Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are complex multifactorial chronic inflammatory disorders affecting nearly 7 million patients worldwide, with an increasing prevalence in Northern Europe and emerging countries. IBD can be associated with a wide spectrum of extra-intestinal manifestations (EIMs) with a significant impact on patients’ quality of life. The pathophysiology of EIMs in IBD is intricate and uncertain. The European Crohn’s and Colitis Organization (ECCO) proposed an operational definition of EIMs’ pathology in IBD patients.
- uveitis
- episcleritis
- scleritis
- inflammatory bowel disease
1. Introduction
2. Pathogenesis
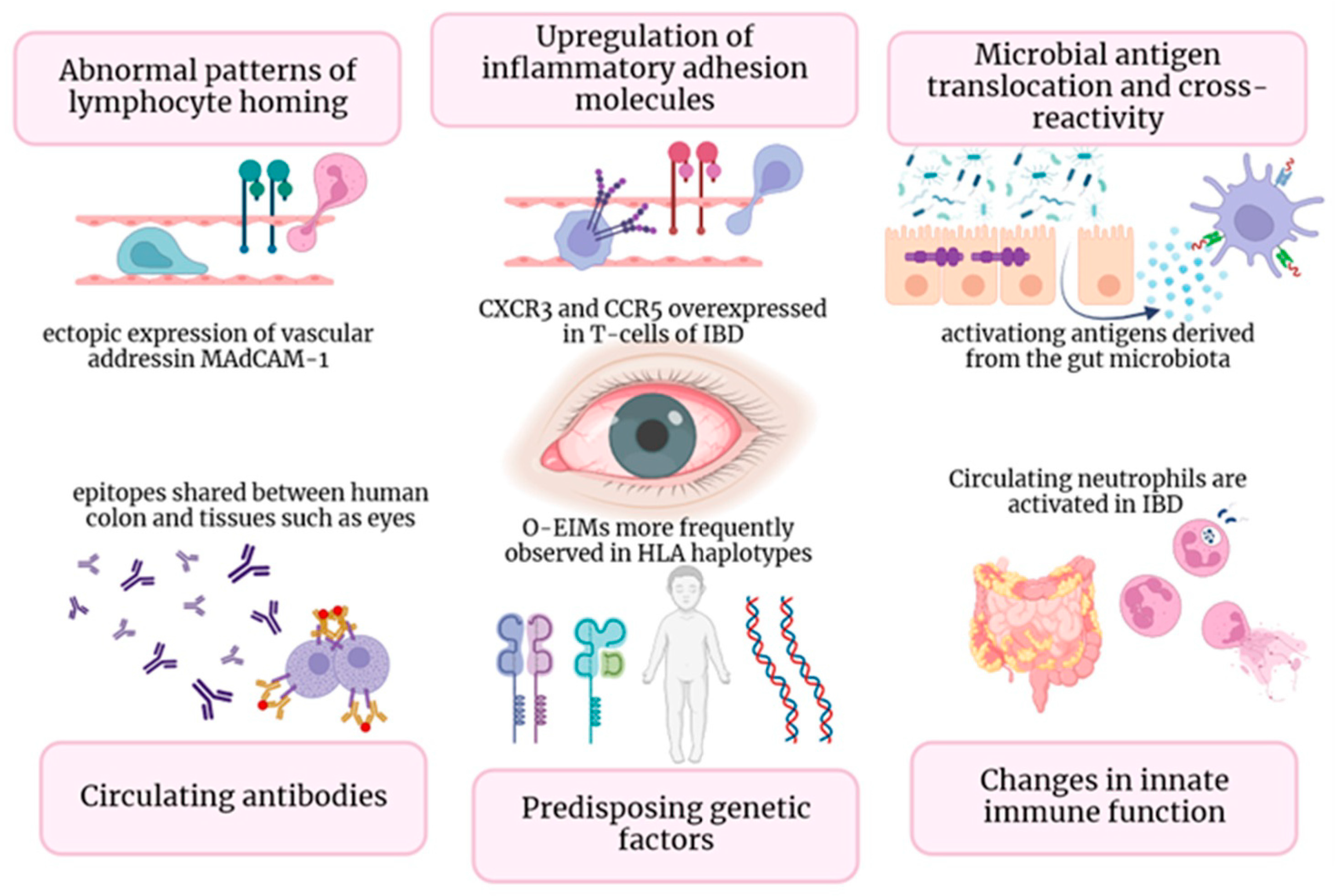
This entry is adapted from the peer-reviewed paper 10.3390/cells13020142
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.; Chan, F.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778.
- Trikudanathan, G.; Venkatesh, P.G.K.; Navaneethan, U. Diagnosis and Therapeutic Management of Extra-Intestinal Manifestations of Inflammatory Bowel Disease. Drugs 2012, 72, 2333–2349.
- Greuter, T.; Vavricka, S.R. Extraintestinal manifestations in inflammatory bowel disease–epidemiology, genetics, and pathogenesis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 307–317.
- Kilic, Y.; Kamal, S.; Jaffar, F.; Sriranganathan, D.; Quraishi, M.N.; Segal, J.P. Prevalence of Extraintestinal Manifestations in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2023, 20, izad061.
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992.
- Vavricka, S.R.; Rogler, G.; Gantenbein, C.; Spoerri, M.; Vavricka, M.P.; Navarini, A.A.; French, L.; Safroneeva, E.; Fournier, N.; Straumann, A.; et al. Chronological order of appearance of extraintestinal manifestations relative to the time of IBD diagnosis in the Swiss Inflammatory Bowel Disease Cohort. Inflamm. Bowel Dis. 2015, 21, 1794–1800.
- Park, S.K.; Wong, Z.; Park, S.H.; Van Vu, K.; Bang, K.B.; Piyachaturawat, P.; Myint, T.; Hilmi, I.; Park, D.I. Extraintestinal manifestation of inflammatory bowel disease in Asian patients: A multinational study. Dig. Liver Dis. 2021, 53, 196–201.
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132.
- Hedin, C.R.H.; Vavricka, S.R.; Stagg, A.J.; Schoepfer, A.; Raine, T.; Puig, L.; Pleyer, U.; Navarini, A.; Van Der Meulen-De Jong, A.E.; Maul, J.; et al. The Pathogenesis of Extraintestinal Manifestations: Implications for IBD Research, Diagnosis, and Therapy. J. Crohns Colitis 2019, 13, 541–554.
- Eksteen, B.; Grant, A.J.; Miles, A.; Curbishley, S.M.; Lalor, P.F.; Hübscher, S.G.; Briskin, M.; Salmon, M.; Adams, D.H. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J. Exp. Med. 2004, 200, 1511–1517.
- Horai, R.; Caspi, R.R. Cytokines in autoimmune uveitis. J. Interferon Cytokine Res. 2011, 31, 733–744.
- Sabino, J.; Vieira-Silva, S.; Machiels, K.; Joossens, M.; Falony, G.; Ballet, V.; Ferrante, M.; Van Assche, G.; Van Der Merwe, S.; Vermeire, S.; et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016, 65, 1681–1689.
- Pytrus, W.; Akutko, K.; Pytrus, T.; Turno-Kręcicka, A. A Review of Ophthalmic Complications in Inflammatory Bowel Diseases. J. Clin. Med. 2022, 11, 7457.
- Troncoso, L.L.; Biancardi, A.L.; Vieira de Moraes, H.J.; Zaltman, C. Ophthalmic manifestations in patients with inflammatory bowel disease: A review. World J. Gastroenterol. 2017, 23, 5836–5848.
- Mintz, R.; Feller, E.R.; Bahr, R.L.; Shah, S.A. Ocular Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2004, 10, 135–139.
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23.
- Li, J.X.; Chiang, C.C.; Chen, S.N.; Lin, J.M.; Tsai, Y.Y. The Prevalence of Ocular Extra-Intestinal Manifestations in Adults Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 15683.
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755.
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619.
- Das, K.M. Relationship of Extraintestinal Involvements in Inflammatory Bowel Disease New Insights into Autoimmune Pathogenesis. Dig. Dis. Sci. 1999, 44, 1–13.
- Meng, Y.; Tan, Z.; Liu, C.; Dong, W.; Chen, C. Association between Inflammatory Bowel Disease and Iridocyclitis: A Mendelian Randomization Study. J. Clin. Med. 2023, 12, 1282.
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27.
- Bhagat, S.; Das, K.M. A Shared and Unique Peptide in the Human Colon, Eye, and Joint Detected by a Monoclonal Antibody. Gastroenterology 1994, 107, 103–108.
- Vavricka, S.R.; Brun, L.; Ballabeni, P.; Pittet, V.; Prinz Vavricka, B.M.; Zeitz, J.; Rogler, G.; Schoepfer, A.M. Frequency and Risk Factors for Extraintestinal Manifestations in the Swiss Inflammatory Bowel Disease Cohort. Am. J. Gastroenterol. 2011, 106, 110–119.
- Taylor, S.R.; McCluskey, P.; Lightman, S. The ocular manifestations of inflammatory bowel disease. Curr. Opin. Ophthalmol. 2006, 17, 538–544.
- Lin, P.; Tessler, H.H.; Goldsterin, D.A. Family history of inflammatory bowel disease in patients with idiopathic ocular inflammation. Am. J. Ophthalmol. 2006, 141, 1097–1104.
- Lanna, C.C.D.; Ferrari, M.D.L.A.; Rocha, S.L.; Nascimento, E.; Carvalho, M.A.P.; Cunha, A.S. A cross-sectional study of 130 Brazilian patients with Crohn’s disease and ulcerative colitis: Analysis of articular and ophthalmologic manifestations. Clin. Rheumatol. 2008, 27, 503–509.
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584.
- Younis, N.; Zarif, R.; Mahfouz, R. Inflammatory bowel disease: Between genetics and microbiota. Mol. Biol. Rep. 2020, 47, 3053–3063.
- Cheema, H.A.; Waheed, N.; Saeed, A.; Fayyaz, Z.; Anjum, M.N.; Alvi, M.A.; Batool, S. Very early onset inflammatory bowel disease: Spectrum of clinical presentation, diagnostic tools and outcome in children. J. Pak. Med. Assoc. 2021, 71, 2350–2354.
- Naser, S.A.; Arce, M.; Khaja, A.; Fernandez, M.; Naser, N.; Elwasila, S.; Thanigachalam, S. Role of ATG16L, NOD2 and IL23R in Crohn’s disease pathogenesis. World J. Gastroenterol. 2012, 18, 412–424.
- Cho, J.H. Basic Science on the Cutting Edge the Nod2 Gene in Crohn’s Disease: Implications for Future Research into the Genetics and Immunology of Crohn’s Disease. Inflamm. Bowel Dis. 2001, 7, 271–275.
- Matsuda, T.; Kambe, N.; Takimoto-Ito, R.; Ueki, Y.; Nakamizo, S.; Saito, M.K.; Takei, S.; Kanazawa, N. Potential Benefits of TNF Targeting Therapy in Blau Syndrome, a NOD2-Associated Systemic Autoinflammatory Granulomatosis. Front. Immunol. 2022, 13, 895765.
- Duerr, R.H.M. The genetics of inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2002, 31, 63–76.
- Wu, Z.; Liu, D.; Deng, F. The Role of Vitamin D in Immune System and Inflammatory Bowel Disease. J. Inflamm. Res. 2022, 15, 3167–3185.
- Orchard, T.R.; Chua, C.N.; Ahmad, T.; Cheng, H.; Welsh, K.I.; Jewell, D.P. Uveitis and erythema nodosum in inflammatory bowel disease: Clinical features and the role of HLA genes. Gastroenterology 2002, 123, 714–718.
- Skaaby, T.; Husemoen, L.L.N.; Thuesen, B.H.; Linneberg, A. Prospective population-based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine 2015, 50, 231–238.
- Santeford, A.; Wiley, L.A.; Park, S.; Bamba, S.; Nakamura, R.; Gdoura, A.; Ferguson, T.A.; Rao, P.K.; Guan, J.L.; Saitoh, T.; et al. Impaired autophagy in macrophages promotes inflammatory eye disease. Autophagy 2016, 12, 1876–1885.
- Horai, R.; Zárate-Bladés, C.R.; Dillenburg-Pilla, P.; Chen, J.; Kielczewski, J.L.; Silver, P.B.; Jittayasothorn, Y.; Chan, C.C.; Yamane, H.; Honda, K.; et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity 2015, 43, 343–353.
- Salvador, R.; Horai, R.; Zhang, A.; Jittayasothorn, Y.; Tang, J.; Gupta, A.; Nagarajan, V.; Caspi, R.R. Too Much of a Good Thing: Extended Duration of Gut Microbiota Depletion Reverses Protection From Experimental Autoimmune Uveitis. Investig. Opthalmol. Vis. Sci. 2023, 64, 43.
