahmed, R.; Malik, K.; Raza, S.; Abbas, A.; Pezzani, R.; et al. Clinical Applications of Artificial Intelligence and Machine Learning in Cancer Diagnosis: Looking into the Future. Cancer Cell Int. 2021, 21, 270.
- Ravishankar, B.; Shukla, V. Indian Systems of Medicine: A Brief Profile. Afr. J. Tradit. Complement. Altern. Med. 2008, 4, 319.
- Singh, M.; Lo, S.-H.; Dubey, R.; Kumar, S.; Chaubey, K.K.; Kumar, S. Plant-Derived Natural Compounds as an Emerging Antiviral in Combating COVID-19. Indian J. Microbiol. 2023, 63, 429–446.
- Vaidya, A.D.B.; Devasagayam, T.P.A. Current Status of Herbal Drugs in India: An Overview. J. Clin. Biochem. Nutr. 2007, 41, 1–11.
- Pirintsos, S.; Panagiotopoulos, A.; Bariotakis, M.; Daskalakis, V.; Lionis, C.; Sourvinos, G.; Karakasiliotis, I.; Kampa, M.; Castanas, E. From Traditional Ethnopharmacology to Modern Natural Drug Discovery: A Methodology Discussion and Specific Examples. Molecules 2022, 27, 4060.
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer Potential of Alkaloids: A Key Emphasis to Colchicine, Vinblastine, Vincristine, Vindesine, Vinorelbine and Vincamine. Cancer Cell Int. 2022, 22, 206.
- Zulkipli, I.N.; David, S.R.; Rajabalaya, R.; Idris, A. Medicinal Plants: A Potential Source of Compounds for Targeting Cell Division. Drug Target Insights 2015, 9, DTI.S24946.
- Kumar, L.; Kumar, S.; Sandeep, K.; Patel, S.K.S. Therapeutic Approaches in Pancreatic Cancer: Recent Updates. Biomedicines 2023, 11, 1611.
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336.
- Li, G.; Lou, H.-X. Strategies to Diversify Natural Products for Drug Discovery. Med. Res. Rev. 2018, 38, 1255–1294.
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59.
- Ponphaiboon, J.; Krongrawa, W.; Aung, W.W.; Chinatangkul, N.; Limmatvapirat, S.; Limmatvapirat, C. Advances in Natural Product Extraction Techniques, Electrospun Fiber Fabrication, and the Integration of Experimental Design: A Comprehensive Review. Molecules 2023, 28, 5163.
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20.
- Mushtaq, S.; Abbasi, B.H.; Uzair, B.; Abbasi, R. Natural Products as Reservoirs of Novel Therapeutic Agents. EXCLI J. 2018, 17, 420–451.
- Abdelmohsen, U.R.; Sayed, A.M.; Elmaidomy, A.H. Natural Products’ Extraction and Isolation-Between Conventional and Modern Techniques. Front. Nat. Prod. 2022, 1, 873808.
- Cech, N.B.; Oberlies, N.H. From Plant to Cancer Drug: Lessons Learned from the Discovery of Taxol. Nat. Prod. Rep. 2023, 40, 1153–1157.
- Shaik, B.B.; Katari, N.K.; Jonnalagadda, S.B. Role of Natural Products in Developing Novel Anticancer Agents: A Perspective. Chem. Biodivers. 2022, 19, e202200535.
- Wani, M.C.; Horwitz, S.B. Nature as a Remarkable Chemist. Anti-Cancer Drugs 2014, 25, 482–487.
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986.
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681.
- Donehower, R.C. The Clinical Development of Paclitaxel: A Successful Collaboration of Academia, Industry and the National Cancer Institute. Oncologist 1996, 1, 240–243.
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559.
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803.
- Ongnok, B.; Chattipakorn, N.; Chattipakorn, S.C. Doxorubicin and Cisplatin Induced Cognitive Impairment: The Possible Mechanisms and Interventions. Exp. Neurol. 2020, 324, 113118.
- Nan, Y.; Su, H.; Zhou, B.; Liu, S. The Function of Natural Compounds in Important Anticancer Mechanisms. Front. Oncol. 2022, 12, 1049888.
- Desilets, A.; Adam, J.-P.; Soulières, D. Management of Cisplatin-Associated Toxicities in Bladder Cancer Patients. Curr. Opin. Support. Palliat. Care 2020, 14, 286–292.
- Cao, K.; Tait, S.W.G. Apoptosis and Cancer: Force Awakens, Phantom Menace, or Both? Int. Rev. Cell Mol. Biol. 2018, 337, 135–152.
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367.
- Zhu, L.; Chen, L. Progress in Research on Paclitaxel and Tumor Immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40.
- Paul, A.; Acharya, K.; Chakraborty, N. Biosynthesis, Extraction, Detection and Pharmacological Attributes of Vinblastine and Vincristine, Two Important Chemotherapeutic Alkaloids of Catharanthus Roseus (L.) G. Don: A Review. S. Afr. J. Bot. 2023, 161, 365–376.
- Avemann, K.; Knippers, R.; Koller, T.; Sogo, J.M. Camptothecin, a Specific Inhibitor of Type I DNA Topoisomerase, Induces DNA Breakage at Replication Forks. Mol. Cell. Biol. 1988, 8, 3026–3034.
- Behera, A.; Padhi, S. Passive and Active Targeting Strategies for the Delivery of the Camptothecin Anticancer Drug: A Review. Environ. Chem. Lett. 2020, 18, 1557–1567.
- Wu, C.-C.; Li, T.-K.; Farh, L.; Lin, L.-Y.; Lin, T.-S.; Yu, Y.-J.; Yen, T.-J.; Chiang, C.-W.; Chan, N.-L. Structural Basis of Type II Topoisomerase Inhibition by the Anticancer Drug Etoposide. Science 2011, 333, 459–462.
- Shahar, N.; Larisch, S. Inhibiting the Inhibitors: Targeting Anti-Apoptotic Proteins in Cancer and Therapy Resistance. Drug Resist. Updates 2020, 52, 100712.
- Zhang, M.; Zhang, L.; Hei, R.; Li, X.; Cai, H.; Wu, X.; Zheng, Q.; Cai, C. CDK Inhibitors in Cancer Therapy, an Overview of Recent Development. Am. J. Cancer Res. 2021, 11, 1913–1935.
- Yang, Y.; Cao, Y. The Impact of VEGF on Cancer Metastasis and Systemic Disease. Semin. Cancer Biol. 2022, 86, 251–261.
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the Link between Inflammation and Cancer. Immunol. Rev. 2012, 246, 379–400.
- Liou, G.-Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic. Res. 2010, 44, 479–496.
- Halder, A.K.; Saha, A.; Jha, T. Exploring QSAR and Pharmacophore Mapping of Structurally Diverse Selective Matrix Metalloproteinase-2 Inhibitors. J. Pharm. Pharmacol. 2013, 65, 1541–1554.
- Li, J.; Li, Y.; Lu, F.; Liu, L.; Ji, Q.; Song, K.; Yin, Q.; Lerner, R.A.; Yang, G.; Xu, H.; et al. A DNA-Encoded Library for the Identification of Natural Product Binders That Modulate Poly (ADP-Ribose) Polymerase 1, a Validated Anti-Cancer Target. Biochem. Biophys. Res. Commun. 2020, 533, 241–248.
- Akone, S.H.; Ntie-Kang, F.; Stuhldreier, F.; Ewonkem, M.B.; Noah, A.M.; Mouelle, S.E.M.; Müller, R. Natural Products Impacting DNA Methyltransferases and Histone Deacetylases. Front. Pharmacol. 2020, 11, 992.
- Malhotra, M.; Suman, S.K. Laccase-Mediated Delignification and Detoxification of Lignocellulosic Biomass: Removing Obstacles in Energy Generation. Environ. Sci. Pollut. Res. 2021, 28, 58929–58944.
- Polivka, J.; Janku, F. Molecular Targets for Cancer Therapy in the PI3K/AKT/MTOR Pathway. Pharmacol. Ther. 2014, 142, 164–175.
- Yuan, Y.; Long, H.; Zhou, Z.; Fu, Y.; Jiang, B. PI3K–AKT-Targeting Breast Cancer Treatments: Natural Products and Synthetic Compounds. Biomolecules 2023, 13, 93.
- Nandi, S.; Dey, R.; Samadder, A.; Saxena, A.; Saxena, A.K. Natural Sourced Inhibitors of EGFR, PDGFR, FGFR and VEGFRMediated Signaling Pathways as Potential Anticancer Agents. Curr. Med. Chem. 2022, 29, 212–234.
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2017, 19, 13.
- Cerella, C.; Radogna, F.; Dicato, M.; Diederich, M. Natural Compounds as Regulators of the Cancer Cell Metabolism. Int. J. Cell Biol. 2013, 2013, 639401.
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer Metabolism: A Therapeutic Perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31.
- Jain, C.; Majumder, H.; Roychoudhury, S. Natural Compounds as Anticancer Agents Targeting DNA Topoisomerases. Curr. Genom. 2016, 18, 75–92.
- Ottaviani, A.; Iacovelli, F.; Fiorani, P.; Desideri, A. Natural Compounds as Therapeutic Agents: The Case of Human Topoisomerase IB. Int. J. Mol. Sci. 2021, 22, 4138.
- Prakobwong, S.; Gupta, S.C.; Kim, J.H.; Sung, B.; Pinlaor, P.; Hiraku, Y.; Wongkham, S.; Sripa, B.; Pinlaor, S.; Aggarwal, B.B. Curcumin Suppresses Proliferation and Induces Apoptosis in Human Biliary Cancer Cells through Modulation of Multiple Cell Signaling Pathways. Carcinogenesis 2011, 32, 1372–1380.
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anti-Cancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447.
- Swamy, M.K.; Vasamsetti, B.M.K. 2-Taxol: Occurrence, Chemistry, and Understanding Its Molecular Mechanisms. In Paclitaxel; Swamy, M.K., Pullaiah, T., Chen, Z.-S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 29–45. ISBN 978-0-323-90951-8.
- Fang, C.-Y.; Wu, C.-C.; Hsu, H.-Y.; Chuang, H.-Y.; Huang, S.-Y.; Tsai, C.-H.; Chang, Y.; Tsao, G.S.-W.; Chen, C.-L.; Chen, J.-Y. EGCG Inhibits Proliferation, Invasiveness and Tumor Growth by up-Regulation of Adhesion Molecules, Suppression of Gelatinases Activity, and Induction of Apoptosis in Nasopharyngeal Carcinoma Cells. Int. J. Mol. Sci. 2015, 16, 2530–2558.
- Yeh, C.-T.; Yen, G.-C. Chemopreventive Functions of Sulforaphane: A Potent Inducer of Antioxidant Enzymes and Apoptosis. J. Funct. Foods 2009, 1, 23–32.
- Varinska, L.; Gal, P.; Mojzisova, G.; Mirossay, L.; Mojzis, J. Soy and Breast Cancer: Focus on Angiogenesis. Int. J. Mol. Sci. 2015, 16, 11728–11749.
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M.H.; Baghbadorani, P.Z.; Eskandari, N. The Potential Anti-Cancer Effects of Quercetin on Blood, Prostate and Lung Cancers: An Update. Front. Immunol. 2023, 14, 1077531.
- Lavorgna, M.; Orlo, E.; Nugnes, R.; Piscitelli, C.; Russo, C.; Isidori, M. Capsaicin in Hot Chili Peppers: In Vitro Evaluation of Its Antiradical, Antiproliferative and Apoptotic Activities. Plant Foods Hum. Nutr. 2019, 74, 164–170.
- Olajide, P.; Adetuyi, B.; Oloke, J.; Omolabi, F. Pharmacological, Biochemical and Therapeutic Potential of Milk Thistle (Silymarin): A Review. World News Nat. Sci. 2022, 37, 75–91.
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. The Natural Alkaloid Berberine Targets Multiple Pathways to Induce Cell Death in Cultured Human Colon Cancer Cells. Eur. J. Pharmacol. 2012, 688, 14–21.
- Ceci, C.; Lacal, P.M.; Tentori, L.; De Martino, M.G.; Miano, R.; Graziani, G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrients 2018, 10, 1756.
- Kelkel, M.; Schumacher, M.; Dicato, M.; Diederich, M. Antioxidant and Anti-Proliferative Properties of Lycopene. Free Radic Res. 2011, 45, 925–940.
- Katz, E.; Nisani, S.; Chamovitz, D.A. Indole-3-Carbinol: A Plant Hormone Combatting Cancer. F1000Research 2018, 7, 689.
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible Mushrooms and Beta-Glucans: Impact on Human Health. Nutrients 2021, 13, 2195.
- Özkan, İ.; Koçak, P.; Yıldırım, M.; Ünsal, N.; Yılmaz, H.; Telci, D.; Şahin, F. Garlic (Allium Sativum)-Derived SEVs Inhibit Cancer Cell Proliferation and Induce Caspase Mediated Apoptosis. Sci. Rep. 2021, 11, 14773.
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Chapter 21—Antioxidant Role of Catechin in Health and Disease. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 267–271. ISBN 978-0-12-398456-2.
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Kanwal, S.; Ali, B.; Khalil, A.T.; Shah, S.A.; Alam, M.M.; Badshah, H. Ursolic Acid a Promising Candidate in the Therapeutics of Breast Cancer: Current Status and Future Implications. Biomed. Pharmacother. 2018, 108, 752–756.
- ALaqeel, N.K. Antioxidants from Different Citrus Peels Provide Protection against Cancer. Braz. J. Biol. 2023, 84, e271619.
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca Alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235.
- Martino, E.; Della Volpe, S.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Sala, A.; Collina, S. The Long Story of Camptothecin: From Traditional Medicine to Drugs. Bioorg. Med. Chem. Lett. 2017, 27, 701–707.
- Basili, S.; Moro, S. Novel Camptothecin Derivatives as Topoisomerase I Inhibitors. Expert Opin. Ther. Pat. 2009, 19, 555–574.
- Zhang, W.; Gou, P.; Dupret, J.-M.; Chomienne, C.; Rodrigues-Lima, F. Etoposide, an Anticancer Drug Involved in Therapy-Related Secondary Leukemia: Enzymes at Play. Transl. Oncol. 2021, 14, 101169.
- Toti, E.; Chen, C.-Y.O.; Palmery, M.; Villaño Valencia, D.; Peluso, I. Non-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxid. Med. Cell Longev. 2018, 2018, 4637861.
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Curcumin: A Review of Clinical Trials. Eur. J. Med. Chem. 2019, 163, 527–545.
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. NPJ Precis. Oncol. 2017, 1, 35.
- Aggarwal, V.; Tuli, H.S.; Tania, M.; Srivastava, S.; Ritzer, E.E.; Pandey, A.; Aggarwal, D.; Barwal, T.S.; Jain, A.; Kaur, G.; et al. Molecular Mechanisms of Action of Epigallocatechin Gallate in Cancer: Recent Trends and Advancement. Semin. Cancer Biol. 2022, 80, 256–275.
- Yang, Y.-H.; Mao, J.-W.; Tan, X.-L. Research Progress on the Source, Production, and Anti-Cancer Mechanisms of Paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897.
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological Basis and New Insights of Quercetin Action in Respect to Its Anti-Cancer Effects. Biomed. Pharmacother. 2020, 121, 109604.
- Goto, K.; Ohe, Y.; Shibata, T.; Seto, T.; Takahashi, T.; Nakagawa, K.; Tanaka, H.; Takeda, K.; Nishio, M.; Mori, K.; et al. Combined Chemotherapy with Cisplatin, Etoposide, and Irinotecan versus Topotecan Alone as Second-Line Treatment for Patients with Sensitive Relapsed Small-Cell Lung Cancer (JCOG0605): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2016, 17, 1147–1157.
- Chen, Q.-H.; Wu, B.-K.; Pan, D.; Sang, L.-X.; Chang, B. Beta-Carotene and Its Protective Effect on Gastric Cancer. World J. Clin. Cases 2021, 9, 6591–6607.
- Mangla, B.; Javed, S.; Sultan, M.H.; Kumar, P.; Kohli, K.; Najmi, A.; Alhazmi, H.A.; Al Bratty, M.; Ahsan, W. Sulforaphane: A Review of Its Therapeutic Potentials, Advances in Its Nanodelivery, Recent Patents, and Clinical Trials. Phytother. Res. 2021, 35, 5440–5458.
- Mashhadi Akbar Boojar, M.; Mashhadi Akbar Boojar, M.; Golmohammad, S. Overview of Silibinin Anti-Tumor Effects. J. Herb. Med. 2020, 23, 100375.
- Thomford, N.; Senthebane, D.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578.
- Singh, I.P.; Ahmad, F.; Chatterjee, D.; Bajpai, R.; Sengar, N. Natural Products: Drug Discovery and Development. In Drug Discovery and Development: From Targets and Molecules to Medicines; Poduri, R., Ed.; Springer: Singapore, 2021; pp. 11–65. ISBN 9789811555343.
- Calixto, J.B. The Role of Natural Products in Modern Drug Discovery. An. Acad. Bras. Ciências 2019, 91, 1–7.
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W. Computational Methods in Drug Discovery. Pharmacol. Rev. 2014, 66, 334–395.
- Huang, Z.; Yao, X.J.; Gu, R.-X. Editorial: Computational Approaches in Drug Discovery and Precision Medicine. Front. Chem. 2021, 8, 639449.
- Ou-Yang, S.; Lu, J.; Kong, X.; Liang, Z.; Luo, C.; Jiang, H. Computational Drug Discovery. Acta Pharmacol. Sin. 2012, 33, 1131–1140.
- Tutone, M.; Almerico, A.M. Computational Approaches: Drug Discovery and Design in Medicinal Chemistry and Bioinformatics. Molecules 2021, 26, 7500.
- Iwaloye, O.; Ottu, P.O.; Olawale, F.; Babalola, O.O.; Elekofehinti, O.O.; Kikiowo, B.; Adegboyega, A.E.; Ogbonna, H.N.; Adeboboye, C.F.; Folorunso, I.M.; et al. Computer-Aided Drug Design in Anti-Cancer Drug Discovery: What Have We Learnt and What Is the Way Forward? Inform. Med. Unlocked 2023, 41, 101332.
- Issa, N.T.; Stathias, V.; Schürer, S.; Dakshanamurthy, S. Machine and Deep Learning Approaches for Cancer Drug Repurposing. Semin. Cancer Biol. 2021, 68, 132–142.
- Prada-Gracia, D.; Huerta-Yépez, S.; Moreno-Vargas, L.M. Application of Computational Methods for Anticancer Drug Discovery, Design, and Optimization. Boletín Médico Hosp. Infant. México 2016, 73, 411–423.
- Li, K.; Du, Y.; Li, L.; Wei, D.-Q. Bioinformatics Approaches for Anti-Cancer Drug Discovery. Curr. Drug Targets 2019, 21, 3–17.
- Goetz, L.H.; Schork, N.J. Personalized Medicine: Motivation, Challenges, and Progress. Fertil. Steril. 2018, 109, 952–963.
- Qureshi, R.; Irfan, M.; Gondal, T.M.; Khan, S.; Wu, J.; Hadi, M.U.; Heymach, J.; Le, X.; Yan, H.; Alam, T. AI in Drug Discovery and Its Clinical Relevance. Heliyon 2023, 9, e17575.
- Hamamoto, R.; Suvarna, K.; Yamada, M.; Kobayashi, K.; Shinkai, N.; Miyake, M.; Takahashi, M.; Jinnai, S.; Shimoyama, R.; Sakai, A.; et al. Application of Artificial Intelligence Technology in Oncology: Towards the Establishment of Precision Medicine. Cancers 2020, 12, 3532.
- Cao, W.; Yu, P.; Yang, S.; Li, Z.; Zhang, Q.; Liu, Z.; Li, H. Discovery of Novel Mono-Carbonyl Curcumin Derivatives as Potential Anti-Hepatoma Agents. Molecules 2023, 28, 6796.
- Nadendla, R.R. Molecular Modeling: A Powerful Tool for Drug Design and Molecular Docking. Resonance 2004, 9, 51–60.
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Aided-Drug Des. 2011, 7, 146–157.
- Adelusi, T.I.; Oyedele, A.-Q.K.; Boyenle, I.D.; Ogunlana, A.T.; Adeyemi, R.O.; Ukachi, C.D.; Idris, M.O.; Olaoba, O.T.; Adedotun, I.O.; Kolawole, O.E.; et al. Molecular Modeling in Drug Discovery. Inform. Med. Unlocked 2022, 29, 100880.
- Salo-Ahen, O.M.H.; Alanko, I.; Bhadane, R.; Bonvin, A.M.J.J.; Honorato, R.V.; Hossain, S.; Juffer, A.H.; Kabedev, A.; Lahtela-Kakkonen, M.; Larsen, A.S.; et al. Molecular Dynamics Simulations in Drug Discovery and Pharmaceutical Development. Processes 2020, 9, 71.
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Bhatia, S.; Al-Harrasi, A.; Zengin, G.; Babes, E.E.; Brisc, C.; Stoicescu, M.; et al. Bioinformatics Accelerates the Major Tetrad: A Real Boost for the Pharmaceutical Industry. Int. J. Mol. Sci. 2021, 22, 6184.
- Panda, S.S.; Tran, Q.L.; Rajpurohit, P.; Pillai, G.G.; Thomas, S.J.; Bridges, A.E.; Capito, J.E.; Thangaraju, M.; Lokeshwar, B.L. Design, Synthesis, and Molecular Docking Studies of Curcumin Hybrid Conjugates as Potential Therapeutics for Breast Cancer. Pharmaceuticals 2022, 15, 451.




