1. Soil Salinity and Global Issues
The accumulation of soluble salts in soil is referred to as salinization, whereas soil salinity is expressed as the concentration of soluble salts in soil solutions or extracts by measuring the
electrical conductivity (EC) in dS m
−1 at 25 °C
[1]. This is one of the most important global issues affecting food security, agricultural production, and environmental sustainability
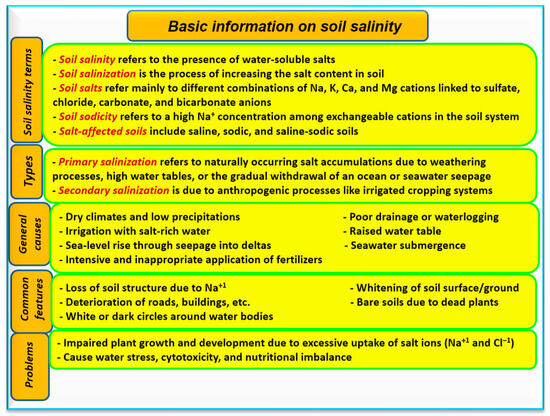
[2]. Basic information regarding soil salinity is shown in
Figure 1. Changing climates can drive soil salinization through processes such as rising sea levels
[3], changing rainfall patterns
[4], increasing air temperature leading to enhanced evaporation
[5], and increased drought events
[6]. Recent studies published on global issues related to soil salinity include a focus on topics such as reducing soil salinization by applying organic materials that increase net carbon sequestration
[7], using drip irrigation
[8], and soil-based technologies
[9] for crop production
[10].
Figure 1. A summary of important information on soil salinity including definition terms, different causes of salinity in soil, main features, and general problems. Sources:
[11][12].
Soil salinity is strongly linked to issues including climate change, soil fertility, carbon sequestration, food security, and the SDGs. Extended drought periods and rapidly melting glaciers causing changes in water dynamics have led to a significant decline in agro-productivity, especially in semi-arid regions
[10]. This impact can reduce crop biomass, soil organic carbon (SOC), microbial biomass carbon (MBC), and the flux of CO
2 and CH
4 under soil salinization
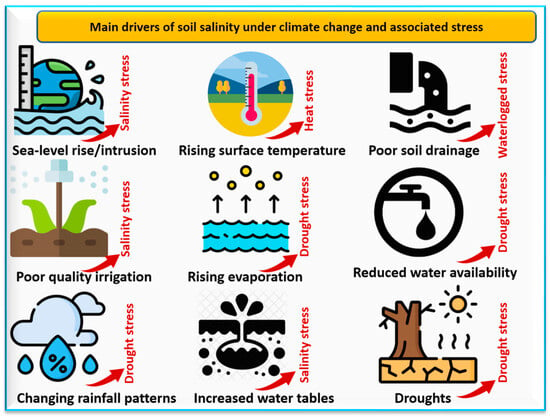
[7]. The main drivers of soil salinity under climate change are presented in
Figure 2. These drivers of salinization may include the low quality of irrigation water
[13], poor soil drainage
[14], increased surface air temperatures
[15], the intrusion of salt water into coastal areas due to global sea level rise
[16], and decreased precipitation rates
[6]. Soil salinity may degrade both soils and vegetation
[6], hindering global food security
[17].
Figure 2. The main drivers of soil salinity under climate change include sea level rise, poor soil drainage, increasing evaporation, poor quality of irrigation water, reduced availability of water, increasing temperatures, droughts, and changing rainfall patterns. Adapted from Eswar et al.
[6]. Images from
https://www.flaticon.com/free-icon/, accessed on 22 September 2023.
Salt-affected soils are most frequently associated with arid and semi-arid climates, where the amount of annual precipitation is not sufficient to leach the ions that create salt-affected soils out of the soil profile. The type of salts that accumulate and where they are found in the soil profile are determined by the amount of annual average precipitation, the presence of a source of the salts through either soil parent materials or some external source (e.g., groundwater, dust deposition, irrigation), and the physical properties of the soil that regulate water infiltration
[18]. However, salt-affected soils can also form in humid environments given the right set of conditions. For example, sodic soils are found across southern Illinois in the USA, an area that sees approximately 1220 mm of precipitation per year. In the case of these soils, it has been proposed that microtopography established by permafrost during the Wisconsinan glaciation established water distribution relationships in the soils that allowed for the accumulation of sodium found in the parent materials
[19].
2. Salt-Affected Soil Classification
Salt-affected soils are often classified according to the system developed by Richards
[20]. This system is based on a combination of soil pH, the electrical conductivity of the soil saturation extract (ECe), and the exchangeable sodium percentage (ESP). Using these indicators,
saline soil has a pH < 8.5, ECe > 4 dS m
−1, and ESP < 15.
Saline–sodic soils have an ECe > 4 dS m
−1 and ESP > 15. And,
sodic soils have an ECe < 4 dS m
−1, ESP > 15, and a pH that is typically between 8.5 and 10. It is important to understand the type of salt-affected soil, because it makes a difference in soil management, mitigation, and reclamation. While the Richards classification is the most commonly used classification for salt-affected soils, it is important to note that other classifications exist. These include the FAO-UNESCO solonchaks and solonetz, which are broadly similar to saline and sodic soils, and the Russian system
[21]. Solonchak (saline) soils have high salinity (ECe > 15 dS m
−1) within 125 cm of the soil surface and are divided into four units (gleyic, orthic, mollic, and takyric), whereas solonetz (sodic) are sodium-rich soils (ESP > 15) that may include gleyic, orthic, or mollic subdivisions. US Soil Taxonomy
[22], the Canadian soil classification system
[23], and the Australian classification system
[24] also include ways of noting salt accumulation in the classified soils.
It is also important to note that several variables determine how a crop will respond to salt-affected soils, including the species and variety of the crop and a number of soil factors
[21]. For example, sugar beet and durum wheat are fairly salt tolerant, with little reduction in yield as ECe increases from 0 to 7 dS m
−1. However, maize, soybean, tomato, and broad bean are much more sensitive to soil salinity, with maize undergoing a rapid decline in yields once ECe reaches about 2 dS m
−1, soybean about 2.5 dS m
−1, tomato about 3 dS m
−1, and broad bean about 3.5 dS m
−1 [25]. Therefore, while 4 dS m
−1 is a commonly used indicator of saline soils, it is not a particularly useful value when estimating the performance of a given crop. Another classification of saline soils is based on electrical conductivity and the expected impact on crop growth given that conductivity (
Table 1).
Table 1. Soil salinity classes based on expected influence on crop yield. Table based on Stavi et al.
[11].
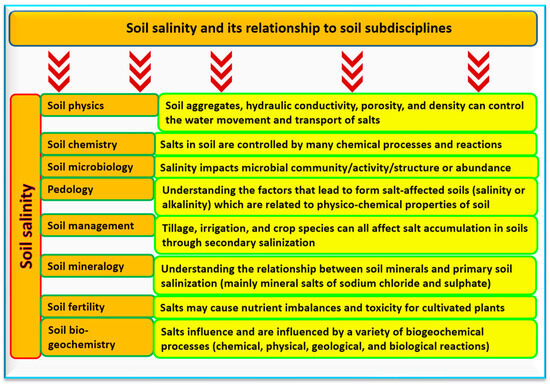
3. Soil Salinity from the Perspective of Different Soil Subdisciplines
All soil subdisciplines can be linked to soil salinity from different points of view. Low levels of salinity (0–2 dS m
−1) are not harmful to many cultivated crops, but higher levels (˃4 dS m
−1) can cause considerable yield loss depending on crop tolerance, and several types of physiological, nutritional, and molecular damage can be realized
[27]. In this section, three of the soil subdisciplines will be explored in detail to understand their links to soil salinity. Other soil science subdisciplines are briefly addressed in
Figure 3.
Soil microbes have a promising role in the mitigation of soil salinity through the alleviation of and reduction in oxidative stress by endophytic and rhizospheric microbes
[29] and in acting as significant selective agents on their host plants
[30] in an eco-friendly approach
[28]. The nutrient uptake by plants under salinity stress is controlled by the salinity level, ions present, plant species, and soil amendments. This depends on soil properties including soil pH and other biological, physical and chemical properties which control the bioavailability of nutrients to be taken up by the plants
[31][32]. This may reflect many approaches related to soil fertility and plant nutrition in the mitigation of soil salinity through integrated nutrient management
[33]. The interplay between different soil science branches and soil salinity can be noted in the biogeochemical perspective of microbial diversity and functions in saline soils
[34]. Planting salt-tolerant crops is an effective approach, but producing new tolerant cultivars is needed
[35].
3.1. Soil Biogeochemistry
Soil biogeochemistry is the science that studies the cycling of elements in the rhizosphere or the agroecosystem through chemical, physical, biological, and geological processes and the interactions between living and non-living components of soils
[36]. This discipline studies the effects of soil salinity on agricultural productivity through biogeochemical influences on soil organic carbon, soil microorganisms, land desertification, greenhouse gas (GHG) emissions, and biodiversity
[2]. Topics mainly focus on the impact of biological, chemical, and geological processes in soil on controlling the dynamics, distribution, and behavior of salts in the rhizosphere and groundwater
[37], on one side, and on cultivated plants on the other
[12]. These processes have a large impact on soil productivity, quality, and degradation
[38]. It is important to manage soils in agroecosystems so that soil biogeochemical processes promote soil health or quality
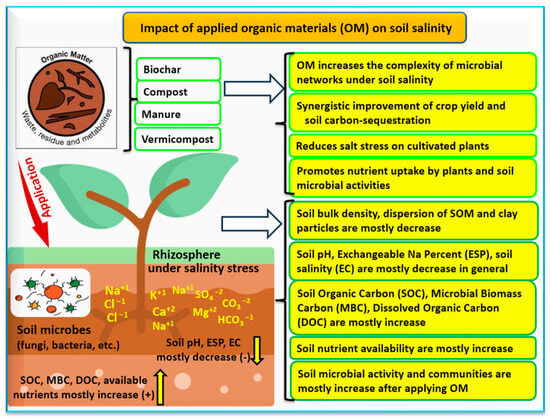
[39]. One common soil management practice that impacts the relationships between soil biogeochemistry and soil salinity is the application of organic amendments that increase the complexity of microbial networks (
Figure 4)
[40].
Figure 4. Applying organic materials to saline/alkaline soils can mitigate salinity/alkalinity stress by reducing ions in the soil solution (measured as electrical conductivity (EC) of the soil), bulk density, and exchangeable sodium percentage (ESP) and increasing nutrient uptake by plants, soil biological activity, soil organic carbon (SOC), microbial biomass carbon (MBC), and dissolved organic carbon (DOC). Adapted from
[7][40].
Several studies have investigated the role of organic amendments in mitigating soil salinity and increasing microbial biomass (MBC), dissolved organic carbon (DOC), the bioavailability of nutrients (NPK and other nutrients), and the activity of many enzymes such as catalase, urease, phosphatase, invertase, and phenol-oxidase
[41]. Studies into this relationship have involved applying compost
[13], biochar
[42], manure
[41], vermicompost
[43], and combinations of biochar and compost
[60], biochar and vermicompost
[44], and titanium, gypsum, and biochar composite
[45]. Effective management of saline soils depends on reducing the soluble salt content and/or the ESP of the soil and the accumulation of sodium ions (Na
+) in cultivated plant tissues
[46]. The influence of OM amendments on soil pH is variable and depends on the specific characteristics of both amendments and soils. For instance, biochar can have alkaline pH values that may increase soil pH
[61][62]. The expectation is that OM amendments will usually lead to an increase in SMB, SOC, DOC and available nutrients, as presented in
Figure 4 [62][63][64][65]. There is still a need for additional studies that investigate soil biogeochemistry and how it interacts with salt-affected soils.
3.2. Soil Microbiology
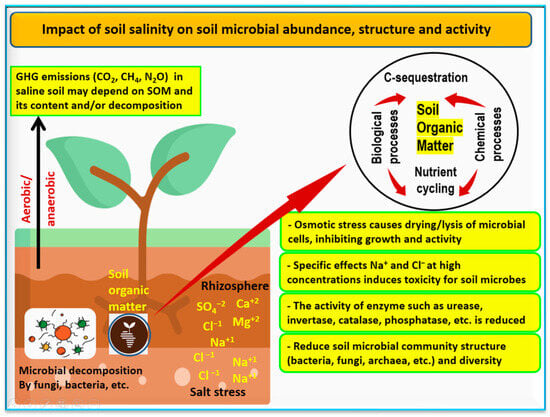
Soil microbes are very important to soil health or quality. Important functions carried out by microbes include the decomposition of organic matter, nutrient cycling, C-sequestration, and promoting soil fertility (
Figure 5). Soil microbiology in saline soils mainly focuses on the relationship between soil salts and microbial structure, abundance, and activities. The mitigative role of microbes on cultivated plants under salinity stress is a very important issue
[12][47][48]. The main soil microbial taxa that enhance the tolerance of cultivated plants under salinity stress include arbuscular mycorrhizal fungi (AMF),
Trichoderma spp.,
Pseudomonas spp.,
Bacillus spp.,
Enterobacter spp., and
Serendipita indica [12]. Plant–microbe interactions in salt-affected soils alter the rhizomicrobiomes in ways that promote plant growth
[49]. This microbial role has been applied successfully under treated wastewater irrigation in saline soils during the cultivation of bioenergy crops
[50]. Building microbial communities able to enhance plant growth under salinity stress through the use of OM is a crucial objective or strategy
[51]. There is still a need for considerable research into the role and function of soil microorganisms in salt-affected soils.
Figure 5. Soil salinity has a negative impact on soil microbial abundance, structure and activity through both osmotic stress and specific ion stress. Sources:
[12][47][48][49][50][51].
3.3. Soil Fertility and Plant Nutrition
Although sodium, chloride, calcium, magnesium, and other ions have important roles in plant nutrition, the high content of Na
+ and/or Cl
− in salt-affected soils can cause stress in cultivated plants. Elevated levels of other nutrients (Ca, K, Mg, etc.) can also cause nutrient imbalances, negatively affecting crop yields
[66]. Salinity can reduce enzyme activity
[52], soil respiration
[53], soil microbial biomass
[54], and the bacterial growth rate
[55], all of which influence biogeochemical cycling
[56] which impacts soil fertility
[57][67]. Soil salinity stress is aggravated in polluted environments, where cultivated plants suffer from soil nutrient and water uptake that are insufficient to meet their needs
[55]. Extra stress on cultivated plants has been documented in saline soils polluted with heavy metals
[58] and organic pollutants
[59]. The combination of salinity and pollution can form high redox potential values, which control the release/uptake/desorption of pollutants (e.g., As, Cd, Cu, Pb, and Zn)
[58]. Polluted saline soils also complicate remediation efforts, as treatments intended to alter microbial biomass/activity, release/degrade pollutants, and change nutrient or contaminant bioavailability may not function the same way as they do in non-polluted or non-saline soils
[59].
4. Crop Response to Soil Salinity and Mechanisms
Salts in soil have detrimental effects on functional processes in both soil and plants. Soil physico-chemical (e.g., BD, infiltration, aeration, soil water potential, soil aggregates, soil fertility) and biological (e.g., soil enzyme and microbial activity and biodiversity) properties are negatively impacted by high soil salt content
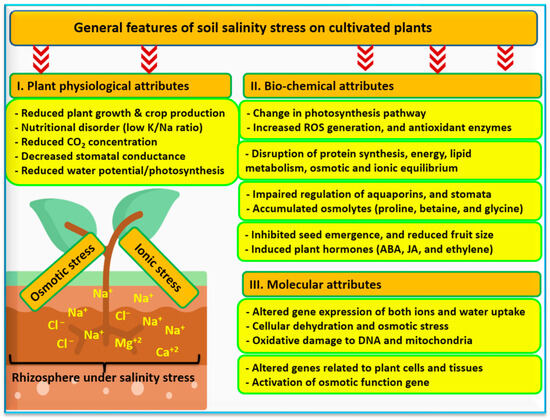
[11][68]. Salt-affected soils cause biochemical, physiological, and molecular alterations in crops (
Figure 6)
[27][69]. The negative impacts of salt-affected soils on crop production can be mitigated through soil management techniques. Many of these techniques are focused on enhancing soil properties, such as soil structure and soil nutrient ratios. Amendments applied to the soil to achieve this include gypsum and related compounds
[41], biochar
[70], compost
[13], earthworms
[71], microbial inoculants
[9], vermicompost
[44], and electro-remediation
[72]. Other approaches to improve plant response to salinity stress include afforestation
[73], seed priming
[74], genetic improvements to crops
[75], using crops that are salt tolerant (halophytes)
[9], and agroforestry
[76]. Some approaches depend on utilizing both soil and plant management in a synergic manner
[9].
Figure 6. Impacts of soil salinity on cultivated plants, including the physiological, biochemical, and genetic attributes. Sources:
[27][69][77].
Several mechanisms have been suggested to explain how plants are able to mitigate stresses imparted by soil salts
[78]. The pathway of any suggested mechanism primarily depends on the applied materials and management approaches used. However, certain groups of physiological, biochemical, and molecular plant attributes are responsible for driving these mechanisms. In general, the mechanisms include activating the osmotic stress pathway, regulating ion homeostasis, mediating plant hormone signaling, and regulating the cell wall composition
[78][79][80][81][82]. Si-NPs have been shown to alleviate salinity stress in rice plants by triggering physiological and genetic repair mechanisms
[83]. The plant gene transporters of both Cl
− and Na
+ are linked with salinity tolerance which may vary from species to species and/or even within cultivars
[84]. The control of Cl
− uptake and its translocation in plants is due to slower loading into the xylem, root efflux, and intracellular compartmentation
[84].
Plant response to salt stress is primarily through ionic and osmotic stress. This leads to the formation of many signals in plants, including the hyperosmolality of Na
+, the accumulation of Ca
2+, the activation of ROS signaling, and the alteration of phospholipid composition. These signals can activate plant adaptive processes to alleviate salt stress through maintaining an ion balance and osmotic homeostasis, inducing phytohormone signaling and regulating cytoskeleton dynamics and the cell wall structure
[82]. High-affinity potassium transporters (HKTs) have been broadly characterized in different plants and have been shown to play a critical role in salt tolerance by excluding Na
+ ions from the sensitive shoots of plants, mediating Na
+ import due to their transport selectivity, and they may mediate Mg
2+/Ca
2+ permeability across the plasma membrane of plant cells
[81]. Therefore, several mechanisms can be illustrated for each applied amendment or approach that are linked to particular genes.
High concentrations of salt ions can change the ion concentrations in the plant cell wall, which are sensed by specific receptors or sensors such as receptor-like kinases (RLKs) and glycosyl inositol phosphoryl ceramide (GIPC). These sensors can activate signaling pathways such as the salt overly sensitive (SOS) pathway to re-distribute ions and achieve homeostasis
[85]. Osmotic potential forms from changes in the balance between ion concentrations inside and outside of plant cells, a process which is monitored by specific sensors such as nicotinamide adenine dinucleotide phosphate (NSCCs), through the high-osmolarity glycerol (HOG) pathway to regulate the synthesis of organic osmolytes (e.g., betaine and proline). Osmotic homeostasis is achieved via the uptake of ions. Plants generate and accumulate ROS through plasma membrane-bound nicotinamide adenine dinucleotide phosphate (NADPH) oxidase under saline conditions, which manages ROS homeostasis through secondary metabolites
[85].