Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Arianna Dal Buono | -- | 1692 | 2024-01-16 09:20:44 | | | |
| 2 | Camila Xu | Meta information modification | 1692 | 2024-01-16 09:30:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Migliorisi, G.; Vella, G.; Dal Buono, A.; Gabbiadini, R.; Busacca, A.; Loy, L.; Bezzio, C.; Vinciguerra, P.; Armuzzi, A. Pathophysiology of Extra-Intestinal Manifestations in Inflammatory Bowel Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/53870 (accessed on 03 March 2026).
Migliorisi G, Vella G, Dal Buono A, Gabbiadini R, Busacca A, Loy L, et al. Pathophysiology of Extra-Intestinal Manifestations in Inflammatory Bowel Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/53870. Accessed March 03, 2026.
Migliorisi, Giulia, Giovanna Vella, Arianna Dal Buono, Roberto Gabbiadini, Anita Busacca, Laura Loy, Cristina Bezzio, Paolo Vinciguerra, Alessandro Armuzzi. "Pathophysiology of Extra-Intestinal Manifestations in Inflammatory Bowel Diseases" Encyclopedia, https://encyclopedia.pub/entry/53870 (accessed March 03, 2026).
Migliorisi, G., Vella, G., Dal Buono, A., Gabbiadini, R., Busacca, A., Loy, L., Bezzio, C., Vinciguerra, P., & Armuzzi, A. (2024, January 16). Pathophysiology of Extra-Intestinal Manifestations in Inflammatory Bowel Diseases. In Encyclopedia. https://encyclopedia.pub/entry/53870
Migliorisi, Giulia, et al. "Pathophysiology of Extra-Intestinal Manifestations in Inflammatory Bowel Diseases." Encyclopedia. Web. 16 January, 2024.
Copy Citation
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are complex multifactorial chronic inflammatory disorders affecting nearly 7 million patients worldwide, with an increasing prevalence in Northern Europe and emerging countries. IBD can be associated with a wide spectrum of extra-intestinal manifestations (EIMs) with a significant impact on patients’ quality of life. The pathophysiology of EIMs in IBD is intricate and uncertain. The European Crohn’s and Colitis Organization (ECCO) proposed an operational definition of EIMs’ pathology in IBD patients.
uveitis
episcleritis
scleritis
inflammatory bowel disease
1. Introduction
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are complex multifactorial chronic inflammatory disorders affecting nearly 7 million patients worldwide, with an increasing prevalence in Northern Europe and emerging countries [1]. IBD can be associated with a wide spectrum of extra-intestinal manifestations (EIMs) with a significant impact on patients’ quality of life [2]. The current definition of EIMs encompasses inflammatory processes associated with IBD’s activity and flares, whether dependent or independent [3][4][5]. The prevalence of EIMs varies from 6% to 47% [5]: a recent systematic review and meta-analysis that examined 52 studies revealed that almost one quarter of IBD patients experienced at least one musculoskeletal, ocular, or skin EIM [4]. Additionally, these manifestations can present prior to IBD diagnosis in up to 25% of the patients [6]. EIMs seem to be more common in CD than UC patients [6][7], and the presence of a singular EIM predisposes to develop a new immune-mediated manifestation in another district [8]. The most commonly affected systems are the joints (i.e., arthritis and enthesitis), the skin (i.e., pyoderma gangrenosum, erythema nodosum, aphthous stomatitis), and the ocular and hepatobiliary districts (such as primary sclerosing cholangitis). EIMs’ underlying mechanisms and pathogenesis are poorly understood; they can either be reliant on or independent of intrinsic intestinal inflammation activity and share common activated immune molecular pathways [9]. An extension of gut inflammation may provide a plausible explanation; for example, gut chemokines, involved in T cell trafficking and migration into the bowel, can be expressed in biliary ducts and are associated with primary sclerosing cholangitis (PSC) in IBD patients [10]. Additionally, an uncontrolled auto-reactive T cell population, namely T-helper (Th1) by producing interferon-gamma (INF-Y) and interleukin 12 (IL-12) and T-helper 17 (Th17) with the release of interleukin 12 (IL-12) and interleukin 17 (IL-17), plays a crucial role in the pathogenesis of EIMs. Thus, patients affected by both IBD and non-infectious uveitis presented high levels of these cytokines [11]. Furthermore, IBD patients may have an intrinsic propensity towards developing independent autoimmune disorders, such as a genetic predisposition, which potentially explains the occurrence of EIMs during IBD remission. Indeed, certain haplotypes, such as HLA-DRB1*0103 or HLA B*58, are reported to be involved in musculoskeletal and eye manifestations [5]. Finally, dysbiosis and the selection of specific gut commensals, with the loss of diversity, have also been linked with the onset of various EIMs [12].
The prevalence of ocular extra-intestinal manifestations (OEIMs) in patients with IBD ranges from 0.3% to 13% [13]. These O-EIMs are mostly represented by episcleritis, scleritis, anterior uveitis, and iridocyclitis. Eye redness and pain or blurred vision are the most common symptoms. Episcleritis is the most frequent manifestation (2–5%), being mostly benign and strongly related to intestinal inflammatory flares, while uveitis (0.5–3.5%) is a more severe condition, potentially leading to vision loss, and independent of IBD activity [14]. O-EIMs are more common in children [15] and women and are associated with arthritis and pyoderma gangrenosum in CD and UC patients, respectively [16]. Other conditions, such as vascular involvement, optic neuritis, papillitis, or myositis are rarer.
2. Pathogenesis
The pathophysiology of EIMs in IBD is intricate and uncertain. The European Crohn’s and Colitis Organization (ECCO) proposed an operational definition of EIMs’ pathology in IBD patients. These manifestations can either result from the translocation of intestinal inflammation or be completely independent of IBD activity, arising from a genetic predisposition to autoimmunity disorders or environmental factors [15]. IBD and EIMs are thought to be the result of the interplay of environmental factors, genetic predisposition, changes to the intestinal microbiota, and immune system dysregulation, which all ultimately cause damage to the mucosa [16][17].
The ultimate disarray of intestinal cells’ tight junctions and the change in the composition of the epithelial mucus film allow luminal antigens to enter bowel epithelial cells. Additionally, molecular pattern-recognition receptors (e.g., Toll-like receptors TLRs) interacting with commensal microbiota can induce the activation of dendritic cells and macrophages. Consequently, the activation of multiple signaling pathways and upregulation of proinflammatory gene transcription resulted in the increased production of proinflammatory cytokines and recruitment of leukocytes, which perpetuates inflammation [18][19]. Another potential pathogenetic mechanism is represented by the formation of circulating antigen–antibody complexes or the production of autoantibodies, directed against common shared antigens by the colon and other tissues, including the eye [20]. In particular, it has been suggested that system-wide inflammation could be caused by the local impact of antigen–antibody complexes generated against bowel walls’ blood vessels and conveyed through the bloodstream [14]. The resulting damaged intestinal barrier allows antigens and microorganisms to penetrate, leading to a reactive lymphoid tissue response. In their initial Mendelian randomization study [21], Meng et al. illustrated that specific cytokines produced in the bowel (namely IL-6, IL-10, and IL-17) may participate in the origin of iridociclytis (IC) and EIMs by circulating to the eye and triggering ocular inflammation (Figure 1), implying that there may be a shared immunopathogenesis among the gut and eye [22]. Moreover, as previously said, shared antigens in the gut and eye may contribute to inflammation in both locations for IBD patients. For example, peptide 7E12H12 is present in both colon epithelial and non-pigmented ciliary epithelial cells and could represent an inflammation target [23]. Additionally, several studies suggest that a human epithelial colonic autoantigen, shared by skin, bile ducts, eyes, and joint cells, may trigger an antibody-mediated immune response in ulcerative colitis (UC) patients [21][22][23][24]. A higher intestinal damage and permeability caused by transmural inflammation in CD, may be a potential explanation for the greater prevalence of o-EIMs in CD patients compared to UC ones. However, there is no strong evidence in the literature confirming this hypothesis, and further studies are warranted.
The pathogenesis of EIMs questions genetic predisposition. Individuals with IBD who have specific haplotypes, mainly HLA-B27 and HLA-DRB1*0103, were reported to be at higher risk of developing an extensive gut disease and the emergence of EIMs, particularly ocular and articular ones [24][25]. In a significant retrospective study, Lin et al. [26] proposed that a family history of IBD could potentially lead to an independent and amplified likelihood of developing ocular inflammation, even when there are no known instances of bowel disease or genetic susceptibility (HLA-B27). However, Lanna et al. [27] analyzed 96 Brazilian IBD patients and did not establish any association between particular HLA and ocular or joint manifestations. This finding suggests that genetic heterogeneity across diverse global populations could explain the different outcomes. The development of IBD is assumed to be attributable to around 150 unique genetic mutations [13][28][29]. The majority of these genes are involved in the primary immune response, specifically related to the autophagic function of leukocytes and the production of pro-inflammatory cytokines and chemokines (e.g., NOD2/CARD15, ATG16 L1 or IL23R). Additionally, genes involved in transmembrane signaling systems of intestinal epithelial cells can be involved (HNF4A, GNA12) [30][31]. Certain rare monogenetic alterations may contribute to the early onset of IBD [30]. Specific alterations of the Nod2 gene on chromosome 16, can induce an abnormal activity of the innate immunity in response to gut microbiota, leading to a higher risk of developing CD [32]. Furthermore, the mutation of NODd2/CARD15 is associated with a systemic autoinflammatory granulomatous disease, Blau syndrome, characterized by uveitis, arthritis, and dermatitis [33]. However, Nod2 is just a minor component of the complex genetics involved in IBD and EIMs [34].
Environmental factors may represent additional pathogenic factors. Vitamin D deficiency could have a shared role in the development of IBD and ocular inflammation. Several studies have shown that vitamin D has an anti-inflammatory effect, achieved through the suppression of B and T cells’ proliferation and differentiation [35]. An inverse correlation has been established between vitamin D levels and the risk of developing IBD and iridocyclitis (IC), indicating that higher vitamin D levels play a protective role [21][36]. Low vitamin D levels, resulting from inadequate sunlight exposure, are risk factors for all types of uveitis (including IC), UC, and CD [37]. These results suggest that vitamin D supplementation should be considered as an option for the prevention of uveitis relapse in high-risk patients.
Impaired autophagy in macrophages may considered another potential pathogenetic mechanism. Therefore, autophagy seems to play a vital role in maintaining ocular immune homeostasis, as the deletion of autophagy genes has been associated with worsening ocular inflammation severity due to inflammasome-mediated IL1B secretion. In their study using a mouse model, Santeford et al. found that a particular polymorphism (Thr300Ala or T300A) of the autophagy gene ATG16L1 was linked to an increased risk of developing both CD and uveitis, suggesting a potential connection between IBD and o-EIMs [38].
Additionally, gut microbiota and dysbiosis may contribute to the pathogenesis of o-EIMs through molecular mimicry, although current data are inconclusive. Therefore, gut commensals may have a dual role. On one hand, they can participate in autoimmune ocular processes. For instance, a 2015 study using mice models found that gut bacteria antigens directly activate auto-reactive T cells that are involved in autoimmune uveitis [9][39]. Conversely, particular species such as Lactobacillus reuterii can provide a protective function by strengthening gut intraepithelial lymphocytes (IELs) that regulate autoreactive T cells [40]. Moreover, the elimination of gut commensals was correlated with an attenuation of the severity of ocular inflammation [39].
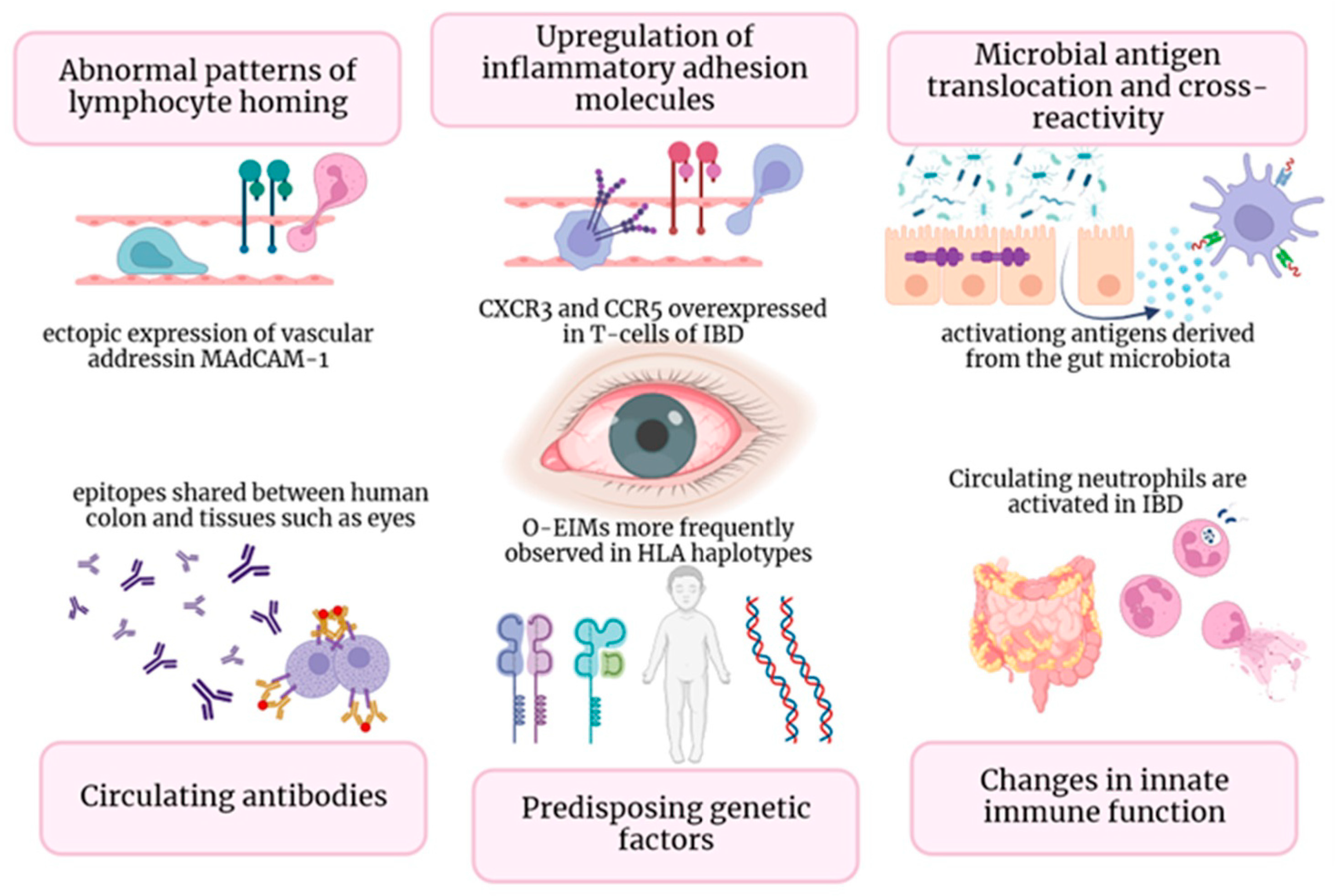
Figure 1. In Figure 1, diverse immune mechanisms that underlie O-EIMs are elucidated: ectopic ocular expression of gut-specific chemokines and adhesion molecules (gut-specific chemokines and adhesion molecules (i.e., MAdCAM-1, CCL25, CCR9); T cell trafficking driven by non-specific adhesion molecules (i.e., VAP-1, CXCR3, and CCR5); common peptide sequence shared by enteric bacteria and host molecules (in a mice-model study, retina-specific T cells involved in uveitis pathogenesis are activated in the gut by a microbiota-dependent activation [39]); circulating antibodies-antigens complexes or autoantibodies directed against shared epitopes could expand inflammation outside the gut (i.e., eye); genetic predisposition (specific HLA complexes have been associated with specific EIM); activated neutrophils and macrophages leads to uncontrolled innate immune response in non-intestinal districts.
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.; Chan, F.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778.
- Trikudanathan, G.; Venkatesh, P.G.K.; Navaneethan, U. Diagnosis and Therapeutic Management of Extra-Intestinal Manifestations of Inflammatory Bowel Disease. Drugs 2012, 72, 2333–2349.
- Greuter, T.; Vavricka, S.R. Extraintestinal manifestations in inflammatory bowel disease–epidemiology, genetics, and pathogenesis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 307–317.
- Kilic, Y.; Kamal, S.; Jaffar, F.; Sriranganathan, D.; Quraishi, M.N.; Segal, J.P. Prevalence of Extraintestinal Manifestations in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2023, 20, izad061.
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992.
- Vavricka, S.R.; Rogler, G.; Gantenbein, C.; Spoerri, M.; Vavricka, M.P.; Navarini, A.A.; French, L.; Safroneeva, E.; Fournier, N.; Straumann, A.; et al. Chronological order of appearance of extraintestinal manifestations relative to the time of IBD diagnosis in the Swiss Inflammatory Bowel Disease Cohort. Inflamm. Bowel Dis. 2015, 21, 1794–1800.
- Park, S.K.; Wong, Z.; Park, S.H.; Van Vu, K.; Bang, K.B.; Piyachaturawat, P.; Myint, T.; Hilmi, I.; Park, D.I. Extraintestinal manifestation of inflammatory bowel disease in Asian patients: A multinational study. Dig. Liver Dis. 2021, 53, 196–201.
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132.
- Hedin, C.R.H.; Vavricka, S.R.; Stagg, A.J.; Schoepfer, A.; Raine, T.; Puig, L.; Pleyer, U.; Navarini, A.; Van Der Meulen-De Jong, A.E.; Maul, J.; et al. The Pathogenesis of Extraintestinal Manifestations: Implications for IBD Research, Diagnosis, and Therapy. J. Crohns Colitis 2019, 13, 541–554.
- Eksteen, B.; Grant, A.J.; Miles, A.; Curbishley, S.M.; Lalor, P.F.; Hübscher, S.G.; Briskin, M.; Salmon, M.; Adams, D.H. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J. Exp. Med. 2004, 200, 1511–1517.
- Horai, R.; Caspi, R.R. Cytokines in autoimmune uveitis. J. Interferon Cytokine Res. 2011, 31, 733–744.
- Sabino, J.; Vieira-Silva, S.; Machiels, K.; Joossens, M.; Falony, G.; Ballet, V.; Ferrante, M.; Van Assche, G.; Van Der Merwe, S.; Vermeire, S.; et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016, 65, 1681–1689.
- Pytrus, W.; Akutko, K.; Pytrus, T.; Turno-Kręcicka, A. A Review of Ophthalmic Complications in Inflammatory Bowel Diseases. J. Clin. Med. 2022, 11, 7457.
- Troncoso, L.L.; Biancardi, A.L.; Vieira de Moraes, H.J.; Zaltman, C. Ophthalmic manifestations in patients with inflammatory bowel disease: A review. World J. Gastroenterol. 2017, 23, 5836–5848.
- Mintz, R.; Feller, E.R.; Bahr, R.L.; Shah, S.A. Ocular Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2004, 10, 135–139.
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23.
- Li, J.X.; Chiang, C.C.; Chen, S.N.; Lin, J.M.; Tsai, Y.Y. The Prevalence of Ocular Extra-Intestinal Manifestations in Adults Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 15683.
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755.
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619.
- Das, K.M. Relationship of Extraintestinal Involvements in Inflammatory Bowel Disease New Insights into Autoimmune Pathogenesis. Dig. Dis. Sci. 1999, 44, 1–13.
- Meng, Y.; Tan, Z.; Liu, C.; Dong, W.; Chen, C. Association between Inflammatory Bowel Disease and Iridocyclitis: A Mendelian Randomization Study. J. Clin. Med. 2023, 12, 1282.
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27.
- Bhagat, S.; Das, K.M. A Shared and Unique Peptide in the Human Colon, Eye, and Joint Detected by a Monoclonal Antibody. Gastroenterology 1994, 107, 103–108.
- Vavricka, S.R.; Brun, L.; Ballabeni, P.; Pittet, V.; Prinz Vavricka, B.M.; Zeitz, J.; Rogler, G.; Schoepfer, A.M. Frequency and Risk Factors for Extraintestinal Manifestations in the Swiss Inflammatory Bowel Disease Cohort. Am. J. Gastroenterol. 2011, 106, 110–119.
- Taylor, S.R.; McCluskey, P.; Lightman, S. The ocular manifestations of inflammatory bowel disease. Curr. Opin. Ophthalmol. 2006, 17, 538–544.
- Lin, P.; Tessler, H.H.; Goldsterin, D.A. Family history of inflammatory bowel disease in patients with idiopathic ocular inflammation. Am. J. Ophthalmol. 2006, 141, 1097–1104.
- Lanna, C.C.D.; Ferrari, M.D.L.A.; Rocha, S.L.; Nascimento, E.; Carvalho, M.A.P.; Cunha, A.S. A cross-sectional study of 130 Brazilian patients with Crohn’s disease and ulcerative colitis: Analysis of articular and ophthalmologic manifestations. Clin. Rheumatol. 2008, 27, 503–509.
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584.
- Younis, N.; Zarif, R.; Mahfouz, R. Inflammatory bowel disease: Between genetics and microbiota. Mol. Biol. Rep. 2020, 47, 3053–3063.
- Cheema, H.A.; Waheed, N.; Saeed, A.; Fayyaz, Z.; Anjum, M.N.; Alvi, M.A.; Batool, S. Very early onset inflammatory bowel disease: Spectrum of clinical presentation, diagnostic tools and outcome in children. J. Pak. Med. Assoc. 2021, 71, 2350–2354.
- Naser, S.A.; Arce, M.; Khaja, A.; Fernandez, M.; Naser, N.; Elwasila, S.; Thanigachalam, S. Role of ATG16L, NOD2 and IL23R in Crohn’s disease pathogenesis. World J. Gastroenterol. 2012, 18, 412–424.
- Cho, J.H. Basic Science on the Cutting Edge the Nod2 Gene in Crohn’s Disease: Implications for Future Research into the Genetics and Immunology of Crohn’s Disease. Inflamm. Bowel Dis. 2001, 7, 271–275.
- Matsuda, T.; Kambe, N.; Takimoto-Ito, R.; Ueki, Y.; Nakamizo, S.; Saito, M.K.; Takei, S.; Kanazawa, N. Potential Benefits of TNF Targeting Therapy in Blau Syndrome, a NOD2-Associated Systemic Autoinflammatory Granulomatosis. Front. Immunol. 2022, 13, 895765.
- Duerr, R.H.M. The genetics of inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2002, 31, 63–76.
- Wu, Z.; Liu, D.; Deng, F. The Role of Vitamin D in Immune System and Inflammatory Bowel Disease. J. Inflamm. Res. 2022, 15, 3167–3185.
- Orchard, T.R.; Chua, C.N.; Ahmad, T.; Cheng, H.; Welsh, K.I.; Jewell, D.P. Uveitis and erythema nodosum in inflammatory bowel disease: Clinical features and the role of HLA genes. Gastroenterology 2002, 123, 714–718.
- Skaaby, T.; Husemoen, L.L.N.; Thuesen, B.H.; Linneberg, A. Prospective population-based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine 2015, 50, 231–238.
- Santeford, A.; Wiley, L.A.; Park, S.; Bamba, S.; Nakamura, R.; Gdoura, A.; Ferguson, T.A.; Rao, P.K.; Guan, J.L.; Saitoh, T.; et al. Impaired autophagy in macrophages promotes inflammatory eye disease. Autophagy 2016, 12, 1876–1885.
- Horai, R.; Zárate-Bladés, C.R.; Dillenburg-Pilla, P.; Chen, J.; Kielczewski, J.L.; Silver, P.B.; Jittayasothorn, Y.; Chan, C.C.; Yamane, H.; Honda, K.; et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity 2015, 43, 343–353.
- Salvador, R.; Horai, R.; Zhang, A.; Jittayasothorn, Y.; Tang, J.; Gupta, A.; Nagarajan, V.; Caspi, R.R. Too Much of a Good Thing: Extended Duration of Gut Microbiota Depletion Reverses Protection From Experimental Autoimmune Uveitis. Investig. Opthalmol. Vis. Sci. 2023, 64, 43.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
573
Revisions:
2 times
(View History)
Update Date:
16 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





