
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lorenzo Monti | -- | 2919 | 2024-01-17 17:39:39 | | | |
| 2 | Mona Zou | Meta information modification | 2919 | 2024-01-18 08:37:24 | | |
Video Upload Options
Cardiac magnetic resonance (CMR) imaging has witnessed substantial progress with the advent of parametric mapping techniques, most notably T1 and T2 mapping. These advanced techniques provide valuable insights into a wide range of cardiac conditions, including ischemic heart disease, cardiomyopathies, inflammatory cardiomyopathies, heart valve disease, and athlete’s heart. Mapping could be the first sign of myocardial injury and oftentimes precedes symptoms, changes in ejection fraction, and irreversible myocardial remodeling. The ability of parametric mapping to offer a quantitative assessment of myocardial tissue properties addresses the limitations of conventional CMR methods, which often rely on qualitative or semiquantitative data. However, challenges persist, especially in terms of standardization and reference value establishment, hindering the wider clinical adoption of parametric mapping. Future developments should prioritize the standardization of techniques to enhance their clinical applicability, ultimately optimizing patient care pathways and outcomes.
1. CMR Mapping and Ischemic Heart Disease
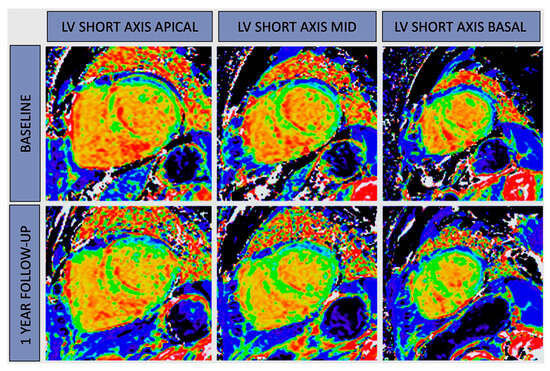
2. CMR Mapping and Cardiomyopathies
3. CMR Mapping and Myocarditis
- (a)
-
Edema: this is detected as high signal intensity in the myocardium on STIR T2w images.
- (b)
-
Hyperemia: This is characterized by increased regional gadolinium contrast agent uptake in the abnormal myocardium during the initial minutes following the injection, commonly referred to as early gadolinium enhancement (EGE). While EGE was initially part of the LLC criteria, later studies demonstrated that its exclusion from the original criteria does not seem to significantly affect their diagnostic accuracy [27]. Alternatively, hyperemia can be assessed using traditional cine steady-state free precession images acquired shortly after contrast administration.
- (c)
-
Fibrosis/Necrosis: this is depicted as myocardial contrast deposition with subepicardial/intramyocardial distribution on LGE images.
4. CMR Mapping and Inflammatory Cardiomyopathies
References
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2 and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imagin. J. Cardiovasc. Magn. Reson. 2017, 19, 75.
- Pontone, G.; Guaricci, A.I.; Fusini, L.; Baggiano, A.; Guglielmo, M.; Muscogiuri, G.; Volpe, A.; Abete, R.; Aquaro, G.; Barison, A.; et al. Cardiac Magnetic Resonance for Prophylactic Implantable-Cardioverter Defibrillator Therapy in Ischemic Cardiomyopathy: The DERIVATE–ICM International Registry. JACC Cardiovasc. Imaging 2023, 16, 1387–1400.
- Abdel-Aty, H.; Boyé, P.; Zagrosek, A.; Wassmuth, R.; Kumar, A.; Messroghli, D.; Bock, P.; Dietz, R.; Friedrich, M.G.; Schulz-Menger, J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: Comparison of different approaches. J. Am. Coll. Cardiol. 2005, 45, 1815–1822.
- Röttgen, R.; Christiani, R.; Freyhardt, P.; Gutberlet, M.; Schultheiss, H.P.; Hamm, B.; Kühl, U. Magnetic resonance imaging findings in acute myocarditis and correlation with immunohistological parameters. Eur. Radiol. 2011, 21, 1259–1266.
- Carbone, I.; Childs, H.; Aljizeeri, A.; Merchant, N.; Friedrich, M.G. Importance of Reference Muscle Selection in Quantitative Signal Intensity Analysis of T2-Weighted Images of Myocardial Edema Using a T2 Ratio Method. Biomed Res. Int. 2015, 2015, 232649.
- McCann, G.P.; Khan, J.N.; Greenwood, J.P.; Nazir, S.; Dalby, M.; Curzen, N.; Hetherington, S.; Kelly, D.J.; Blackman, D.J.; Ring, A.; et al. Complete Versus Lesion-Only Primary PCI the Randomized Cardiovascular MR CvLPRIT Substudy. J. Am. Coll. Cardiol. 2015, 66, 2713–2724.
- Ferreira, V.M.; Piechnik, S.K. CMR parametric mapping as a tool for myocardial tissue characterization. Korean Circ. J. 2020, 50, 658–676.
- Salerno, M.; Kramer, C.M. Advances in parametric mapping with CMR imaging. JACC Cardiovasc. Imaging 2013, 6, 806–822.
- O’Brien, A.T.; Gil, K.E.; Varghese, J.; Simonetti, O.P.; Zareba, K.M. T2 mapping in myocardial disease: A comprehensive review. J. Cardiovasc. Magn. Reson. 2022, 24, 33.
- Tahir, E.; Sinn, M.; Bohnen, S.; Avanesov, M.; Säring, D.; Stehning, C.; Schnackenburg, B.; Eulenburg, C.; Wien, J.; Radunski, U.K.; et al. Acute versus chronic myocardial infarction: Diagnostic accuracy of quantitative Native T1 and T2 mapping versus assessment of edema on Standard T2-weighted cardiovascular MR images for differentiation. Radiology 2017, 285, 83–91.
- Layland, J.; Rauhalammi, S.; Lee, M.M.Y.; Ahmed, N.; Carberry, J.; May, V.T.Y.; Watkins, S.; McComb, C.; Mangion, K.; McClure, J.D.; et al. Diagnostic accuracy of 3.0-T magnetic resonance T1 and T2 mapping and T2-weighted dark-blood imaging for the infarct-related coronary artery in Non-ST-segment elevation myocardial infarction. J. Am. Heart Assoc. 2017, 6, 210–219.
- Bulluck, H.; White, S.K.; Rosmini, S.; Bhuva, A.; Treibel, T.A.; Fontana, M.; Abdel-Gadir, A.; Herrey, A.; Manisty, C.; Wan, S.M.Y.; et al. T1 mapping and T2 mapping at 3T for quantifying the area-at-risk in reperfused STEMI patients. J. Cardiovasc. Magn. Reson. 2015, 17, 73.
- Carrabba, N.; Parodi, G.; Maehara, A.; Pradella, S.; Migliorini, A.; Valenti, R.; Colagrande, S.; Mintz, G.; Antoniucci, D. Effects of rheolytic thrombectomy and manual thrombus aspiration on infarct size and microvascular obstruction during primary angioplasty: Smart–mri substudy. J. Am. Coll. Cardiol. 2013, 61, E1849.
- Dastidar, A.G.; Harries, I.; Pontecorboli, G.; Bruno, V.D.; Garate, E.; De Moret, C.; Baritussio, A.; Johnson, T.W.; McAlindon, E.; Bucciarelli-Ducci, C. Native T1 mapping to detect extent of acute and chronic myocardial infarction: Comparison with late gadolinium enhancement technique. Int. J. Cardiovasc. Imaging 2019, 35, 517–527.
- Schwitter, J.; Arai, A.E. Assessment of cardiac ischaemia and viability: Role of cardiovascular magnetic resonance. Eur. Heart J. 2011, 32, 799–809.
- Weingärtner, S.; Akçakaya, M.; Basha, T.; Kissinger, K.V.; Goddu, B.; Berg, S.; Manning, W.J.; Nezafat, R. Combined saturation/inversion recovery sequences for improved evaluation of scar and diffuse fibrosis in patients with arrhythmia or heart rate variability. Magn. Reson. Med. 2013, 71, 1024–1034.
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626.
- Guglielmo, M.; Fusini, L.; Muscogiuri, G.; Baessato, F.; Loffreno, A.; Cavaliere, A.; Rizzon, G.; Baggiano, A.; Rabbat, M.G.; Muratori, M.; et al. T1 mapping and cardiac magnetic resonance feature tracking in mitral valve prolapse. Eur. Radiol. 2020, 31, 1100–1109.
- Huang, L.; Ran, L.; Zhao, P.; Tang, D.; Han, R.; Ai, T.; Xia, L.; Tao, Q. MRI native T1 and T2 mapping of myocardial segments in hypertrophic cardiomyopathy: Tissue remodeling manifested prior to structure changes. Br. J. Radiol. 2019, 92, 20190634.
- Nordin, S.; Kozor, R.; Medina-Menacho, K.; Abdel-Gadir, A.; Baig, S.; Sado, D.M.; Lobascio, I.; Murphy, E.; Lachmann, R.H.; Mehta, A.; et al. Proposed Stages of Myocardial Phenotype Development in Fabry Disease. JACC Cardiovasc. Imaging 2019, 12, 1673–1683.
- Fontana, M.; Pica, S.; Reant, P.; Abdel-Gadir, A.; Treibel, T.A.; Banypersad, S.M.; Maestrini, V.; Barcella, W.; Rosmini, S.; Bulluck, H.; et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2015, 132, 1570–1579.
- Dowd, R.; Dhanjal, T.; Schmucki, M.; Kanagala, P.; Khan, J.N. Unique role of cardiovascular magnetic resonance imaging parametric mapping in the diagnosis of arrhythmogenic left ventricular cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2020, 22, e96.
- Spieker, M.; Katsianos, E.; Gastl, M.; Behm, P.; Horn, P.; Jacoby, C.; Schnackenburg, B.; Reinecke, P.; Kelm, M.; Westenfeld, R.; et al. T2 mapping cardiovascular magnetic resonance identifies the presence of myocardial inflammation in patients with dilated cardiomyopathy as compared to endomyocardial biopsy. Eur. Heart J. Cardiovasc. Imaging 2017, 19, 574–582.
- Guaricci, A.I.; Masci, P.G.; Muscogiuri, G.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Lorenzoni, V.; Martini, C.; Andreini, D.; Pavon, A.G.; et al. CarDiac magnEtic Resonance for prophylactic Implantable-cardioVerter defibrillAtor ThErapy in Non-Ischaemic dilated CardioMyopathy: An international Registry. Europace 2021, 23, 1072–1083.
- Mitropoulou, P.; Georgiopoulos, G.; Figliozzi, S.; Klettas, D.; Nicoli, F.; Masci, P.G. Multi-Modality Imaging in Dilated Cardiomyopathy: With a Focus on the Role of Cardiac Magnetic Resonance. Front. Cardiovasc. Med. 2020, 7, 97.
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Alakija, P.; Cooper, L.T.; White, J.A.; Gutberlet, M.; Prasad, S.; Aletras, A. Cardiovascular MRI in myocarditis. J. Am. Coll. Cardiol. 2009, 53, 1475–1487.
- Chu, G.C.W.; Flewitt, J.A.; Mikami, Y.; Vermes, E.; Friedrich, M.G. Assessment of acute myocarditis by cardiovascular MR: Diagnostic performance of shortened protocols. Int. J. Cardiovasc. Imaging 2013, 29, 1077–1083.
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176.
- Eichhorn, C.; Greulich, S.; Bucciarelli-Ducci, C.; Sznitman, R.; Kwong, R.Y.; Gräni, C. Multiparametric Cardiovascular Magnetic Resonance Approach in Diagnosing, Monitoring, and Prognostication of Myocarditis. JACC Cardiovasc. Imaging 2022, 15, 1325–1338.
- Lurz, P.; Luecke, C.; Eitel, I.; Föhrenbach, F.; Frank, C.; Grothoff, M.; Waha SDe Rommel, K.P.; Lurz, J.A.; Klingel, K.; Kandolf, R.; et al. Comprehensive Cardiac Magnetic Resonance Imaging in Patients with Suspected Myocarditis the MyoRacer-Trial. J. Am. Coll. Cardiol. 2016, 67, 1800–1811.
- Spieker, M.; Aberkorn, S.; Gastl, M.; Behm, P.; Katsianos, S.; Horn, P.; Jacoby, C.; Schnackenburg, B.; Reinecke, P.; Kelm, M.; et al. Abnormal T2 mapping cardiovascular magnetic resonance correlates with adverse clinical outcome in patients with suspected acute myocarditis. J. Cardiovasc. Magn. Reson. 2017, 19, 38.
- Thomas, K.E.; Fotaki, A.; Botnar, R.M. Europe PMC Funders Group Imaging methods: Magnetic resonance imaging. Circ. Cardiovasc. Imaging 2023, 16, e014068.
- Tymińska, A.; Ozierański, K.; Skwarek, A.; Kapłon-Cieślicka, A.; Baritussio, A.; Grabowski, M.; Marcolongo, R.; Caforio, A.L.P. Personalized Management of Myocarditis and Inflammatory Cardiomyopathy in Clinical Practice. J. Pers. Med. 2022, 12, 183.
- Barison, A.; Ricci, F.; Pavon, A.G.; Muscogiuri, G.; Bisaccia, G.; Camastra, G.; Lazzari, M.; De Lanzillo, C.; Raguso, M.; Monti, L.; et al. Cardiovascular Magnetic Resonance in Patients with Cardiac Electronic Devices: Evidence from a Multicenter Study. J. Clin. Med. 2023, 12, 6673.





