Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Villen-Guzman | -- | 2579 | 2024-01-09 12:22:11 | | | |
| 2 | Rita Xu | Meta information modification | 2579 | 2024-01-10 03:12:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cerrillo-Gonzalez, M.D.M.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Electrodialysis Separation. Encyclopedia. Available online: https://encyclopedia.pub/entry/53605 (accessed on 08 February 2026).
Cerrillo-Gonzalez MDM, Villen-Guzman M, Rodriguez-Maroto JM, Paz-Garcia JM. Electrodialysis Separation. Encyclopedia. Available at: https://encyclopedia.pub/entry/53605. Accessed February 08, 2026.
Cerrillo-Gonzalez, Maria Del Mar, Maria Villen-Guzman, Jose Miguel Rodriguez-Maroto, Juan Manuel Paz-Garcia. "Electrodialysis Separation" Encyclopedia, https://encyclopedia.pub/entry/53605 (accessed February 08, 2026).
Cerrillo-Gonzalez, M.D.M., Villen-Guzman, M., Rodriguez-Maroto, J.M., & Paz-Garcia, J.M. (2024, January 09). Electrodialysis Separation. In Encyclopedia. https://encyclopedia.pub/entry/53605
Cerrillo-Gonzalez, Maria Del Mar, et al. "Electrodialysis Separation." Encyclopedia. Web. 09 January, 2024.
Copy Citation
Electrodialysis is classified as a membrane separation process in which ions are transferred through selective ion-exchange membranes from one solution to another using an electric field as the driving force. Electrodialysis is a mature technology in the field of brackish water desalination, but in recent decades the development of new membranes has made it possible to extend their application in the food, drug, and chemical process industries, including wastewater treatment.
metal separation
membrane
selectivity
1. Introduction
During recent decades, the rising population and industrialization have increased demand for the Earth’s natural resources and the occurrence of subsequent environmental problems. One of these problems is the generation of large amounts of wastewater containing metals that are a risk to the population and ecosystems due to their toxicity [1]. Some metals affect biological function and can accumulate in different organs, causing hazardous effects [2]. The most prevalent metals found in wastewater are Al, As Cd, Cr, Cu, Hg, Pb, Ni, Zn, Co, Fe, and Mn, and their composition in wastewater differs according to the type of industry. The main sectors that contribute to this problem are mining, smelting, foundries, and other chemistries such as textiles and refineries [2].
The negative effect of some metals in wastewater makes it necessary to treat these industrial effluents before discharging to reduce the pollution. The conventional method to remove metals from industrial effluents has been chemical precipitation, adjusting the pH of the effluents, and removing the precipitated particles through sedimentation or filtration [3]. Other chemical-based separation methods include coagulation/flocculation and flotation. These methods can be applied to treat industrial effluents with high metal concentrations. Although these methods are simple and require inexpensive equipment, they involve a large amount of chemicals to reduce metal concentrations and a large-volume sludge formation which requires post-treatment [4]. As an alternative method, adsorption is widely selected to remove metal from aqueous effluents. Several studies have focused on developing cheaper and more efficient adsorbent materials in the last decade, for example, natural materials, industrial by-products, or biological and agricultural waste [5]. However, biosorption (i.e., adsorption with biological materials) has some shortcomings, such as a lack of specificity in metal binding, large amounts of biomass required if the biosorption capacity is low, and a limited reusability of biomass after desorption for real applications in industrial effluents [6]. Another method applied to remove metals from aqueous effluents is ion exchange due to its higher ion selectivity and the reusability of ion-exchange material. The main drawbacks of this technology are high operational costs, which limit application at industrial scale, and the formation of fouling by solids and organic compounds contained in industrial effluents [6]. Electrochemical processes based on passing a direct current through an aqueous solution containing metal between electrodes have also been widely studied. Metal selectivity, no consumption of additional chemicals, high efficiency, and lower amounts of sludge produced are the main advantages of these processes. However, electrochemical technologies possess some drawbacks, such as a high dependence on the pH values of aqueous effluents and high operational costs related to electrical energy requirements and electrode replacement [3].
Some alternative technologies to conventional methods are based on pressure-driven membrane processes, such as ultrafiltration, nanofiltration, and reverse osmosis [7]. Another membrane technology is electrodialysis (ED), which involves the migration of cations and anions through ion-exchange membranes under the effect of an electric field. This technique has been employed industrially to treat saline waters and brines. However, its selective separation of charged species offers excellent advantages, increasing its interest for treating water containing metals. Some advantages related to the selective separation of ions are the high separation efficiency, low operating pressure, small operating footprint, no need for adding chemicals, and reduced sludge formation. Moreover, this technology can treat effluents with low concentrations of metal ions. All these advantages make electrodialysis one of the most effective and promising technologies for treating industrial effluents [8].
2. Fundamentals of Electrodialysis Processes
2.1. Operational Principle
The working principle of ED consists of the migration of cations and anions through cation-exchange membranes (CEMs) and anion-exchange membranes (AEMs), respectively, induced by an applied electric field set between a pair of electrodes. In a simple electrodialysis cell (Figure 1), a pair of membranes is used. Anions migrate towards the anode, and cations towards the cathode, resulting in an overall decrease in the feed stream salt concentration. The membranes are separated by channels ending with the electrode compartments.

Figure 1. Schematic of a simple (two-membrane) electrodialysis cell.
The two-membrane ED cell results in the production of an acid and a base from the fed salt. The unit cell is formed by an AEM, a CEM, and a channel in-between where the concentrated salt solution is fed. The application of an electrical current between the electrodes promotes the dissociation of water at the electrodes [9].
Multichannel cells consist of a system of alternative membranes creating channels which produce diluted and concentrated outflows (Figure 2). The flow containing ions is fed in the channels between membranes. Anions migrate toward the anode crossing the AEM but they are retained in the compartment due to the presence of an adjacent CEM. In this channel, there are also cations coming from the opposite directions, migrating to the cathode through the CEM, but they are blocked by the AEM. This means the migrated anions and cations remain blocked in the same compartment, creating a concentrated channel. On the other hand, the adjacent channels to the concentrate are denoted as dilute channels due to the reduction in both anion and cation concentrations. The combination of an AEM, a concentrated channel, a CEM, and a dilute spacer is known as a cell pair.
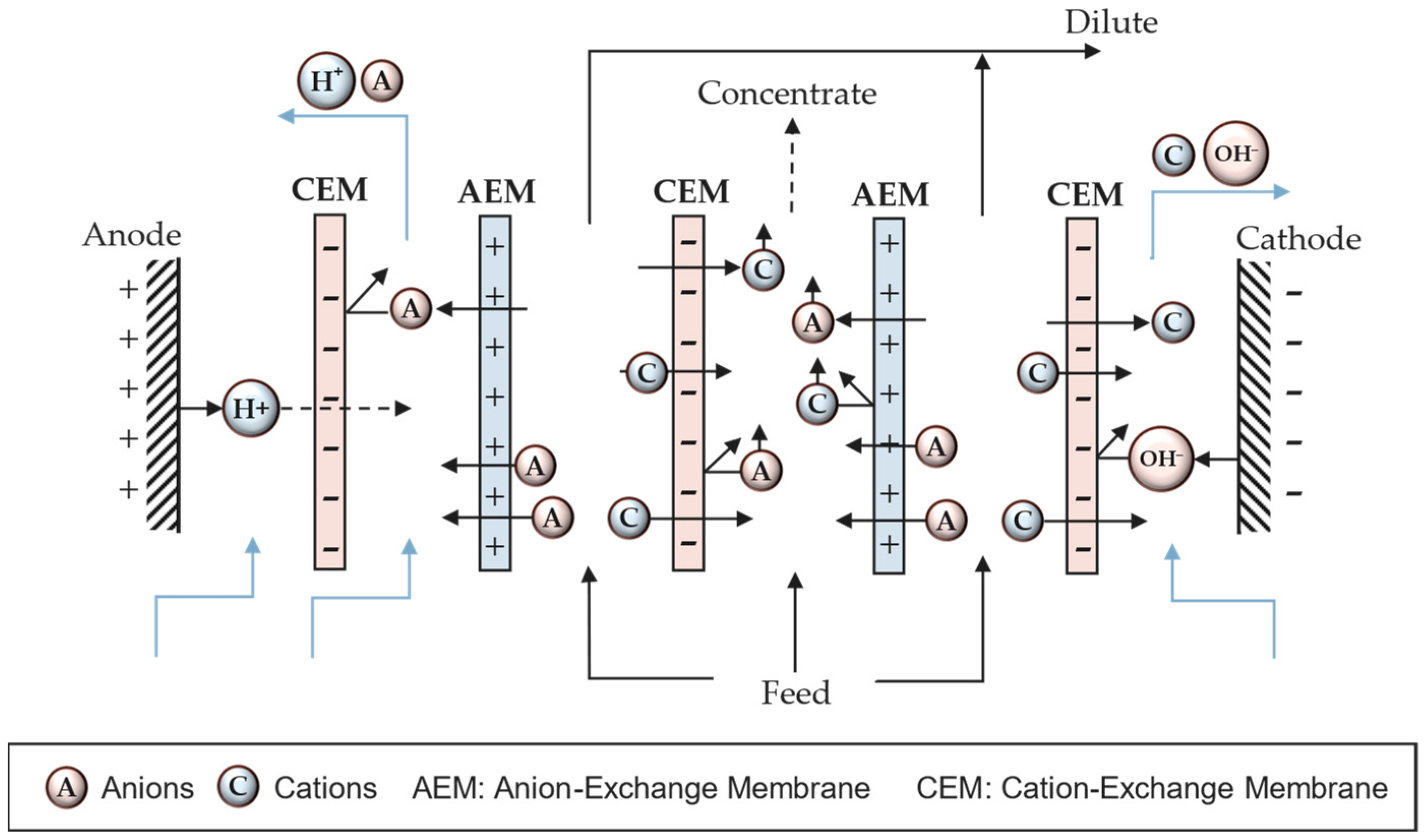
Figure 2. Depiction of a multichannel electrodialysis stack.
In aqueous solution, water electrolysis reactions are expected to take place: namely, water oxidation at the anode, Equation (1), and water reduction at the cathode, Equation (2).
However, different electrochemical reactions may occur depending on the nature of the involved ions. For example, in the presence of chloride ions, chlorine gas can be formed at the anode. Similarly, certain metal ions may electrodeposit at the cathode surface. Different cell configurations can be used to prevent certain electrochemical reactions. Figure 2 shows a CEM at the anode end to prevent the anion, assumed to be Cl−, from reaching the electrode surface and forming Cl2 gas.
The development of membrane technologies has allowed new cell configurations, expanding the application of electrodialysis [10]. An interesting enhancement is the use of bipolar membranes in the cell stack (Figure 3). A bipolar membrane (BM) is composed of an anion-exchange layer (AEL) and a cation-exchange layer (CEL), and does not allow anions and cations through it. This phenomenon promotes the dissociation of water available within the thin layer between AEL and CEL into protons (H+) and hydroxyl (OH−). This cell configuration generates an acidic and alkaline compartment on both sides of the bipolar membrane due to H+ and OH− crossing the CEL and AEL, respectively [11].
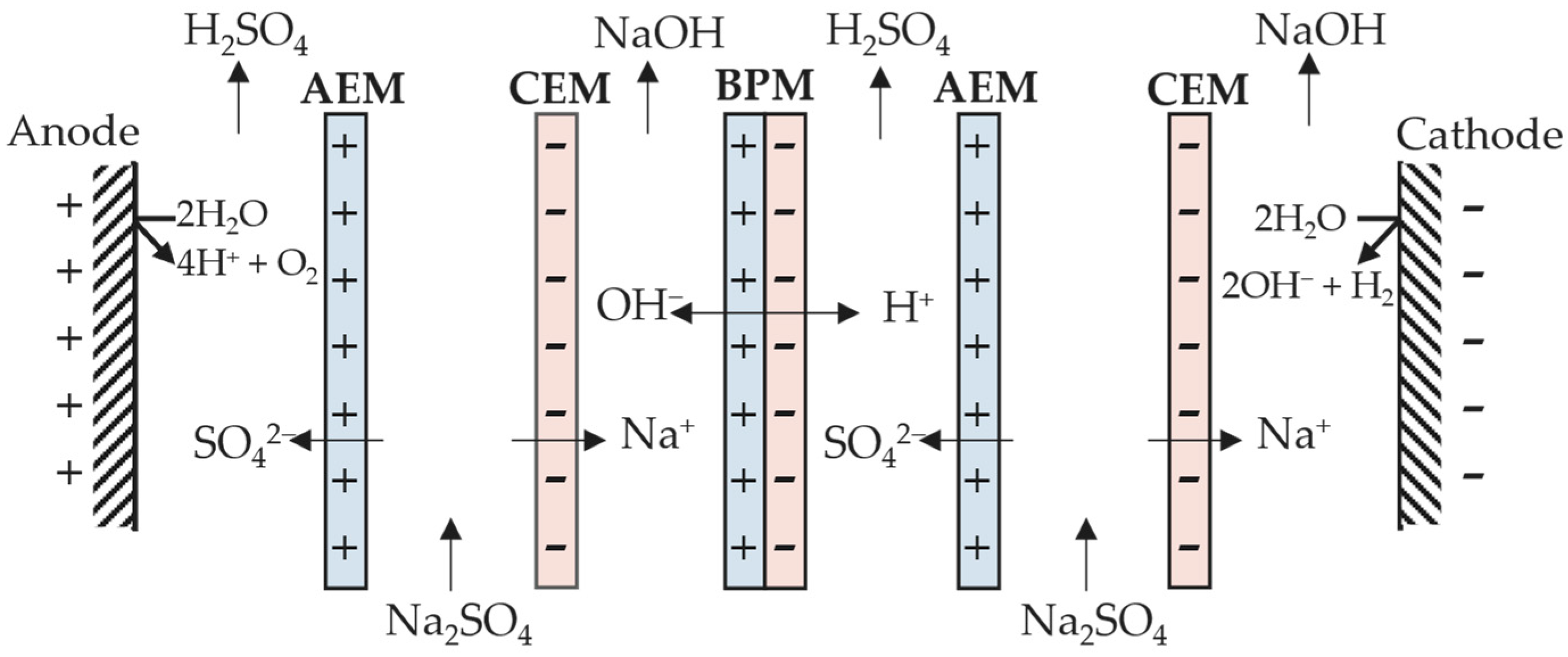
Figure 3. Depiction of a three-compartment electrodialytic cell with a bipolar membrane.
The use of monovalent anion-exchange membranes (MVAs) and monovalent cation-exchange membranes (MVCs) has been recently proposed to selectively separate ions in different ED compartments [12]. In this case, an MVA is used in the cell, which allows only the migration of monovalent anions, producing two compartments: one with multivalent anions retained and the other with monovalent ions (Figure 4). This process is classified as “selectrodialysis”. In addition to using MVAs and MVCs, selectrodialysis can be achieved using different pH and chelating/complexation agents.
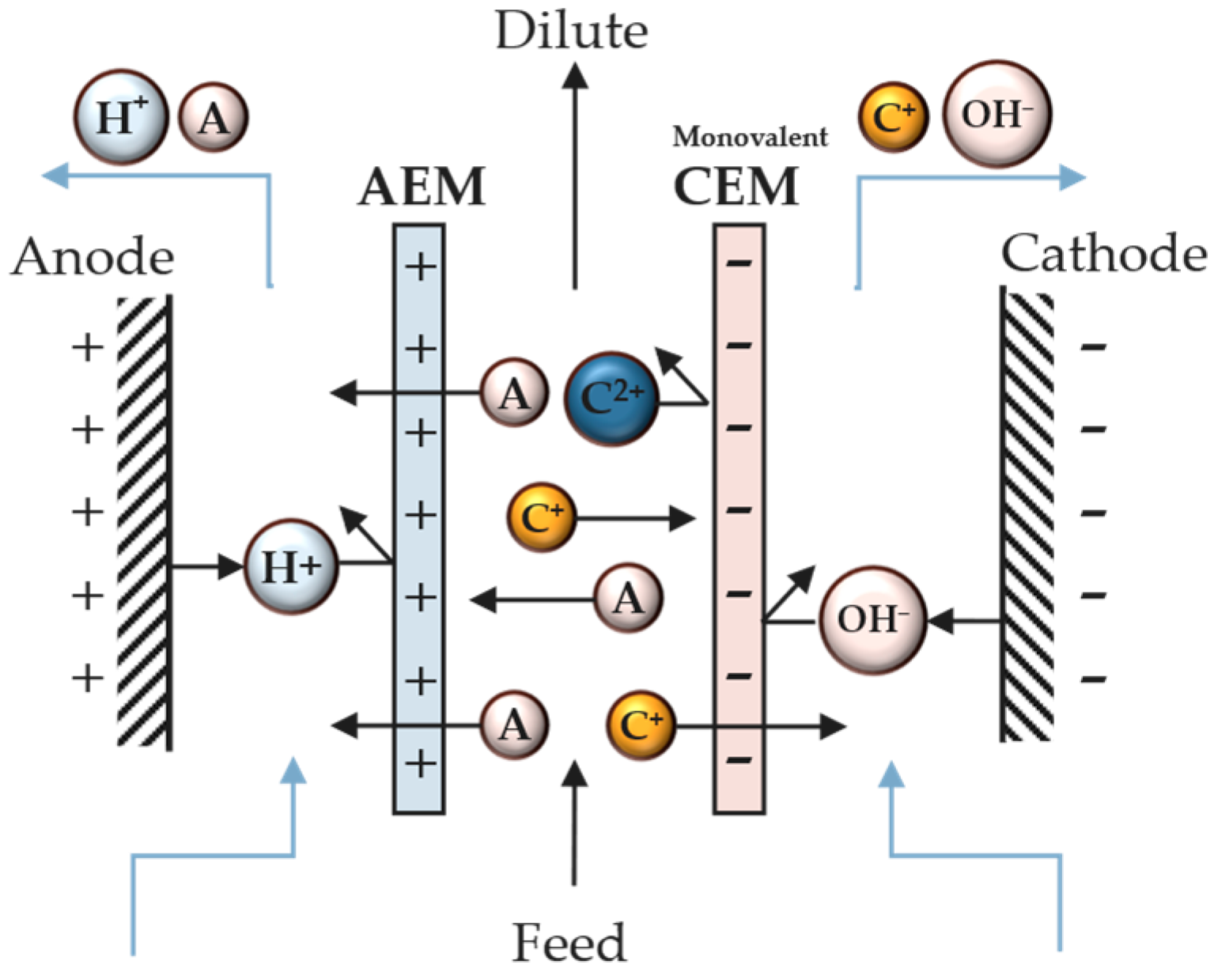
Figure 4. Scheme of a unit cell with a monovalent cation-exchange membrane.
One of the main drawbacks in the membrane processes is the fouling of their surfaces, caused by suspended particles or the precipitation of dissolved solids. Solid particles cause the deterioration of the membranes, increasing their electrical resistance with a subsequent lowering of the ED performance. In addition to performing pretreatments and cleaning procedures to prevent surface fouling, electrodialysis reversal (EDR) has been proposed to mitigate the fouling problem [13]. The technique consists of switching periodically the polarity of electrodes to force the migration of charged components to the opposite direction [14]. Several studies reported the success of EDR systems due to their self-cleaning mechanism.
2.2. Mass Transport Mechanism
Mass and charge transport in electrolyte solutions and ion-exchange membranes is described with the same set of equations. Under an electric field, ions are mainly transported by electromigration. Diffusion transport becomes significant at the boundary layers, near the membranes and the electrodes, due to high concentration gradients. The coupling of these transport mechanisms is known as electrodiffusion and can be described by the Nernst–Plank transport equation, which considers the diffusive, electromigration, and convective flux to calculate the total flux of ions, (mol/m2/s):
where 𝑐𝑖 (mM) is the concentration, 𝐷∗𝑖 (m2/s) is the effective diffusion coefficient, 𝑧𝑖 is the ionic charge, F (≈95,485 A/mol) is the Faraday constant, φ (V) is the electric potential, and 𝒖 (m/s) is the velocity of water. The term 𝑧𝑖𝐹𝐷𝑖/𝑅𝑇 (m2/s/V) is known as the ionic migration coefficient. The set of equations is completed with the following electroneutrality condition:
At the submicro scale, at the interface of the electrically charged membranes, the electroneutrality condition does not hold, and electrical double layers form. This can be described by means of the Poisson equation:
The performance of an electrodialysis treatment depends on numerous variables, such as the membrane properties, the cell configuration, and the operational conditions. Regarding the transport mechanisms, the most important parameters are the electric current applied, the electrical conductivity of the electrolytes and the membranes, the concentration and pH of the electrolyte solutions, and the stream flow rate. All these parameters are inter-related between each other, and the study of their effects is essential to design and optimize the process.
Ion-exchange membranes (IEMs) are crucial elements of any ED process, and their characteristics play an important role in the performance of the process. Membrane properties can be divided into physicochemical (e.g., thickness) and electrochemical properties (e.g., permeability, selectivity, charge density, and area resistance) [15]. IEMs are dense polymeric membranes containing fixed charges in the polymer matrix, which can selectively enable the passage of oppositely charged ions (counter ions) while obstructing similarly charge ions (co-ions). The enhancement in the permselectivity between counter and co-ions as well as between counter ions with different (monovalent and multivalent) or equal valences (Cl− and NO3−) has allowed the expansion of IEMs to multiple applications [16]. However, improving the permselectivity involves an increase in cross-linking in the polymer matrix, which also results in an increase in the area resistance, which is not desirable from the point of view of energy efficiency. Thus, membrane designers must find a balance between permselectiviy and area resistance. Lu et al. [16] elaborated a comprehensive review of IEMs, focused on the progress of membrane manufacturing techniques, ion transport mechanisms, and experimental approaches to determine ion selectivity. In the same line, Tekinalp et al. [17] published a review on cation-exchange membranes and their properties for selective metal separation by electrodialysis. They discussed the counter ion selectivity based on the membrane properties and the operational conditions, emphasizing the implication of the boundary layer at the membrane surface in the transport ratio of competing counter ions.
The Intensity of the applied electric current is also one of the key factors in an electrical separation process. By default, operating with the highest possible current density is desirable to increase the driving force and achieve the maximum ion flows. However, high electric current values have drawbacks, such as increased energy requirements, possible membrane damage, and increased concentration polarization at the membrane surfaces. [8]. Concentration polarization appears due to the difference in transport numbers of the ions (i.e., the fraction of the current carried by each ionic species) in the solution and the membranes. In the solution electrolyte, the ionic current derives from the transport of both cations and anions. In the membrane, the ionic current is only transported by the counter ions inside the membranes, resulting in a higher transport number through the membranes than in the solution. This phenomenon leads to the formation of concentration gradients at the solution–membrane interface, decreasing the concentration with respect to the bulk in the dilute compartment and increasing it in the concentrate compartment.
Depending on the applied current density, the concentration of specific ions can deplete near the surface membrane of the dilute region, which means there may not be any available ions to transport the current in that region. The current density that generates the depletion of the ion concentration to zero is known as the limiting current density. Working over the limiting current density is not recommended due to the increased electrical resistance and voltage drop, negatively affecting the process efficiency [18]. Furthermore, when approaching the limiting current density, the lack of ions in the system promotes the migration of protons and hydroxides, which are replenished through the water self-ionization reaction. This phenomenon is referred to as water splitting [19].
The ion concentration in the feed solution plays an important role in the process’s efficiency. A low concentration in the solutions involves low ionic conductivity, affecting ions’ migration across the IEMs due to lower diffusion- and electromigration-driven forces. On the other hand, high concentrations of ions can cause some problems. First, the recovery rate of the target ions can be reduced because the residence time is insufficient to separate the desirable number of ions. Second, although electrical resistance decreases, applying a high value of electric current is necessary to mobilize the ions to the recovery compartment. Finally, in the case of metallic ions, a high concentration can cause their precipitation if the pH is not appropriate. Hence, one of the first parameters that should be decided in designing an ED stack is the feed flow, and then, the concentration of the dilute and concentrate output flows.
The pH is also an important parameter, especially when the feed flow contains metallic ions, in which the ED treatment is typically carried out at low pH to avoid metal precipitation. However, low pH affects ED variables such as concentration polarization, current efficiency, and energy consumption [20]. Protons are ions with the highest molar conductivity (350.1 Ω−1 cm2 mol−1 at 25 °C [21]). So, when the pH is low, protons are the predominant current transporter in the systems, affecting the recovery efficiency because the current is used to mobilize the protons instead the other cations.
Controlling the pH and optimizing its value is crucial to the excellent performance of ED treatment. According to Abou-Shady et al. [20], who studied the effect of pH on the separation of Pb(II) and NO3− from aqueous solution by electrodialysis, the optimal pH range for metallic ion solutions is between 3 and 5. Operating at pH < 3 makes the technique noneconomical due to high energy consumption, while operating at pH > 5 has undesirable effects, such as metal precipitation.
Water electrolysis tends to generate an acidic medium and an alkaline medium in the anode and cathode compartments, respectively. The alkalization of the catholyte may produce the precipitation of salts and hydroxides, while the acidification of anolyte leads to an increase in the energy consumption due to the high ionic conductivity of protons. Furthermore, water electrolysis reactions involve the formation of O2 and H2 at the anode and cathode, respectively, which may produce polarization at the electrodes due to attached bubbles and overpressure in the compartments.
References
- Ahmed, J.; Thakur, A.; Goyal, A. Chapter 1: Industrial Wastewater and Its Toxic Effects. In Biological Treatment of Industrial Wastewater; The Royal Society of Chemistry: London, UK, 2021; pp. 1–14.
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691.
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological Trends in Heavy Metals Removal from Industrial Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105688.
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377.
- Villen-Guzman, M.; Cerrillo-Gonzalez, M.M.; Paz-Garcia, J.M.; Rodriguez-Maroto, J.M.; Arhoun, B. Valorization of Lemon Peel Waste as Biosorbent for the Simultaneous Removal of Nickel and Cadmium from Industrial Effluents. Environ. Technol. Innov. 2021, 21, 101380.
- Doble, M.; Kumar, A. Treatment of Waste from Metal Processing and Electrochemical Industries. In Biotreatment of Industrial Effluents; Butterworth-Heinemann: Oxford, UK, 2005; pp. 145–155.
- Goh, P.S.; Wong, K.C.; Ismail, A.F. Membrane Technology: A Versatile Tool for Saline Wastewater Treatment and Resource Recovery. Desalination 2022, 521, 115377.
- Arana Juve, J.M.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for Metal Removal and Recovery: A Review. Chem. Eng. J. 2022, 435, 134857.
- Gurreri, L.; Cipollina, A.; Tamburini, A.; Micale, G. Electrodialysis for Wastewater Treatment—Part I: Fundamentals and Municipal Effluents. In Current Trends and Future Developments on (Bio-) Membranes: Membrane Technology for Water and Wastewater Treatment—Advances and Emerging Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–192. ISBN 9780128168240.
- Van der Bruggen, B. Ion-Exchange Membrane Systems—Electrodialysis and Other Electromembrane Processes. In Fundamental Modeling of Membrane Systems: Membrane and Process Performance; Elsevier: Amsterdam, The Netherlands, 2018; pp. 251–300.
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar Membranes: A Review on Principles, Latest Developments, and Applications. J. Membr. Sci. 2021, 617, 118538.
- Kitchenham, B. Procedures for Performing Systematic Reviews; Keele University: Keele, UK, 2004; Volume 33.
- Hansima, M.A.C.K.; Makehelwala, M.; Jinadasa, K.B.S.N.; Wei, Y.; Nanayakkara, K.G.N.; Herath, A.C.; Weerasooriya, R. Fouling of Ion Exchange Membranes Used in the Electrodialysis Reversal Advanced Water Treatment: A Review. Chemosphere 2021, 263, 127951.
- Tanaka, Y. Chapter 2: Electrodialysis Reversal. In Membrane Science and Technology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 12, pp. 383–404.
- Güler, E.; Elizen, R.; Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Performance-Determining Membrane Properties in Reverse Electrodialysis. J. Membr. Sci. 2013, 446, 266–276.
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of Ion Exchange Membranes: A Review. J. Membr. Sci. 2018, 555, 429–454.
- Tekinalp, Ö.; Zimmermann, P.; Holdcroft, S.; Burheim, O.S.; Deng, L. Cation Exchange Membranes and Process Optimizations in Electrodialysis for Selective Metal Separation: A Review. Membranes 2023, 13, 566.
- Moura Bernardes, A.; Zoppas Ferreira, J.; Siqueira Rodrigues, M.A. Electrodialysis and Water Reuse: Novel Approaches; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783642402494.
- Ebbers, B.; Ottosen, L.M.; Jensen, P.E. Electrodialytic Treatment of Municipal Wastewater and Sludge for the Removal of Heavy Metals and Recovery of Phosphorus. Electrochim. Acta 2015, 181, 90–99.
- Abou-Shady, A.; Peng, C.; Almeria, O.J.; Xu, H. Effect of PH on Separation of Pb (II) and NO3- from Aqueous Solutions Using Electrodialysis. Desalination 2012, 285, 46–53.
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill Professional Publishing: New York, NY, USA, 1999; ISBN 0-07-016384-7.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
750
Revisions:
2 times
(View History)
Update Date:
10 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




