Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Suryang Kwak | -- | 2650 | 2024-01-18 12:20:23 | | | |
| 2 | Jessie Wu | Meta information modification | 2650 | 2024-01-19 03:59:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kwak, S. Sb and Sc, and Their Modes of Action. Encyclopedia. Available online: https://encyclopedia.pub/entry/54047 (accessed on 07 February 2026).
Kwak S. Sb and Sc, and Their Modes of Action. Encyclopedia. Available at: https://encyclopedia.pub/entry/54047. Accessed February 07, 2026.
Kwak, Suryang. "Sb and Sc, and Their Modes of Action" Encyclopedia, https://encyclopedia.pub/entry/54047 (accessed February 07, 2026).
Kwak, S. (2024, January 18). Sb and Sc, and Their Modes of Action. In Encyclopedia. https://encyclopedia.pub/entry/54047
Kwak, Suryang. "Sb and Sc, and Their Modes of Action." Encyclopedia. Web. 18 January, 2024.
Copy Citation
Saccharomyces cerevisiae var. boulardii (Sb) is currently receiving significant attention as a synthetic probiotic platform due to its ease of manipulation and inherent effectiveness in promoting digestive health.
probiotic
parabiotic
prebiotic
synthetic biology
1. Introduction
Saccharomyces cerevisiae var. boulardii (Sb) was obtained from tea made with peels of tropical fruits and described by Henri Boulard in 1920. Sb is recognized as nonpathogenic, is generally regarded as safe (GRAS), and has been employed for managing various gastrointestinal disorders [1]. Recent molecular typing technologies and phylogenetic analyses categorized Sb into the same species sharing very similar karyotypes with brewer’s yeast S. cerevisiae (Sc) but a different strain [2]. Early studies have reported Sb as a distinct yeast species from Sc, considering the differences between the two yeasts on a number of key physiologic and metabolic traits [3][4]. First of all, better thermotolerance and acid tolerance have been considered to represent the phenotypic distinction of Sb as a probiotic yeast strain because they permit better viability through the host digestive tract, while the bile salt tolerance of Sb is weaker than Sc [5][6]. In addition, galactose utilization by Sb is significantly inefficient compared to Sc [3][7], albeit the culture pattern on galactose is slightly varied among Sb strains [8]. The truncation of PGM2 encoding phosphoglucomutase, which likely led to its loss of function, was the major cause of impaired galactose utilization by Sb. Intriguingly, recovery of the full length of PGM2 resulted in a detriment to the growth rate on glucose, the universal carbon source for Saccharomyces, at human body temperature, connoting that phosphoglucomutase could play a pivotal role in the thermotolerance of Sb [7]. It is also known that Sb cannot produce ascospores, which wild-type Sc produces [3]. In addition, the Sb cell wall composition has more mannan but less glucan compared to that of Sc. Transmission electron microscopy also demonstrated that Sb carries a thicker and coarser mannan layer and thinner glucan layer on its cell wall than Sc [9][10].
Sb is the only commercialized probiotic yeast to date and has been prescribed in the past 40 years as an effective prophylactic or therapeutic avenue in a wide range of gastrointestinal disorders including infectious diseases [1][4][11]. Sb has been believed to encompass pathogen exclusion, enhancement of gut barrier function, immune modulation, and trophic effects. Although most of these efficacies have been validated in animal models or humans through placebo-controlled clinical trials [12], the intrinsic mechanisms behind the efficacies are not entirely understood yet [1][12]. Also, investigations on Sb have predominantly aimed at uncovering potential mechanisms behind its beneficial properties and exploring its applications as a probiotic strain only [4].
Due to its recognition as a eukaryotic host system with robust viability at human body temperature and the ease with which it undergoes genetic transformation, Sb emerges as a synthetic probiotic chassis with the capacity to deliver therapeutic molecules within the host intestinal environment as well [13]. Early Sb engineering studies had faced significant inefficiencies, primarily due to the absence of auxotrophic mutants [14], concerns surrounding the use of genetic markers for drug resistance [15], and the low efficiencies of classic genome editing systems, such as UV random mutagenesis and the Cre-loxP system [16][17][18], before CRISPR-mediated genome editing arose in the yeast engineering field.
2. Health Benefits of Sb and Sc, and Their Modes of Action
Previous studies have identified diverse functionalities of Sb against the host and pathogens including control of the balance of intestinal microbes, disruption of the colonization and infection of pathogens on the mucosa, local and systemic immune response adjustment, and stabilization of the gastrointestinal barrier function. It has been reported that lyophilized Sb products carry a higher number of viable cells and outperform heat-killed Sb products regarding the pharmacokinetics and probiotic stability at room temperature [1], but the efficacy difference between the two product types may vary depending on whether the mechanism of action is probiotic or parabiotic. In the case of Sc, a few health benefits of its intake have been reported but mostly from a nutritional perspective [19]. Despite the considerable genetic similarity, the efficacies of Sc as a prophylactic or therapeutic avenue against gastrointestinal disorders have not been studied as thoroughly as those of Sb. Considering their genotypic and phenotypic similarities, however, Sc may also provide some of the reported benefits of Sb. The following subsections introduce detailed examples of the probiotic and non-probiotic mechanisms of the health benefits of the Saccharomyces yeasts (described in Figure 1 and Table 1).
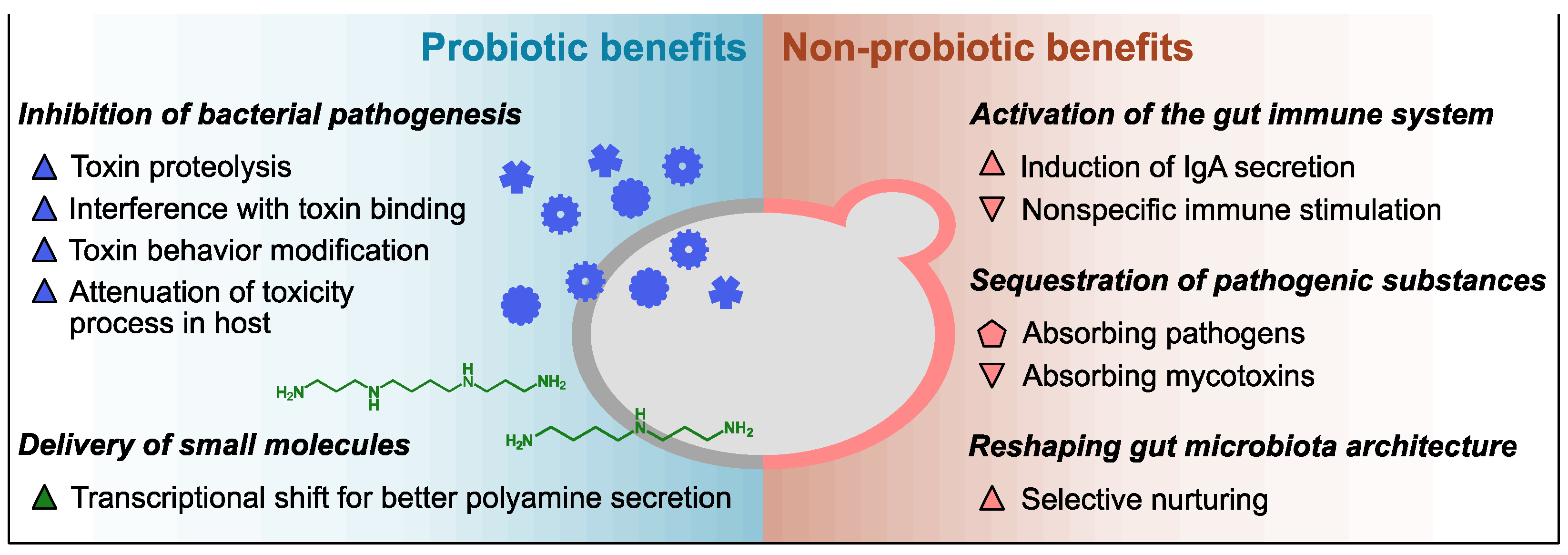
Figure 1. Overview of innate health benefits of Sb and Sc. Up-pointing triangle, benefits demonstrated only in Sb to date; down-pointing triangle, benefits demonstrated only in Sc to date; pentagon, benefits demonstrated in both Sc and Sb. Blue, benefits associated with secreted proteins; green, benefits associated with small molecules; pink, benefits associated with cell wall polysaccharides.
Table 1. Studies demonstrating key health benefits of Sb and Sc and their mechanisms.
| Health Benefit | Study Design and Methodology | Outcome | Ref. |
|---|---|---|---|
| Protection against C. difficile infection | Randomized placebo-controlled clinical trial, the combination of Sb and antibiotics | Lower relative risk of recurrent C. difficile infection in Sb recipients than placebo | [20][21] |
| In vivo (mice), Sb administration | Dose- and viability-dependent prophylactic effect of Sb decreasing lethality | [22] | |
| In vivo (rats), Sb administration | 54 kDa protease digested TcdA and inhibited its binding to rat ileal brush border | [23] | |
| In vitro (human colonic mucosa), functional validation of 54 kDa protease of Sb | Attenuation of toxin-induced electrophysiologic and cytotoxic effects | [24] | |
| Potential protection from anthrax | In vitro, biochemical assay of B. anthracis lethal toxin and Sb cells | Trapping and proteolysis of protective antigens of lethal toxin by Sb | [25] |
| Inactivation of E. coli endotoxin | Isolation of phosphatases from rat small intestines after Sb administration | Dephosphorylation and inhibition of E. coli O55:B5 LPS toxicity by 63 kDa protein | [26] |
| Protection against cholera pathogenesis | In vitro (rat small intestine epithelial and human colon cells), Sb or Sb product treatment | Modulation of cAMP levels by 120 kDa protein in Sb-conditioned medium | [27] |
| Recovery from proximal enterectomy | In vivo (60% proximal enterectomy rats), Sb administration | Improvement of functional adaptation of remnant ileum via polyamine metabolites | [28] |
| Activation of host immune system | In vivo (rats), Sb administration | Enhanced secretory IgA in the duodenal fluid of rats after Sb administration | [29] |
| In vitro (murine macrophage and fibroblast cells), Sc cell wall fraction treatment | Nonspecific immune stimulation (higher NO secretion and macrophage activity) | [30] | |
| Absorbing enteric pathogens | In vitro, binding assays of Sb and enteric pathogens | Adhesion and sedimentation with S. enterica Typhimurium and enterohemorrhagic E. coli | [31] |
| In vivo (gnotobiotic mice), evaluation of Sb–pathogen adhesion | Adhesion between Sb and S. enterica Typhimurium on intestinal epithelium | [32] | |
| Absorbing mycotoxins | In vivo (broiler chicks), Sc administration after aflatoxicosis | Positive protection effect of Sc administration on liver weight, histopathology, and growth | [33] |
| In vivo (rats), MOS, thermolyzed Sc, and dehydrated Sc treatment after aflatoxicosis | Attenuation of the toxicity and liver damage only by dehydrated Sc administration | [34] | |
| Obesity and type 2 diabetes | In vivo (obese and type 2 diabetic mice), Sb administration | Reduction of fat mass, hepatic steatosis, and inflammation with shift in host gut microbiome | [35] |
LPS, lipopolysaccharide; cAMP, cyclic adenosine monophosphate; MOS, manno-oligosaccharide.
2.1. Innate Probiotic Benefits
The inhibitory activity against the pathogenic mechanisms of varied bacterial toxins has been thoroughly investigated as a representative probiotic capability of Sb. For instance, colitis associated with C. difficile infection has been a major target ailment of the probiotic application of Sb; the protective effect of Sb administration against Clostridioides difficile infection has been proven not only in animal models but also in placebo-controlled clinical trials [20][21][22][36][37][38]. A gnotobiotic murine model demonstrated that the protective effect was associated with the viability of administered Sb as well as its dose [22]. In vivo investigation using a rat model and in vitro assessment employing human colonic cells substantiated that a 54 kDa serine protease secreted by Sb possesses the capacity to attenuate the pathogenicity of C. difficile by proteolyzing its two exotoxins, toxins A and B (TcdA and TcdB) [23][24]. In addition, the serine protease inhibited the binding of TcdA to its receptor on the brush border epithelium in rats [39]. Similarly, Sb exhibits a prophylactic effect on gastrointestinal anthrax by inactivating the lethal toxin from Bacillus anthracis, the causative pathogen of anthrax [40]. As its major virulence factor, B. anthracis synthesizes the lethal toxin consisting of protective antigens and the lethal factor. In vitro tests using human intestinal epithelial cells determined two mechanisms of Sb inactivating the lethal toxin, namely absorbing the protective antigens on its cell wall and inducing its cleavage [25]. However, the molecules exerting the binding and proteolytic actions against the B. anthracis lethal toxin have not been demonstrated from Sb yet.
In addition, the inhibition of bacterial endotoxin by Sb was also demonstrated with Escherichia coli O55:B5 as a model pathogen in a rat model. The key element of the inhibitory activity was a 63 kDa protein phosphatase catalyzing the dephosphorylation of two phosphorylation sites of the lipopolysaccharide of E. coli O55:B5. In vivo tests revealed that the intraperitoneal injection of intact E. coli O55:B5 lipopolysaccharide into rats resulted in 100 ng/mL of circulating tumor necrosis factor-α, along with inflammatory lesions and apoptotic bodies in the liver and heart after 9 h. In contrast, rats injected with dephosphorylated lipopolysaccharide had 40 ng/mL of tumor necrosis factor-α without any observable organic lesions [26].
Sb also attenuates the morphological damage caused by Vibrio cholerae. It was demonstrated in multiple rat model studies that Sb decreased cholera toxin-induced fluid and sodium secretion [41]. Cholera toxin increases cyclic adenosine monophosphate levels by activating adenylate cyclase. The elevation of cyclic adenosine monophosphate levels prompts the secretion of chloride and bicarbonate in crypt cells while inhibiting chloride absorption in villi [42]. In a rat intestinal cell model, the inhibitory effect of Sb on cyclic adenosine monophosphate was abolished when Sb was heat-inactivated. A 120 kDa protein identified from an Sb-conditioned medium has been proposed as the factor mediating the protective efficacy of Sb toward V. cholerae. The 120 kDa protein neutralized the cholera toxin-induced secretion by not exerting proteolytic or protein modification activities on cholera toxin but reducing cyclic adenosine monophosphate levels [27].
While these specific 54 kDa, 63 kDa, and 120 kDa proteins have been proposed to play pivotal roles in the probiotic activities of Sb, genes encoding those proteins have not been identified in the Sb genome [2][43]. Accordingly, their existence in genomes of Sc or other Saccharomyces species has also not been confirmed yet.
Another probiotic capability of Sb is the in situ delivery of advantageous small molecules. In a simulated gastrointestinal tract environment, Sb and Sc showed different transcriptional patterns of genes encoding enzymes involved in the production and secretion of polyamines, such as spermidine and spermine. Specifically, Sb exhibited higher expression levels of the synthetic pathway of ornithine, the precursor of spermidine and spermine, and the polyamine exporter Tpo2p compared to Sc. On the other hand, Sb down-regulated the expression of the ornithine catabolic pathway, the polyamine importer Tpo1p, and the positive regulator of spermine uptake Ptk1p [2][44]. In a rat model featuring a 60% proximal small bowel resection, an elevation in mucosal polyamine concentrations attributable to the influence of Sb was discerned [28]. Polyamines promote the expression of digestive enzymes and nutrient transporters in gut epithelial cells, maintain the integrity of the gut epithelium, and regulate macrophage differentiation for anti-inflammatory effects [2][28][45][46].
2.2. Innate Non-Probiotic Benefits
Saccharomyces yeast cell biomass is reported to interact with the host via cell wall oligosaccharides, such as mannan and glucan, regardless of cell viability. The administration of cell wall polysaccharide fractions of Sb or its whole cells triggers the gut mucosal immune system by stimulating enterocytes and gastrointestinal-associated immune cells via β-glucan and mannose receptors in various animal models [29][47][48][49][50]. In vivo (mice) and in vitro (human colonic cells) assays demonstrated that the induction by Sb cell wall components leads to immunomodulatory responses including the secretion of immunoglobulins, which protects intestinal epithelium from pathogenic bacteria and their toxins [20][51][52]. In addition, the cell wall mannoprotein and β-glucan of Sc were also documented as nonspecific immune stimulators demonstrating interactions with macrophages, neutrophils, and eosinophils in an in vitro evaluation employing murine cell lines [30].
Also, in vitro assays have demonstrated that the mannan oligosaccharide on the surface of both Sc and Sb is a biomaterial that traps enteric pathogens carrying mannose-specific adhesins or receptors, such as Salmonella enterica Typhimurium and Escherichia coli O157, and form yeast–bacteria clusters [9][31][32][53]. Importantly, the binding affinity between representative Saccharomyces strains and gut commensal bacteria has not been reported except for the Sc UFMG 905 strain and Bacteroides fragilis [32]. The trapping capability of Sc and Sb is independent of their viability but prominent in the stationary phase compared to other growth phases [31][32][53]. As the adhesive interaction is dependent on the mannan and mannan-specific adhesion factors, the presence of other sugars and bile salts can interfere with the trapping mechanism [32][54]. The adhesive interaction between the pathogenic bacteria and the Saccharomyces yeast surface can contribute to the therapeutic efficacy of Sb against enteric diseases, as the rivalry between yeast cell wall mannan and oligomannoside chains on enterocytes reduces the colonization and infection chances of the pathogenic bacteria [32][55][56]. Because Saccharomyces yeasts stay in the host gut transiently, yeast cells pass through the host gut, capturing pathogenic bacteria and ultimately diminishing the intestinal population of the pathogens [12][50]. Nevertheless, the in vivo substantiation of the parabiotic protective efficacy of Sb predicated on adhesive interactions with pathogens remains unestablished.
Yeast cell wall polysaccharides absorb not only pathogenic bacteria but also mycotoxins. Aflatoxin B1 is a representative mycotoxin, demonstrating a binding affinity with the majority of Sc strains. In poultry farming, Sc has therefore been utilized as a performance-promoting ingredient with an ameliorating effect against aflatoxin B1 [33]. An in vitro binding test manifested the dose-dependent binding of the Sc cell wall fraction and aflatoxin B1, and the binding affinity was affected by the cell wall mannan condition [57]. On the other hand, thermolyzed Sc and pure mannan oligosaccharide could not successfully attenuate liver damage by aflatoxins, while dehydrated active Sc maintained efficacy against aflatoxins during an in vivo bioassay with rats [34]. Together, these results suggest that the aflatoxin-absorbing capacity of Sc is a parabiotic property but thermosensitive and probably requires all cell wall components [58]. The in vitro binding assay utilizing Sc cell wall materials indicated a notable binding affinity between zearalenone and fumonisin B1 with Sc cell wall polysaccharides, while deoxynivalenol did not exhibit a noticeable binding affinity [57]. However, there is currently no substantiation of the mycotoxin-absorbing www in human subjects.
Furthermore, the administration of cell wall mannan can reshape the architecture of gut microbiota as a selective carbon source. Sb administration increased relative abundances of Bacteroidetes but decreased those of Firmicutes in the mouse gut at the phylum level, and the genus Bacteroides was one of the major momenta of the increase in the Bacteroidetes phylum [35][59]. This taxonomic reconstruction of gut microbiota is connected to the efficacy of Sb administration in multiple disorders including obesity, inflammation, skin dryness, and infectious diseases [9][35][59]. In vitro competition between Bacteroides thetaiotaomicron and C. difficile for quenched Saccharomyces yeast cells demonstrated that the selective nurturing effect is a non-probiotic characteristic of yeast biomass [9]. Bacteroides is a representative genus that efficiently metabolizes various polysaccharides, including yeast cell wall mannan, via a large number of carbohydrate-active enzymes [60]. In particular, B. thetaiotaomicron, one of the dominant members of the commensal gut microbiota, is well known for its capacity to utilize Saccharomyces cell wall mannan through a selfish mechanism. B. thetaiotaomicron does not break down extracellular mannan into small oligosaccharides or mannose monomers. Instead, it produces complex mannan chunks that are not readily usable by many bacteria in the gut [60][61]. B. thetaiotaomicron imports the complex mannan chunks into its periplasmic space through the sus-like transport system and then digests them further to mannan monomers. The selfish mechanism has also been overserved in Bacteroides ovatus, another example of commensal Bacteroides [60][62]. The administration of Saccharomyces cell wall mannan enhanced the relative abundances of both B. thetaiotaomicron and B. ovatus in a human feces fermentation system, and a positive correlation was noted in the relative ratio of B. thetaiotaomicron and B. ovatus. This indicates a coordinated utilization of Saccharomyces cell wall mannan by the two Bacteroides species [62].
References
- Kelesidis, T.; Pothoulakis, C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Ther. Adv. Gastroenterol. 2012, 5, 111–125.
- Pais, P.; Oliveira, J.; Almeida, V.; Yilmaz, M.; Monteiro, P.T.; Teixeira, M.C. Transcriptome-wide differences between Saccharomyces cerevisiae and Saccharomyces cerevisiae var. boulardii: Clues on host survival and probiotic activity based on promoter sequence variability. Genomics 2021, 113, 530–539.
- McFarland, L.V. Saccharomyces boulardii is not Saccharomyces cerevisiae. Clin. Infect. Dis. 1996, 22, 200–201.
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 78.
- Edwards-Ingram, L.; Gitsham, P.; Burton, N.; Warhurst, G.; Clarke, I.; Hoyle, D.; Oliver, S.G.; Stateva, L. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2007, 73, 2458–2467.
- Fietto, J.L.R.; Araújo, R.S.; Valadão, F.N.; Fietto, L.G.; Brandão, R.L.; Neves, M.J.; Gomes, F.C.O.; Nicoli, J.R.; Castro, I.M. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can. J. Microbiol. 2004, 50, 615–621.
- Liu, J.-J.; Zhang, G.-C.; Kong, I.I.; Yun, E.J.; Zheng, J.-Q.; Kweon, D.-H.; Jin, Y.-S. A Mutation in PGM2 Causing Inefficient Galactose Metabolism in the Probiotic Yeast Saccharomyces boulardii. Appl. Environ. Microbiol. 2018, 84, 2280–2287.
- Kwak, S.; Mahmud, B.; Dantas, G. A Tunable and Expandable Transactivation System in Probiotic Yeast Saccharomyces boulardii. ACS Synth. Biol. 2021, 11, 508–514.
- Kwak, S.; Robinson, S.J.; Lee, J.W.; Lim, H.; Wallace, C.L.; Jin, Y.-S. Dissection and enhancement of prebiotic properties of yeast cell wall oligosaccharides through metabolic engineering. Biomaterials 2022, 282, 121379.
- Hudson, L.E.; McDermott, C.D.; Stewart, T.P.; Hudson, W.H.; Rios, D.; Fasken, M.B.; Corbett, A.H.; Lamb, T.J. Characterization of the Probiotic Yeast Saccharomyces boulardii in the Healthy Mucosal Immune System. PLoS ONE 2016, 11, e0153351.
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66.
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics—Saccharomyces boulardii, Aliment. Pharmacol. Ther. 2007, 26, 767–778.
- Nielsen, J. Yeast Systems Biology: Model Organism and Cell Factory. Biotechnol. J. 2019, 14, e1800421.
- Liu, J.-J.; Kong, I.I.; Zhang, G.-C.; Jayakody, L.N.; Kim, H.; Xia, P.-F.; Kwak, S.; Sung, B.H.; Sohn, J.-H.; Walukiewicz, H.E.; et al. Metabolic Engineering of Probiotic Saccharomyces boulardii. Appl. Environ. Microbiol. 2016, 82, 2280–2287.
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020, 12, 82.
- Hudson, L.E.; Fasken, M.B.; McDermott, C.D.; McBride, S.M.; Kuiper, E.G.; Guiliano, D.B.; Corbett, A.H.; Lamb, T.J. Functional heterologous protein expression by genetically engineered probiotic yeast Saccharomyces boulardii. PLoS ONE 2014, 9, e112660.
- Hamedi, H.; Misaghi, A.; Modarressi, M.H.; Salehi, T.Z.; Khorasanizadeh, D.; Khalaj, V. Generation of a Uracil Auxotroph Strain of the Probiotic Yeast Saccharomyces boulardii as a Host for the Recombinant Protein Production. Avicenna J. Med. Biotechnol. 2013, 5, 29–34.
- Wang, L.; Sun, H.; Zhang, J.; Liu, Q.; Wang, T.; Chen, P.; Li, H.; Xiao, Y.; Wang, F.; Zhao, X. Establishment and application of target gene disruption system in Saccharomyces boulardii. Biotechnol. Bioprocess Eng. 2015, 20, 26–36.
- Moslehi-Jenabian, S.; Pedersen, L.L.; Jespersen, L. Beneficial effects of probiotic and food borne yeasts on human health. Nutrients 2010, 2, 449–473.
- McFarland, L.V.; Surawicz, C.M.; Greenberg, R.N.; Fekety, R.; Elmer, G.W.; Moyer, K.A.; Melcher, S.A.; Bowen, K.E.; Cox, J.L.; Noorani, Z. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994, 271, 1913–1918.
- Surawicz, C.M.; McFarland, L.V.; Greenberg, R.N.; Rubin, M.; Fekety, R.; Mulligan, M.E.; Garcia, R.J.; Brandmarker, S.; Bowen, K.; Borjal, D.; et al. The search for a better treatment for recurrent Clostridium difficile disease: Use of high-dose vancomycin combined with Saccharomyces boulardii. Clin. Infect. Dis. 2000, 31, 1012–1017.
- Elmer, G.W.; Corthier, G. Modulation of Clostridium difficile induced mortality as a function of the dose and the viability of the Saccharomyces boulardii used as a preventative agent in gnotobiotic mice. Can. J. Microbiol. 1991, 37, 315–317.
- Castagliuolo, I.; LaMont, J.T.; Nikulasson, S.T.; Pothoulakis, C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect. Immun. 1996, 64, 5225–5232.
- Castagliuolo, I.; Riegler, M.F.; Valenick, L.; LaMont, J.T.; Pothoulakis, C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect. Immun. 1999, 67, 302–307.
- Pontier-Bres, R.; Rampal, P.; Peyron, J.-F.; Munro, P.; Lemichez, E.; Czerucka, D. The Saccharomyces boulardii CNCM I-745 strain shows protective effects against the B. anthracis LT toxin. Toxins 2015, 7, 4455–4467.
- Buts, J.-P.; Dekeyser, N.; Stilmant, C.; Delem, E.; Smets, F.; Sokal, E. Saccharomyces boulardii produces in rat small intestine a novel protein phosphatase that inhibits Escherichia coli endotoxin by dephosphorylation. Pediatr. Res. 2006, 60, 24–29.
- Czerucka, D.; Roux, I.; Rampal, P. Saccharomyces boulardii inhibits secretagogue-mediated adenosine 3′,5′-cyclic monophosphate induction in intestinal cells. Gastroenterology 1994, 106, 65–72.
- Buts, J.P.; De Keyser, N.; Marandi, S.; Hermans, D.; Sokal, E.M.; Chae, Y.H.; Lambotte, L.; Chanteux, H.; Tulkens, P.M. Saccharomyces boulardii upgrades cellular adaptation after proximal enterectomy in rats. Gut 1999, 45, 89–96.
- Buts, J.P.; Bernasconi, P.; Vaerman, J.P.; Dive, C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig. Dis. Sci. 1990, 35, 251–256.
- Ha, C.H.; Yun, C.W.; Paik, H.D.; Kim, S.W.; Kang, C.W.; Hwang, H.J.; Chang, H.I. Preparation and analysis of yeast cell wall mannoproteins, immune enhancing materials, from cell wall mutant Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2006, 16, 247–255.
- Gedek, B.R. Adherence of Escherichia coli serogroup O 157 and the Salmonella typhimurium mutant DT 104 to the surface of Saccharomyces boulardii. Mycoses 1999, 42, 261–264.
- Tiago, F.C.P.; Martins, F.S.; Souza, E.L.S.; Pimenta, P.F.P.; Araujo, H.R.C.; Castro, I.M.; Brandão, R.L.; Nicoli, J.R. Adhesion to the yeast cell surface as a mechanism for trapping pathogenic bacteria by Saccharomyces probiotics. J. Med. Microbiol. 2012, 61, 1194–1207.
- Pizzolitto, R.P.; Armando, M.R.; Salvano, M.A.; Dalcero, A.M.; Rosa, C.A. Evaluation of Saccharomyces cerevisiae as an antiaflatoxicogenic agent in broiler feedstuffs. Poult. Sci. 2013, 92, 1655–1663.
- Baptista, A.S.; Horii, J.; Calori-Domingues, M.A.; da Glória, E.M.; Salgado, J.M.; Vizioli, M.R. The capacity of mannooligosaccharides thermolysed yeast and active yeast to attenuate aflatoxicosis. World J. Microbiol. Biotechnol. 2004, 20, 475–481.
- Everard, A.; Matamoros, S.; Geurts, L.; Delzenne, N.M.; Cani, P.D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. MBio 2014, 5, e01011–e01014.
- Toothaker, R.D.; Elmer, G.W. Prevention of clindamycin-induced mortality in hamsters by Saccharomyces boulardii. Antimicrob. Agents Chemother. 1984, 26, 552–556.
- Corthier, G.; Dubos, F.; Ducluzeau, R. Prevention of Clostridium difficile induced mortality in gnotobiotic mice by Saccharomyces boulardii. Can. J. Microbiol. 1986, 32, 894–896.
- Castex, F.; Corthier, G.; Jouvert, S.; Elmer, G.W.; Lucas, F.; Bastide, M. Prevention of Clostridium difficile-induced experimental pseudomembranous colitis by Saccharomyces boulardii: A scanning electron microscopic and microbiological study. J. Gen. Microbiol. 1990, 136, 1085–1089.
- Pothoulakis, C.; Kelly, C.P.; Joshi, M.A.; Gao, N.; O’Keane, C.J.; Castagliuolo, I.; Lamont, J.T. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology 1993, 104, 1108–1115.
- Beatty, M.E.; Ashford, D.A.; Griffin, P.M.; Tauxe, R.V.; Sobel, J. Gastrointestinal anthrax: Review of the literature. Arch. Intern. Med. 2003, 163, 2527–2531.
- Czerucka, D.; Rampal, P. Experimental effects of Saccharomyces boulardii on diarrheal pathogens. Microbes Infect. 2002, 4, 733–739.
- Conner, J.G.; Teschler, J.K.; Jones, C.J.; Yildiz, F.H. Staying Alive: Vibrio cholerae’s Cycle of Environmental Survival, Transmission, and Dissemination. Microbiol. Spectr. 2016, 4, 593–633.
- Khatri, I.; Akhtar, A.; Kaur, K.; Tomar, R.; Prasad, G.S.; Ramya, T.N.C.; Subramanian, S. Gleaning evolutionary insights from the genome sequence of a probiotic yeast Saccharomyces boulardii. Gut Pathog. 2013, 5, 30.
- Buts, J.P.; De Keyser, N.; De Raedemaeker, L. Saccharomyces boulardii enhances rat intestinal enzyme expression by endoluminal release of polyamines. Pediatr. Res. 1994, 36, 522–527.
- Rao, J.N.; Xiao, L.; Wang, J.-Y. Polyamines in Gut Epithelial Renewal and Barrier Function. Physiology 2020, 35, 328–337.
- Ray, R.M.; McCormack, S.A.; Covington, C.; Viar, M.J.; Zheng, Y.; Johnson, L.R. The requirement for polyamines for intestinal epithelial cell migration is mediated through Rac1. J. Biol. Chem. 2003, 278, 13039–13046.
- Gómez-Verduzco, G.; Cortes-Cuevas, A.; López-Coello, C.; Avila-González, E.; Nava, G.M. Dietary supplementation of mannan-oligosaccharide enhances neonatal immune responses in chickens during natural exposure to Eimeria spp. Acta Vet. Scand. 2009, 51, 11.
- Kudoh, K.; Shimizu, J.; Ishiyama, A.; Wada, M.; Takita, T.; Kanke, Y.; Innami, S. Secretion and excretion of immunoglobulin A to cecum and feces differ with type of indigestible saccharides. J. Nutr. Sci. Vitaminol. 1999, 45, 173–181.
- Swanson, K.S.; Grieshop, C.M.; Flickinger, E.A.; Bauer, L.L.; Healy, H.-P.; Dawson, K.A.; Merchen, N.R.; Fahey, G.C. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr. 2002, 132, 980–989.
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Salmonella infection—Prevention and treatment by antibiotics and probiotic yeasts: A review. Microbiology 2018, 164, 1327–1344.
- Qamar, A.; Aboudola, S.; Warny, M.; Michetti, P.; Pothoulakis, C.; LaMont, J.T.; Kelly, C.P. Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect. Immun. 2001, 69, 2762–2765.
- Pontier-Bres, R.; Munro, P.; Boyer, L.; Anty, R.; Imbert, V.; Terciolo, C.; André, F.; Rampal, P.; Lemichez, E.; Peyron, J.-F.; et al. Saccharomyces boulardii modifies Salmonella typhimurium traffic and host immune responses along the intestinal tract. PLoS ONE 2014, 9, e103069.
- Posadas, G.A.; Broadway, P.R.; Thornton, J.A.; Carroll, J.A.; Lawrence, A.; Corley, J.R.; Thompson, A.; Donaldson, J.R. Yeast pro- and paraprobiotics have the capability to bind pathogenic bacteria associated with animal disease. Transl. Anim. Sci. 2017, 1, 60–68.
- Martins, F.S.; Dalmasso, G.; Arantes, R.M.E.; Doye, A.; Lemichez, E.; Lagadec, P.; Imbert, V.; Peyron, J.-F.; Rampal, P.; Nicoli, J.R.; et al. Interaction of Saccharomyces boulardii with Salmonella enterica serovar Typhimurium protects mice and modifies T84 cell response to the infection. PLoS ONE 2010, 5, e8925.
- Fàbrega, A.; Vila, J. Salmonella enterica serovar Typhimurium skills to succeed in the host: Virulence and regulation. Clin. Microbiol. Rev. 2013, 26, 308–341.
- Sharon, N.; Eshdat, Y.; Silverblatt, F.J.; Ofek, I. Bacterial adherence to cell surface sugars. Ciba Found. Symp. 1981, 80, 119–141.
- Shetty, P.H.; Jespersen, L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 2006, 17, 48–55.
- Hassan, Z.U.; Al Thani, R.; Atia, F.A.; Alsafran, M.; Migheli, Q.; Jaoua, S. Application of yeasts and yeast derivatives for the biological control of toxigenic fungi and their toxic metabolites. Environ. Technol. Innov. 2021, 22, 101447.
- Tanihiro, R.; Sakano, K.; Oba, S.; Nakamura, C.; Ohki, K.; Hirota, T.; Sugiyama, H.; Ebihara, S.; Nakamura, Y. Effects of Yeast Mannan Which Promotes Beneficial Bacteroides on the Intestinal Environment and Skin Condition: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, E3673.
- Abbott, D.W.; Martens, E.C.; Gilbert, H.J.; Cuskin, F.; Lowe, E.C. Coevolution of yeast mannan digestion: Convergence of the civilized human diet, distal gut microbiome, and host immunity. Gut Microbes 2015, 6, 334–339.
- Cuskin, F.; Lowe, E.C.; Temple, M.J.; Zhu, Y.; Cameron, E.A.; Pudlo, N.A.; Porter, N.T.; Urs, K.; Thompson, A.J.; Cartmell, A.; et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 2015, 517, 165–169.
- Oba, S.; Sunagawa, T.; Tanihiro, R.; Awashima, K.; Sugiyama, H.; Odani, T.; Nakamura, Y.; Kondo, A.; Sasaki, D.; Sasaki, K. Prebiotic effects of yeast mannan, which selectively promotes Bacteroides thetaiotaomicron and Bacteroides ovatus in a human colonic microbiota model. Sci. Rep. 2020, 10, 17351.
More
Information
Subjects:
Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
455
Revisions:
2 times
(View History)
Update Date:
19 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




