Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pingyang Zhu | -- | 2498 | 2024-01-16 17:05:35 | | | |
| 2 | Sirius Huang | Meta information modification | 2498 | 2024-01-17 02:36:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Qian, Q.; Cui, J.; Miao, Y.; Xu, X.; Gao, H.; Xu, H.; Lu, Z.; Zhu, P. Plant Volatile-Sensing Mechanism of Insects. Encyclopedia. Available online: https://encyclopedia.pub/entry/53918 (accessed on 07 February 2026).
Qian Q, Cui J, Miao Y, Xu X, Gao H, Xu H, et al. Plant Volatile-Sensing Mechanism of Insects. Encyclopedia. Available at: https://encyclopedia.pub/entry/53918. Accessed February 07, 2026.
Qian, Qi, Jiarong Cui, Yuanyuan Miao, Xiaofang Xu, Huiying Gao, Hongxing Xu, Zhongxian Lu, Pingyang Zhu. "Plant Volatile-Sensing Mechanism of Insects" Encyclopedia, https://encyclopedia.pub/entry/53918 (accessed February 07, 2026).
Qian, Q., Cui, J., Miao, Y., Xu, X., Gao, H., Xu, H., Lu, Z., & Zhu, P. (2024, January 16). Plant Volatile-Sensing Mechanism of Insects. In Encyclopedia. https://encyclopedia.pub/entry/53918
Qian, Qi, et al. "Plant Volatile-Sensing Mechanism of Insects." Encyclopedia. Web. 16 January, 2024.
Copy Citation
Plants and insects are engaged in a tight relationship, with phytophagous insects often utilizing volatile organic substances released by host plants to find food and egg-laying sites. Using plant volatiles as attractants for integrated pest management is vital due to its high efficacy and low environmental toxicity. Using naturally occurring plant volatiles combined with insect olfactory mechanisms to select volatile molecules for screening has proved an effective method for developing plant volatile-based attractant technologies.
plant volatiles
insect
olfactory system
attractant
pest management
1. Introduction
Insects and plants have co-evolved for hundreds of millions of years [1]. During this evolutionary process, insects have developed a unique olfactory system that distinguishes plant volatiles from other environmental odors [2][3]. Plant volatile organic chemicals (PVOCs) are essential chemical information links for insect-searching host plants and locating habitat, and play critical roles in the interdependent relationship between plants and insects. Insects can use these volatile compounds to collect information about plants, such as assisting bark beetles in the location of stressed host trees [4]. Research on the olfactory sensory mechanism of insects on PVOCs can help reveal the coevolutionary relationship between insects and plants and provide a theoretical basis for developing ecological technologies for preventing and curing pests.
Plant volatiles meet several key prerequisites for modern pest management, including being species-specific and environmentally benign [5]. Methods that interfere with an insect’s normal sense of smell fulfill these conditions and have been implemented on a large scale in the field. For example, physicochemical trap technology utilizing insect pheromones and plant volatiles to attract pests is highly targeted and provides efficacious pest management, reducing the need for traditional chemical applications [6][7]. As modern molecular and behavioral biology techniques are applied to further study the interactions of plant volatiles on pest behavior, the capabilities of olfaction-based pest management will continue to advance. High-throughput screening methods are particularly promising and will enable the identification and testing of highly efficacious, natural plant volatiles to alter the behavior of or trap agronomic pests. This has the potential to continue to improve our pest management technology in a sustainable and environmentally friendly manner in line with modern goals of ecological agriculture [8][9][10][11].
2. Plant Volatiles
PVOCs are a mixture of multiple volatile plant secondary metabolites. Plant volatiles can be divided into green leaf, floral, and fruit volatiles according to the different organs of the plant, in which straight-chain alcohols and aldehydes containing six carbon atoms as well as their esters are the primary source of green leaf odors. Terpenes such as monoterpenes, sesquiterpenes, and sesquiterpenes together with aromatic compounds are the main constituents of the floral odors of the plant. The short-chained acetic esters formed by the degradation pathway of carbohydrates are the main source of the fruit aroma (Table 1). The metabolic pathways of different plants can produce specific odors, such as the cystine and cysteine metabolic pathways of the lily family and the methionine metabolic pathway of the cruciferous family [12][13].
| Plant Organs | Types of Volatiles |
|---|---|
| Green leaf | C6/C9 aldehydes, alcohols, and esters |
| Flower | Terpenoids, phenylpropanoids/benzenoids, and fatty acid derivatives |
| Root | Alcohols, ketones, aldehydes, esters, terpenes, furans, organic acids, aromatic compounds, and sulfur compounds |
The production of PVOCs by plants, including both the diversity and abundance of the compounds, is strongly influenced by biotic and abiotic factors [14][15], and they also play essential functions in plant–insect interactions. The study of PVOCs is complicated by the number of compounds involved and the complexity of the interactions that they facilitate. For example, multiple PVOCs with different functions often originate from the same plant tissue or organ, all of which can be involved in interactions with numerous insect species. In just flowers, Martel et al. [16] found that not only do floral cuticle hydrocarbons play an important role in plant–pollinator interactions, but these same hydrocarbons are also critical for interspecies communication among insects.
PVOCs directly affect insect mating and reproductive behavior and help insects search, locate, and select suitable host plants. Different classes of PVOCs have different attractant properties to insects. Pests often use plant green leaf odor to locate hosts, while floral and fermented molasses odors are the main signals for non-pest insects to locate pollen, nectar, and other food sources. However, these are generalizations and not rules. For example, the floral scent substances attractive to Lepidoptera insects include phenylacetaldehyde, β-geranylene, and methyl salicylate. However, phenylacetaldehyde is also used by a variety of Lepidoptera pest species to locate suitable hosts [17]. Additionally, Feng et al. [18] found that floral odor, color, and nectar secretion would change independently throughout flowering in Lonicera japonica, suggesting that the combination of both visual and olfactory cues may play a role in attracting or filtering different visitors.
Arthropod pests evolve resistance to insecticides at varying rates, averaging only 60–78 generations [19]. Therefore, there is a need to find safer and more reliable methods to achieve sustainable pest control. Using volatile signals between plants and pests can be part of this more holistic and ecological approach to pest management. Yan et al. [20] found in field experiments that trans-2-hexen-1-ol and isopropyl isothiocyanate from cruciferous plants were highly attractive to Plutella xylostella adults, especially when used in conjunction with yellow and green sticky traps. Field trials confirmed the synergistic effect of phenylacetaldehyde and linalool on the trapping of Anticarsia gemmatalis [21]. In conclusion, although many monomers or simple formulations of plant volatile attractants have been reported [22][23], the ratio of pest population control, as well as the selectivity of attraction to target pests and beneficial insects, still needs to be improved. The following problems are highlighted: First, although several studies on field screening of pest-regulating PVOCs have been carried out, the scope of field trials is necessarily limited to the systematic and comprehensive screening of plant volatiles. Second, background levels of green leaf odorants can interfere with deployed green leaf odorants, thus limiting the effectiveness of trapping and plant masking. Third, the unknown mechanisms of pest attraction and the variable effects of PVOCs complicate screening. Clarifying the molecular mechanisms underlying the attraction of pests by plant volatiles will help improve the speed and reliability of PVOC screening.
3. Molecular Perception of PVOCs by Insects
3.1. The Role of Insect Antennal Olfactory Sensors in the Recognition of PVOCs
Insect antennae sensors have more than ten types based on the structural characteristics of the sensilla’s epidermis and its mode of attachment [24]. Different kinds of sensilla have their own roles, e.g., the sensilla squamiformia is a receptor that senses mechanical stimuli [25]; the sensilla trichodea is associated with plant volatile recognition [26]; the sensilla chaetica is associated with taste [27]; and most sensilla basiconca are a class of olfactory receptors which can function in capturing plant volatile molecules using a large number of pore structures on their surfaces which contain a large number of neuronal cells (Figure 1) [28].
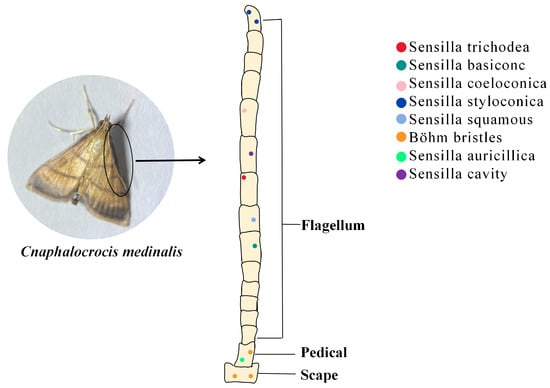
Figure 1. The role of insect antennal olfactory sensors in the recognition of PVOCs. Sensilla trichodea (red dot): mainly distributed in the center of the ventral surface of the antenna; Sensilla basiconc (dark green dot): distributed on the ventral and dorsal surface; Sensilla coeloconica (pink dot): distributed on the ventral surface; Sensilla styloconica (blue dot): distributed on the ventral surface and the end of the antennae; Sensilla squamous (light blue dot): distributed on the dorsal surface; Böhm bristles (orange dot): distributed on the scape and pedicel; Sensilla auricillica (green dot): distributed on the scape; Sensilla cavity (purple dot): distributed on all antenna surfaces.
Insects use special olfactory sensors to capture hydrophobic lipid-soluble small molecules in the air. Olfactory receptors can then differentiate between many complex odorant molecules and generate olfactory signals [29], which regulate behaviors such as feeding, courtship, and defense against predators [10][30]. Insect olfactory sensors, the main organs insects use to perceive the outside world, are overwhelmingly distributed in their antennae [31]. These olfactory sensors are in the form of sensilla, which exist in different forms according to their function. For example, the sensilla trichodea in the antennae of C. medinalis may play an important role in host localization, whereas the sensilla basiconca exists in a sexually dimorphic pattern and plays different roles in olfactory perception in male and female adults [32].
Insect olfactory receptors show similarities between phylogenetically related species. For example, the type and distribution of larval antennal receptors in two Zygoptera species, Ischnura elegans and Calopteryx haemorrhoidalis, are similar to those of other species in the order [33]. However, the number, type, and localization of sensilla vary significantly among species. Callosamia promethea have more than 60,000 sensilla trichodea on their antennae, while Choristoneura fumiferana have only 2300 [34]. Sensilla campaniform are present only in the antennae of male Hepialus yulongensis and not the females [35].
Currently, the dominant research method for constructing insect olfactory sensors is electron microscopy [36][37]. With the updating of technical means, the ultramicrostructure of the sensory organs on the antennae of insects continues to become clearer [38][39][40]. Using scanning and transmission electron microscopy techniques, Sun et al. [32] observed eight morphological types of the sensilla of C. medinalis: sensilla trichodea, sensilla basiconc, sensilla coeloconica, sensilla styloconica, sensilla squamous, sensilla auricillica, Böhm bristles, and sensilla cavity. Du et al. [41] combined scanning electron microscopy techniques with electroantennography to discover alarm pheromone-specific receptors on the antennae of Aphis glycines. However, structural analysis of olfactory sensors is important; ultimately, it is molecular interactions that govern odorant detection.
3.2. The Role of Insect Antennal Olfaction-Related Proteins in the Recognition of PVOCs
Odor molecules enter the receptor lymphatic fluid through micropores in the olfactory sensory epidermis. However, hydrophobic odorants cannot cross the hydrophilic lymphatic fluid to reach the odorant receptor neuron (ORN) and must be carried by a transport protein. These odorant-transporting proteins fall into two categories known as odorant-binding proteins (OBPs) or chemosensory proteins (CSPs) [10]. After being wrapped by a transport protein in the lymphatic fluid to form a complex, the odorant is transported to the ORN and activates the odorant receptors (ORs), ionotropic receptors (IRs), or sensory neuron membrane proteins (SNMPs) on the dendritic membrane. Subsequent excitation of the olfactory neurons is induced, converting chemical signals into electrical signals, which are transmitted as action potentials to higher nerve centers (i.e., antennal lobes and mushroom bodies). Finally, the higher nerve centers integrate the electrical signals and release nerve impulses that direct the insect to produce specific physiological and behavioral responses (Figure 2) [10][42][43].
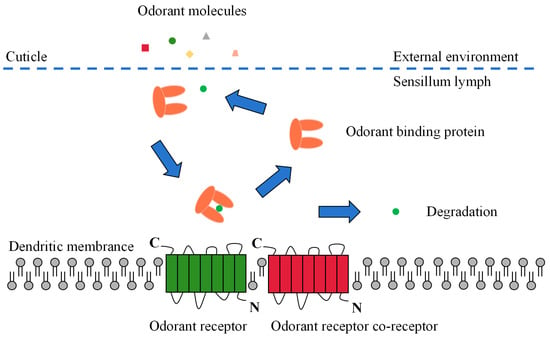
Figure 2. Schematic view of the odorant perception process in insects.
OBPs are a class of small molecule water-soluble proteins with molecular weights of about 15–17 kDa and a signal peptide of approximately 20 amino acids at the N-terminus, which are present in high concentrations in the lymphatic fluid of receptors [44]. Insect OBPs generally contain six conserved cysteines that form three disulfide bonds for supporting and maintaining the stability of the protein structure and have hydrophobic binding cavities formed by folding six α-helical structures. Due to their small molecular weight, high water solubility, good stability, ease of modification, and ease of obtaining in vitro, OBPs were one of the first classes of carrier proteins to be studied in insects. In addition, they are also an essential class of lymphatic olfactory proteins, and their functions are best known due to their ease of study [45][46]. Comprehensive studies have shown that insect OBPs perform several physiological functions in both olfactory and non-olfactory tissues, including (1) transporting plant volatiles to receptor proteins, (2) assisting in activating the receptor, (3) transporting and releasing insect pheromones, (4) protecting PVOCs from degradation by odorant-degrading enzymes during transport, (5) degrading high concentrations of PVOCs to avoid excessive stimulation of olfactory neurons, (6) removing extraneous substances from the sensory lymph fluid, (7) participating in insect physiological development and tissue regeneration, (8) acting as blood anticoagulants in blood-sucking insects, (9) playing a role in the development of drug resistance in insects, and (10) participating in insect feeding and nutrient uptake [44][47][48].
The genes for OBPs exercising olfactory functions are highly expressed in the antennae [49]. With the wide application of various bioinformatic techniques, the research on OBPs has developed rapidly. The number of odorant-binding proteins identified in insects continues to increase [10][50][51]. For example, more than 507 genes encoding OBP in Lepidoptera, 102 in Hymenoptera, 8 in Homoptera, 104 in Hemiptera, 299 in Diptera, 295 in Coleoptera, and 48 in Blattodea have yet to be identified [52]. At present, some progress has been made in understanding the binding mechanism of OBPs through crystal structure analysis [53][54][55]. It has been found that the C-terminus fragments of some OBPs can control ligand binding and release using pH-dependent conformational changes [56][57]. Additionally, further investigation of the binding mechanisms between OBPs and their ligands was conducted using homology modeling combined with molecular docking technology to create 3D protein models [58][59]. Based on this technique, several key binding sites in the binding cavity of OBPs were found. For example, in the OBP McinOBP4 of Macrocentrus cingulum, the Met119 site forms a hydrophobic bond with the functional group of limonene, trans-3-hexen-1-ol-acetate, which can then be transported in the lymph [60]. In the OBP HarmPBP1 of Helicoverpa armigera, the sites Phe12, Trp37, and Phe119 may be involved in the binding to the main components of the insect’s sex pheromones, cis-11-hexadecenal and cis-9-hexadecenal [61].
Another important class of olfactory protein is the chemosensory proteins (CSPs), which are small, compact, soluble polypeptides. CSPs typically consist of 100 to 120 amino acids with molecular weights of about 10 to 15 kDa. These proteins bind and transport hydrophobic information chemicals. CSPs across different species of insects contain four conserved cysteines, forming two disulfide bonds that maintain the stability of the three-dimensional protein structure. This represents a decrease in conserved cysteines compared to OBPs [10].
Olfactory receptors (ORs), critical to insect olfaction, are generally composed of 300–350 amino acid residues and are a class of membrane proteins located on ORNs. ORs can be divided into two main groups: (1) the common odorant-binding protein (ORx), which is highly differentiated across species of insects, and (2) the odorant co-receptor (ORco), which is highly conserved across different insect species. Although ORcos do not directly bind odor molecules, ORcos assist ORxs in binding specificity and recognizing the correct odorants [62]. ORcos represent one of several classes of olfactory proteins critical to the functioning of insect olfactory systems but do not bind directly to odorant molecules, such as SNMPs and IRs. SNMPs are insect-specific membrane proteins comprising approximately 520 amino acids and are highly expressed in antennae. SNMPs are thought to contribute to insect odor recognition and are divided into three subfamilies (SNMP 1–3) [63]. IRs contain two or more co-receptors, where each co-receptor is co-expressed with one or two other IRs. These IRs have complex interactions and work together to sense acids, amino acids, and other compounds. However, studies on the functions and mechanisms of IRs have primarily been conducted on Drosophila, and data from other insects, such as Lepidoptera, are rare [64]. Insect olfactory sensitivity is closely related to the expression of several types of olfactory proteins. These proteins determine the recognition of different odors through specific binding sites and co-factors that regulate the sensitivity of the olfactory system to specific odorant molecules. Through new bioinformatics technologies, the research on olfactory proteins has been developing rapidly [65]. This in-depth knowledge of the mechanisms of the insect olfactory system plays a crucial role in exploiting insect attraction with plant volatiles for agronomic purposes.
References
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24.
- Plettner, E. Insect pheromone olfaction: New targets for the design of species-selective pest control agents. Curr. Med. Chem. 2002, 9, 1075–1085.
- Zu, P.; Zhang, D.Y.; Luo, Y.B. Chemical communication between plants and insects. J. Syst. Evol. 2023, 61, 441–444.
- Fang, J.X.; Chen, D.F.; Shi, X.; Zhang, S.F.; Liu, F.; Shen, W.X.; Jia, C.Y.; Ma, S.C.; Zhang, Z.; Kong, X.B. Solid-phase microextraction and cuticular hydrocarbon differences related to reproductive activity in juniper bark borer Semanotus bifasciatus Motschulsky. J. Syst. Evol. 2023, 61, 498–505.
- Rizvi, S.A.H.; George, J.; Reddy, G.V.P.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484.
- Wyckhuys, K.A.G.; Lu, Y.; Zhou, W.; Cock, M.J.W.; Naranjo, S.E.; Fereti, A.; Williams, F.E.; Furlong, M.J. Ecological pest control fortifies agricultural growth in Asia–Pacific economies. Nat. Ecol. Evol. 2020, 4, 1522–1530.
- Zhao, J.; Cai, W.L.; Shen, Y.Y.; Zhu, H.Y.; Pu, L.; Xie, M.Q.; Zou, Y.L.; Hua, H.X. Current situation and prospect of green rice pest control technology. J. Huazhong Agric. Univ. 2022, 41, 92–104. (In Chinese)
- Beck, J.J.; Torto, B.; Vannette, R.L. Eavesdropping on plant-insect-microbe chemical communications in agricultural ecology: A virtual issue on semiochemicals. J. Agric. Food Chem. 2017, 65, 5101–5103.
- Liu, H.; Sun, X.; Shi, Z.; An, X.; Khashaveh, A.; Li, Y.; Gu, S.; Zhang, Y. Identification and functional analysis of odorant-binding proteins provide new control strategies for Apolygus lucorum. Int. J. Biol. Macromol. 2023, 224, 1129–1141.
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.; Dani, F.R. Beyond chemoreception: Diverse tasks of soluble olfactory proteins in insects. Biol. Rev. Camb. Philos. Soc. 2018, 93, 184–200.
- Zeng, L.T.; Zhou, X.C.; Fu, X.M.; Hu, Y.L.; Gu, D.C.; Hou, X.L.; Dong, F.; Yang, Z.Y. Effect of the biosynthesis of the volatile compound phenylacetaldehyde on chloroplast modifications in tea (Camellia sinensis) plants. Hortic. Res. 2023, 10, uhad003.
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32.
- Laothawornkitkul, J.; Taylor, J.E.; Paul, N.D.; Hewitt, C.N. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009, 183, 27–51.
- Schiestl, F.; Ayasse, M. Post-pollination emission of a repellent compound in a sexually deceptive orchid: A new mechanism for maximising reproductive success? Oecologia 2001, 126, 531–534.
- Majetic, C.J.; Raguso, R.A.; Ashman, T.L. Sources of floral scent variation: Can environment define floral scent phenotype? Plant Signal. Behav. 2009, 4, 129–131.
- Martel, C.; Rakosy, D.; Romero, P.E.; Jersáková, J.; Ayasse, M. The evolution of tachinid pollination in Neotinea ustulata is related to floral cuticular composition and the combined high relative production of (Z)-11-C23/C25enes. J. Syst. Evol. 2021, 61, 487–497.
- Guo, M.; Du, L.; Chen, Q.; Feng, Y.; Zhang, J.; Zhang, X.; Tian, K.; Cao, S.; Huang, T.; Jacquin-Joly, E.; et al. Odorant receptors for detecting flowering plant cues are functionally conserved across moths and butterflies. Mol. Biol. Evol. 2021, 38, 1413–1427.
- Feng, H.H.; Wang, X.Y.; Luo, Y.B.; Huang, S.Q. Floral scent emission is the highest at the second night of anthesis in Lonicera japonica (Caprifoliaceae). J. Syst. Evol. 2023, 61, 530–537.
- Brevik, K.; Schoville, S.D.; Mota-Sanchez, D.; Chen, Y.H. Pesticide durability and the evolution of resistance: A novel application of survival analysis. Pest Manag. Sci. 2018, 74, 1953–1963.
- Yan, X.Z.; Ma, L.; Li, X.F.; Chang, L.; Liu, Q.Z.; Song, C.F.; Zhao, J.Y.; Qie, X.T.; Deng, C.P.; Wang, C.Z.; et al. Identification and evaluation of cruciferous plant volatiles attractive to Plutella xylostella L. (Lepidoptera: Plutellidae). Pest Manag. Sci. 2023, 79, 5270–5282, Accepted Author Manuscript.
- Meagher, R.L.; Landolt, P.J. Binary floral lure attractive to velvetbean caterpillar adults (Lepidoptera: Noctuidae). Fla. Entomol. 2010, 93, 73–79.
- Desurmont, G.A.; Arx, M.V.; Turlings, T.C.J.; Schiestl, F.P. Floral odors can interfere with the foraging behavior of parasitoids searching for hosts. Front. Ecol. Evol. 2020, 8, 148.
- Wu, S.; Liu, F.; Zeng, W.; Xiao, Z.; Li, J.; Teng, K.; Guo, Q.; Zhao, J.; Du, Y. Evaluation of floral-derived volatile blend for attracting aphid parasitoids and lady beetles in the tobacco fields. Biol. Control 2022, 172, 104979.
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122.
- Mclver, S.B. Structure of cuticular mechanoreceptors of arthropods. Annu. Rev. Entomol. 1975, 20, 381–397.
- Roh, H.S.; Park, K.C.; Oh, H.W.; Park, C.G. Morphology and distribution of antennal sensilla of two tortricid moths, Cydia pomonella and C. succedana (Lepidoptera). Microsc. Res. Tech. 2016, 79, 1069–1081.
- Zacharuk, R.Y. Antennae and sensilla. Compr. Insect Physiol. Biochem. Pharmacol. 1985, 17, 1283–1284.
- Pophof, B.; Stange, G.; Abrell, L. Volatile organic compounds as signals in a plant-herbivore system: Electrophysiological responses in olfactory sensilla of the moth Cactoblastis cactorum. Chem. Senses 2005, 30, 51–68.
- Khallaf, A.; Knaden, M. Evolutionary neuroecology of olfactory-mediated sexual communication and host specialization in Drosophila—A review. Entomol. Exp. Appl. 2022, 170, 289–302.
- Brilli, F.; Loreto, F.; Baccelli, I. Exploiting plant volatile organic compounds (VOCs) in agriculture to improve sustainable defense strategies and productivity of crops. Front. Plant Sci. 2019, 10, 264.
- Kwon, H.W.; Lu, T.; Rützler, M.; Zwiebel, L.J. Olfactory responses in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2006, 103, 13526–13531.
- Sun, X.; Wang, M.Q.; Zhang, G.A. Ultrastructural observations on antennal sensilla of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Microsc. Res. Tech. 2011, 74, 113–121.
- Piersanti, S.; Rebora, M. The antennae of damselfly larvae. Arthropod Struct. Dev. 2018, 47, 36–44.
- Ma, R.Y.; Du, J.W. Antennal sensilla of insect. Chin. J. Appl. Entomol. 2000, 37, 179–183. (In Chinese)
- Li, C.D.; Yang, D.R.; Shen, F.R.; Yang, Y.X. Scanning electron microscopic observation on antennal sensilla of Hepialus yulongcnsis. Zool. Res. 1994, 11, 83–86. (In Chinese)
- Liébanas, G.; Sáez, Á.; Luna, Á.; Romero-Vidal, P.; Palma, A.; Pérez, J.M. The morphology of Colpocephalum pectinatum (Phthiraptera: Amblycera: Menoponidae) under scanning electron microscopy. Arthropod Struct. Dev. 2021, 64, 101085.
- Dong, W.Y.; Wang, B.; Wang, G.R. Morphological and ultrastructural characterization of antennal sensilla and the detection of floral scent volatiles in Eupeodes corollae (Diptera: Syrphidae). Front. Neuroanat. 2021, 15, 791900.
- Chen, Q.; Liu, X.; Cao, S.; Ma, B.; Guo, M.; Shen, J.; Wang, G. Fine structure and olfactory reception of the labial palps of Spodoptera frugiperda. Front. Physiol. 2021, 12, 680697.
- Liu, Y.Q.; Li, J.; Ban, L.P. Morphology and distribution of antennal sensilla in three species of Thripidae (Thysanoptera) infesting alfalfa Medicago sativa. Insects 2021, 12, 81.
- Shi, X.; Zhang, S.F.; Liu, F.; Xu, F.Y.; Zhang, F.B.; Guo, X.B.; Zhang, Z.; Kong, X.B. SEM analysis of sensilla on the mouthparts and antennae of Asian larch bark beetle Ips subelongatus. Micron 2021, 140, 102976.
- Du, Y.J.; Yan, F.S.; Tang, J. Structure and function of olfactory receptors in Aphis glycines antennae. Acta Entomol. Sin. 1995, 38, 1–7. (In Chinese)
- Brito, N.F.; Moreira, M.F.; Melo, A.C. A look inside odorant-binding proteins in insect chemoreception. J. Insect Physiol. 2016, 95, 51–65.
- Krieger, J.; Breer, H. Olfactory reception in invertebrates. Science 1999, 286, 720–723.
- Fan, J.; Francis, F.; Liu, Y.; Chen, J.L.; Cheng, D.F. An overview of odorant-binding protein functions in insect peripheral olfactory reception. Genet. Mol. Res. 2011, 10, 3056–3069.
- Breer, H.; Krieger, J.; Raming, K. A novel class of binding proteins in the antennae of the silk moth Antheraea pernyi. Insect Biochem. 1990, 20, 735–740.
- Rihani, K.; Ferveur, J.F.; Briand, L. The 40-year mystery of insect odorant-binding proteins. Biomolecules 2021, 11, 509.
- Zhang, Y.; Yang, B.; Wang, G.R. Research progress of soluble proteins on chemosensationin insects. J. Environ. Entomol. 2019, 41, 229–240. (In Chinese)
- Wu, F.; Zhang, L.; Qiu, Y.L.; Li, H.L. Research progress of olfactory binding proteins in insects. Acta Entomol. Sin. 2021, 64, 523–535. (In Chinese)
- Sun, Y.L.; Dong, J.F.; Song, Y.Q.; Wang, S.L. GOBP1 from the variegated cutworm Peridroma saucia (Hübner) (Lepidoptera: Noctuidae) displays high binding affinities to the behavioral attractant (Z)-3-Hexenyl acetate. Insects 2021, 12, 939.
- Zhou, J.J. Odorant-binding proteins in insects. Vitam. Horm. 2010, 83, 241–272.
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391.
- Venthur, H.; Zhou, J.J. Odorant receptors and odorant-binding proteins as insect pest control targets: A comparative analysis. Front. Physiol. 2018, 9, 1163.
- Li, T.T.; Liu, W.C.; Zhu, J.; Yang, Y.H.; Ma, C.; Lu, C.; Zhang, K.X. Crystal structure and ligand identification of odorant binding protein 4 in the natural predator Chrysopa pallens. Int. J. Biol. Macromol. 2019, 141, 1004–1012.
- Northey, T.; Venthur, H.; De, B.F.; Chauviac, F.X.; Cole, A.; Ribeiro, K.A.J.; Grossi, G.; Falabella, P.; Field, L.M.; Keep, N.H.; et al. Crystal structures and binding dynamics of odorant-binging protein 3 form two aphid species Megoura viciae and Nasonovia ribisniari. Sci. Rep. 2016, 6, 24739.
- Gonzalez, D.; Rihani, K.; Neiers, F.; Poirier, N.; Fraichard, S.; Gotthard, G.; Chertemps, T.; Maïbèche, M.; Ferveur, J.F.; Briand, L. The Drosophila odorant-binding protein 28a is involved in the detection of the floral odour ß-ionone. Cell. Mol. Life Sci. 2020, 77, 2565–2577.
- Sandler, B.H.; Nikonova, L.; Leal, W.S.; Clardy, J. Sexual attraction in the silkworm moth: Structure of the pheromone-binding-protein-bombykol complex. Chem. Biol. 2000, 7, 143–151.
- Horst, R.; Damberger, F.; Luginbühl, P.; Güntert, P.; Peng, G.; Nikonova, L.; Leal, W.S.; Wüthrich, K. NMR structure reveals intramolecular regulation mechanism for pheromone binding and release. Proc. Natl. Acad. Sci. USA 2001, 98, 14374–14379.
- Li, G.; Chen, X.; Li, B.; Zhang, G.; Li, Y.; Wu, J. Binding properties of general odorant binding proteins from the oriental fruit moth, Grapholita molesta (Busck) (Lepidoptera: Tortricidae). PLoS ONE 2016, 11, e0155096.
- Nagnan-Le, M.P.; Huet, J.C.; Maibeche, M.; Pernollet, J.C.; Descoins, C. Purification and characterization of multiple forms of odorant/pheromone binding proteins in the antennae of Mamestra brassicae (Noctuidae). Insect Biochem. Mol. Biol. 1996, 26, 59–67.
- Ahmed, T.; Zhang, T.T.; Wang, Z.Y.; He, K.L.; Bai, S.X. C-terminus methionene specifically involved in binding corn odorants to odorant binding protein 4 in Macrocentrus cingulum. Front. Physiol. 2017, 8, 62.
- Dong, K.; Duan, H.X.; Liu, J.T.; Sun, L.; Gu, S.H.; Yang, R.N.; Dhiloo, K.H.; Gao, X.W.; Zhang, Y.J.; Guo, Y.Y. Key site residues of pheromone-binding protein 1 involved in interacting with sex pheromone components of Helicoverpa armigera. Sci. Rep. 2017, 7, 16859.
- del Mármol, J.; Yedlin, M.A.; Ruta, V. The structural basis of odorant recognition in insect olfactory receptors. Nature 2021, 597, 126–131.
- Liu, S.; Wang, W.L.; Zhang, Y.X.; Zhang, B.X.; Rao, X.J.; Liu, X.M.; Wang, D.M.; Li, S.G. Transcriptome sequencing reveals abundant olfactory genes in the antennae of the rice leaffolder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Entomol. Sci. 2017, 20, 177–188.
- Zhang, Y.; Yang, B.; Yu, J.; Pang, B.P.; Wang, G.R. Expression profiles and functional prediction of ionotropic receptors in Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae). J. Integr. Agric. 2022, 21, 474–485.
- Jiang, X.; Jiang, J.; Yu, M.; Zhang, S.; Qin, Y.; Xu, Y.; Francis, F.; Fan, J.; Chen, J. Functional analysis of odorant-binding proteins for the parasitic host location to implicate convergent evolution between the grain aphid and its parasitoid Aphidius gifuensis. Int. J. Biol. Macromol. 2023, 226, 510–524.
More
Information
Subjects:
Entomology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
568
Revisions:
2 times
(View History)
Update Date:
17 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




