Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dania Haddad | -- | 2649 | 2024-01-09 07:11:13 | | | |
| 2 | Catherine Yang | Meta information modification | 2649 | 2024-01-09 07:25:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Haddad, D.; Dsouza, V.S.; Al-Mulla, F.; Al Madhoun, A. Dorzagliatin in Type 2 Diabetes Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/53578 (accessed on 07 February 2026).
Haddad D, Dsouza VS, Al-Mulla F, Al Madhoun A. Dorzagliatin in Type 2 Diabetes Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/53578. Accessed February 07, 2026.
Haddad, Dania, Vanessa Sybil Dsouza, Fahd Al-Mulla, Ashraf Al Madhoun. "Dorzagliatin in Type 2 Diabetes Treatment" Encyclopedia, https://encyclopedia.pub/entry/53578 (accessed February 07, 2026).
Haddad, D., Dsouza, V.S., Al-Mulla, F., & Al Madhoun, A. (2024, January 09). Dorzagliatin in Type 2 Diabetes Treatment. In Encyclopedia. https://encyclopedia.pub/entry/53578
Haddad, Dania, et al. "Dorzagliatin in Type 2 Diabetes Treatment." Encyclopedia. Web. 09 January, 2024.
Copy Citation
Achieving glycemic control and sustaining functional pancreatic β-cell activity remains an unmet medical need in the treatment of type 2 diabetes mellitus (T2DM). Glucokinase activators (GKAs) constitute a class of anti-diabetic drugs designed to regulate blood sugar levels and enhance β-cell function in patients with diabetes. A significant progression in GKA development is underway to address the limitations of earlier generations. Dorzagliatin, a dual-acting GKA, targets both the liver and pancreas and has successfully completed two phase III trials, demonstrating favorable results in diabetes treatment.
dorzagliatin
TTP399
glucokinase activator
type 2 diabetes
1. Dorzagliatin: Mechanisms of Action
Dorzagliatin is a dual-acting GKA currently in the clinical developmental phase for treating T2DM. It is postulated that dorzagliatin binds to the allosteric site of GK at the P-loop, a conserved motif critical in regulating GK activity [1]. The P-loop forms a pocket distal to the active site of GK, enhancing its affinity for glucose and lowering the set point for GSIS. This binding stabilizes a high-affinity conformation of the enzyme, increasing its activity and improving its ability to phosphorylate glucose. In the pancreas, dorzagliatin activates GK and exerts glucose-lowering effects by inhibiting IR and increasing insulin sensitivity (see Figure 1). Meanwhile, in the liver, dorzagliatin activates GK and promotes the dissociation of the GK-GKRP complex (see Figure 2) [2].
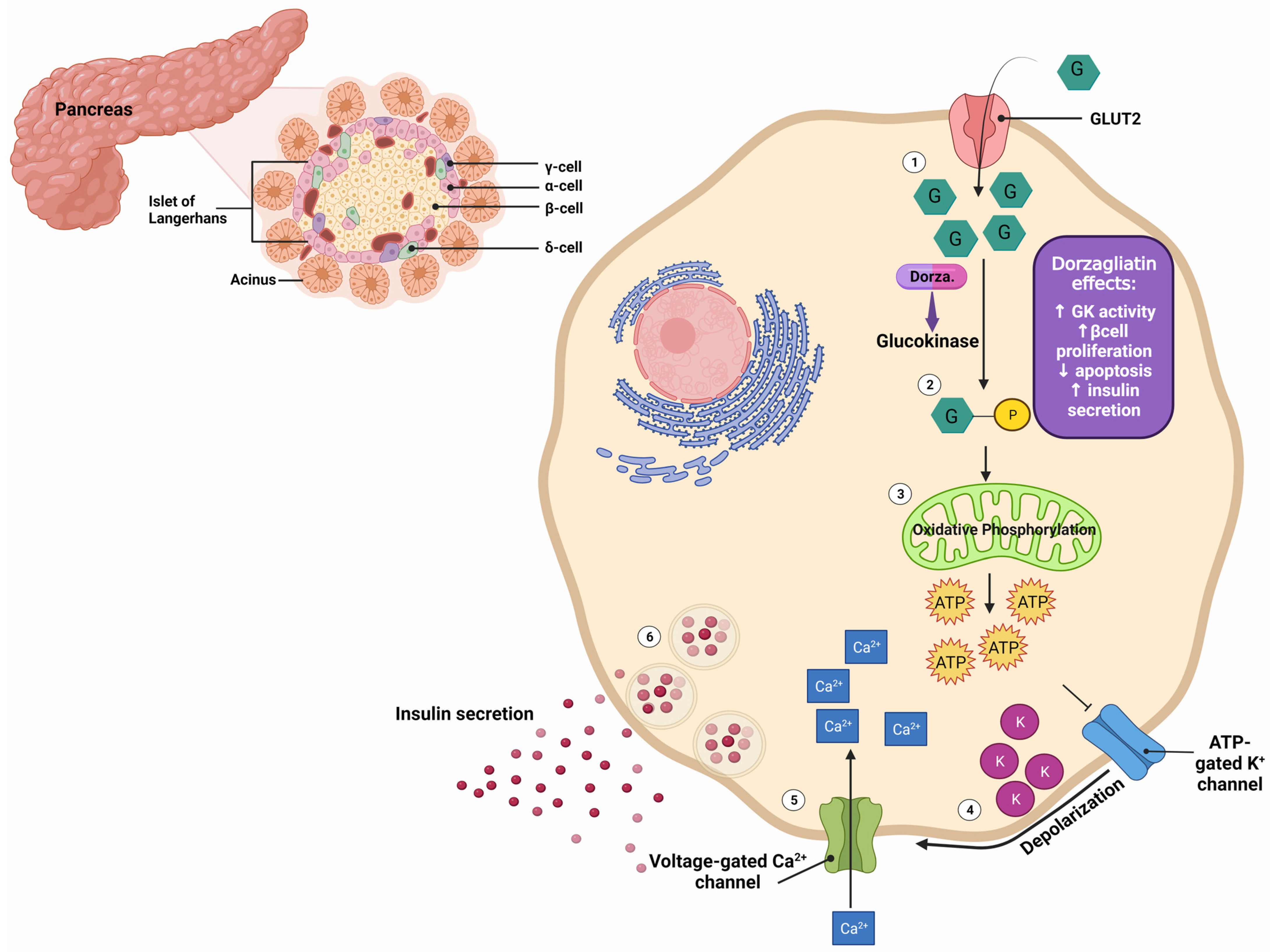
Figure 1. Effects of dorzagliatin on the pancreas: As blood glucose levels increase (1), dorzagliatin directly activates glucokinase (2), a crucial enzyme in the glycolysis pathway. Subsequent oxidative phosphorylation (3) generates ATP. The elevated ATP levels cause the closure of ATP-sensitive potassium channels (4), leading to membrane depolarization. This depolarization triggers the opening of voltage-gated calcium channels (5). The increased intracellular calcium concentration stimulates the exocytosis of insulin-containing secretory vesicles from the β-cells, leading to the release of insulin from the pancreatic β-cells (6). Dorzagliatin stimulates the proliferation and limits the apoptosis of β-cells, thereby improving blood glucose levels. GK, glucokinase; GLUT2, glucose transporter 2; F cells, pancreatic polypeptide cells; α-cell, pancreatic alpha cells; β-cell, pancreatic beta cells; δ-cell, pancreatic delta cells. Created with BioRender.com.
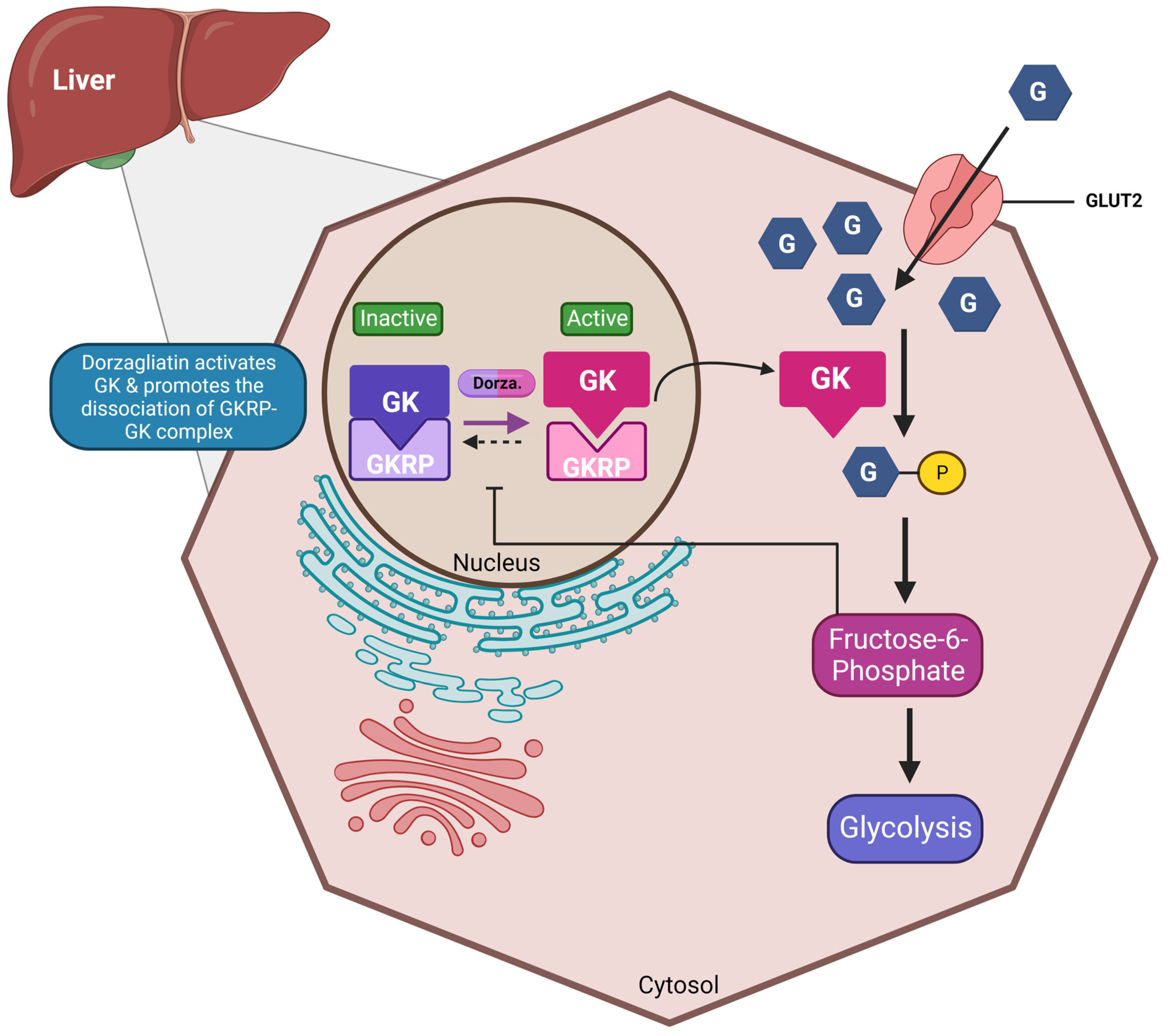
Figure 2. Effects of dorzagliatin on the liver: Under normoglycemic conditions, glucokinase is tightly bound to GKRP in the nucleus. As blood sugar levels rise, dorzagliatin promotes the dissociation of the GK-GKRP complex through the direct activation of GK and translocates it to the cytoplasm, initiating the glycolysis pathway. The energy generated can then be further utilized for glycogenolysis. GKA, glucokinase activator; GK, glucokinase; GKRP: glucokinase regulatory protein; GLUT2, glucose-transporter 2. Created with BioRender.com.
Dorzagliatin is a small-molecule drug with a molecular formula of C20H22N6O2, IUPAC name (2S)-2-(4-(2-Chloro-phenoxy)-2-oxo-2,5-dihydro-1H-pyrrol-1-yl)-N-(1-((2R)-2,3-dihydroxypropyl)-1H-pyrazol-3-yl)-4-methyl-pentanamide, and a molecular weight of 462.9 g/mol, appearing as a white-to-off-white solid with a melting point of 242–244 °C. Dorzagliatin is slightly soluble in water, but exhibits a higher level of solubility in organic solvents, ethanol, and DMSO.
Structurally, dorzagliatin can be divided into three components. The central core comprises a pyrazole ring and a phenyl ring connected by an amide bond, critical for the drug’s binding affinity to GK. The substituent group is attached to the central core’s phenyl ring, consisting of an amino group and a guanidine group, mediating hydrogen bonding and electrostatic interactions with GK. The terminal alkyl chain is linked to the central core’s pyrazole ring, composed of an ethyl group and a methyl group, engaged in hydrophobic interactions with GK [1][3].
Herein, the researchers will delve into preclinical studies and clinical trials employing dorzagliatin, summarizing the outcomes in Table 1.
Table 1. Results from preclinical studies and clinical trials using dorzagliatin.
| Preclinical Studies | |||||
|---|---|---|---|---|---|
| Reference | Animal | Duration (Weeks) | Interventions | Primary Findings | |
| 2017, Wang et al. [2] | Male Sprague–Dawley (SD) rats with T2DM | 4 | Control, diabetic, 10 mg/kg, 30 mg/kg | Reduction in FPG by ∼18% (10 mg/kg) and 23% (30 mg/kg) Reduction in FINS: 28.40 mU/L (10 mg/kg) and 18.74 mU/L (30 mg/kg) Levels of TC and TG unchanged Increase in FG: 43 pg/mL (10 mg/kg) and 51 pg/mL (30 mg/kg) Reduction in OGTT: 9 mmol/L (10 mg/kg) and 7 mmol/L (30 mg/kg) compared to diabetic rats Increased expression of GK-immunopositive cells and insulin (Western blot) |
|
| Clinical Trials | |||||
| Reference | Total Participants (N) | Duration (weeks) | Study design | Interventions | Primary findings |
| 2016, Xu et al. [4] | 60 (31 M, 29 F) | XXX | Phase Ia: randomized, double-blind, placebo-controlled, parallel-group, administered to healthy subjects | Six dose cohorts (5, 10, 15, 25, 35, and 50 mg), 10 randomized subjects (8 receiving HMS5552 and 2 receiving placebo) | HMS5552 at doses up to 50 mg in healthy subjects is safe and well-tolerated Dose-related glucose-lowering effects and post-prandial insulin secretion AEs: belching, dizziness, palpitation, cold sweat, and proteinuria No hypoglycemia |
| 2018, Zhu et al. [5] | 24 (17 M, 7 F) | 4 | T2DM patients randomized at 1:1 ratio to receive two concentrations of dorzagliatin | 75 mg QD, 75 mg BID | Overall, QD treatment was better than BID Decrease in HBA1c, FPG and PPG Increase in C-peptide Reduction in AUC Increase in HOMA2 parameter %B Increase in the dynamic state parameter ΔC30/ΔG30 Hypoglycemia: 17% |
| 2018, Zhu et al. [6][7] | 258 (154 M, 104 F) | 12 | Phase II: multicenter, randomized, double-blind, placebo-controlled T2DM patients were on a diet and exercise regimen; drug-naïve or previously treated with metformin or α-glucosidase inhibitor monotherapy |
75 or 100 mg QD, 50 or 75 mg BID, placebo | Decrease in HbA1c, FPG, and PPG, particularly in patients who received 100 QD or 75 BID AE: URTi, hyperuricemia, dizziness Hypoglycemia 6% in all studied groups |
| 2020–2022, Zhu et al. (SEED trial) [8][9] | 463 (301 M, 162 F) | 52 | Phase III: multicenter, randomized, double-blind, placebo-controlled (24 weeks), open-label (28 weeks) study T2DM drug-naïve patients |
75 mg BID, placebo | Decrease in HbA1c and FPG at week 24, sustained through week 52 Increase in HOMA2-β AEs: URTi, hyperlipidemia, proteinuria, abnormal hepatic function, hypertension Hypoglycemia 0.3% in all studied groups |
| 2022, Yang et al. (DAWN trial) [10] | 767 (475 M, 292 F) | 52 | Phase III: randomized, double-blind, placebo-controlled (24 weeks), open-label (28 weeks) study T2DM add-on therapy to metformin |
75 mg BID, placebo, add-on metformin 1500 mg | Decrease in HbA1c < 7% in 44.4% patients at week 24 Decrease in FPG and HOMA2-IR Increase in HOMA2-β Hypoglycemia 0.3%, no weight gain in all studied groups |
| 2022, Miao et al. [11] | 17 (7 M, 10 F) | ½ | Open-label, single-dose, sequential two-part, parallel-group study 8 non-dialysis ESRD, including 1 T2DM; 9 healthy volunteers |
25 mg QD | End-stage renal insufficiency does not affect dorzagliatin efficacy Dorzagliatin absorption is rapid peak plasma concentration (Cmax) 1.25–2.5 h post-dose Dorzagliatin elimination half-life (t1/2) for dorzagliatin is 4.5–8.6 h |
| 2023, Chow et al. [12] | 18 (6 M, 12 F) | 2 | Phase II: randomized, double-blind, cross-over study 8 GCK-MODY; 10 T2DM |
Single dose First group: 75 mg dorzagliatin (first visit), placebo (second visit) Second group: Placebo (first visit), 75 mg dorzagliatin (second visit) |
GCK-MODY, dorzagliatin significantly increased absolute and incremental second-phase ISRs Dorzagliatin improves β-cell glucose sensitivity in GCK-MODY Dorzagliatin increases basal prehepatic insulin secretion rates in T2DM Dorzagliatin restores GK enzymatic activity |
| 2023, Zeng et al. [13] (DREAM, longitudinal SEED study) | 69 (48 M, 21 F) | 52 | Phase III: randomized, double-blind, placebo-controlled (24 weeks), open-label (28 weeks) study Patients who completed the SEED trial (56), who achieved stable glycemic control with potential to sustain drug-free remission Glycemic control status was assessed at weeks 0, 12, 26, 39, and 52. |
T2DM remission, glycemic control status was assessed at weeks 0, 12, 26, 39, and 52. | Dorzagliatin leads to stable glycemic control and drug-free remission in drug-naïve patients with T2DM Dorzagliatin sustains HbA1c and FPG levels and glucose homeostasis Dorzagliatin maintains the steady-state β-cell function and insulin resistance |
| 2023, Chen et al. [14] | 15 | 2 | Phase I: open-label, single-sequence, multiple-dose, single-center Dorzagliatin + sitagliptin in obese T2DM patients |
Day 1–5: sitagliptin 100 mg QD on Day 1–5 Day 6–10: sitagliptin 100 mg QD and dorzagliatin 75 mg BID Day 11–15: dorzagliatin 75 mg BID alone |
Combination treatment did not increase AEs and was well-tolerated in T2DM No pharmacokinetic interactions between dorzagliatin and sitagliptin Improvement of glycemic control under combination conditions |
ΔC30/ΔG30, dynamic state parameter; %B, steady-state HOMA 2 parameter; AE, adverse event; AUC, last quantifiable concentration; BID, twice a day; Cmax, maximum plasma concentration; F, female; FG, fasting glucagon; FPG, fasting plasma glucose; GK, glucokinase; HOMA2, Homeostatic Model Assessment 2; IR, insulin resistance; M, male; OGTT, oral glucose tolerance test; PPG, post-prandial glucose; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglyceride; URTI, upper respiratory tract infection; QD, once a day.
2. Dorzagliatin: Preclinical Studies
Using male Sprague–Dawley rats, a preclinical study was conducted to evaluate the effectiveness of dorzagliatin in improving glucose metabolism. The rats were first induced to develop T2DM by administering a combination of a high-fat diet and streptozotocin. Subsequently, the diabetic rats were divided into four groups: a control group, a diabetic group, a group receiving 10 mg/kg of dorzagliatin, and a group receiving 30 mg/kg of dorzagliatin. After 27 days of treatment, dorzagliatin significantly reduced FPG levels by approximately 18% and 23% in the 10 mg/kg and 30 mg/kg treatment groups, respectively. Dorzagliatin treatment also modestly increased fasting glucagon (FG) levels while significantly decreasing fasting plasma insulin (FINS) levels. Oral glucose tolerance test (OGTT) results demonstrated a noticeable improvement in glucose tolerance in dorzagliatin-treated rats compared to untreated diabetic rats. Immunohistochemical analyses of dorzagliatin-treated rats showed a notably higher number of GK-immunopositive cells in the liver and insulin-immunopositive cells in the pancreas, as compared to that in untreated animal tissues. Western blot analysis further corroborated these findings, showing a significantly higher expression of GK proteins in the 30 mg/kg dorzagliatin-treated group than in the diabetic group. The results of GK gene expression aligned with those of the Western blot analysis. These findings suggest that dorzagliatin exerts beneficial effects on GK activity, IR, and glucose metabolism in diabetic rats. Further studies are warranted to validate these results in humans [2].
3. Dorzagliatin: Clinical Studies
In a phase I trial involving 60 healthy volunteers, dorzagliatin was found to be safe and well-tolerated at single doses ranging from 5 to 50 mg. The drug exhibited linear pharmacokinetics with minimal urinary metabolites, suggesting that renal excretion is a minor elimination pathway. Dorzagliatin demonstrated a dose-dependent reduction in FPG, reaching up to 31.49% at the maximum tested dose of 50 mg. Preprandial plasma samples showed no insulin secretion, while postprandial samples revealed a dose-dependent rise in insulin levels. Notably, a comparable incidence of AEs was observed in both the dorzagliatin and placebo groups, at 14.6% and 16.7%, respectively. The AEs were mild and included dizziness, palpitation, belching, cold sweats, and proteinuria [4].
In a randomized 4-week study involving Chinese T2DM patients with well-defined disease biomarkers, it was demonstrated that administering 75 mg dorzagliatin once (QD) or twice (BID) daily significantly reduced glycated hemoglobin (HbA1c) levels, FPG, 2-h post-prandial glucose (PPG), and 24-h glucose area under the curve (AUC). Dorzagliatin also substantially increased β-cell function, with HOMA2-B and ΔC30/ΔG30 increasing by approximately 40% and 168%, respectively. The drug was generally safe and well-tolerated, with mild hypoglycemia being the most common AE [5].
A 12-week phase II multicenter, randomized, double-blind, placebo-controlled study in China found that dorzagliatin is a safe and effective treatment for patients with T2DM. Patients who received dorzagliatin had dose-dependent reductions in HbA1c levels, with the 50 mg or 75 mg BID groups experiencing the most significant reductions. Almost half of the patients in these groups achieved the target HbA1c level of <7.0%. The 75 mg BID regimen also showed the highest decrease in FPG levels. Drug-naïve patients had a greater reduction in HbA1c levels than previously treated patients. The study recommended 75 mg BID as the minimum effective dose for achieving glycemic control with a favorable safety profile. After drug withdrawal, there were sustained improvements in the disposition index and HOMA for insulin resistance. Most treatment-emergent adverse events (TEAEs) were mild, including upper respiratory tract infections, hyperuricemia, and dizziness. There were similar rates of mild hypoglycemia across all dorzagliatin subgroups [6][7]
The phase III randomized, double-blind, placebo-controlled SEED clinical trial evaluated the long-term safety and efficacy of dorzagliatin in newly diagnosed, drug-naïve T2DM patients who failed to control their blood glucose levels with diet and exercise. Patients were randomly assigned to either the dorzagliatin or placebo group for 24 weeks, followed by 28 weeks of open-label dorzagliatin treatment for all patients. After 24 weeks of treatment, dorzagliatin significantly reduced HbA1c levels compared to those of the placebo group, and a higher proportion of dorzagliatin-treated patients achieved HbA1c levels of <7.0%. Dorzagliatin significantly reduced FPG and 2-hour postprandial glucose (PPG) levels, while enhancing β-cell function. Throughout the open-label treatment period (weeks 24–52), the decline in HbA1c levels was consistently notable. Furthermore, the transition from placebo to dorzagliatin in the open-label period resulted in a significant reduction in HbA1c levels. The frequency of AEs was similar between the two groups, with no instances of severe hypoglycemia events, only one case, and no drug-related serious AEs observed in the dorzagliatin group. These observations suggest that dorzagliatin is a safe, well-tolerated, and effective long-term treatment for newly diagnosed T2DM patients [8][9].
The phase III randomized double-blind placebo-controlled DAWN clinical trial evaluated the efficacy of dorzagliatin as an add-on therapy in T2DM patients who had inadequate glycemic control with metformin monotherapy. After 24 weeks of treatment, dorzagliatin significantly reduced HbA1c levels compared to those of the placebo group. Approximately 44% of patients treated with dorzagliatin and metformin achieved an HbA1c level of <7%, compared to only 10% of patients treated with placebo and metformin. Dorzagliatin also significantly reduced FPG and PPG levels and improved β-cell function and insulin sensitivity. At the end of the open-label treatment period, weeks 24–52, reduced HbA1c levels were observed in the dorzagliatin and metformin group. Furthermore, switching from the placebo control to dorzagliatin and metformin dual therapy during the open-label period resulted in a significant reduction in HbA1c levels. Dorzagliatin was safe and well-tolerated, with a low incidence of hypoglycemia. These findings suggest that dorzagliatin as an add-on therapy is effective and safe [10].
A study involving patients with end-stage renal disease (ESRD) was conducted to evaluate the effects of renal impairment (RI) on the pharmacokinetics and safety of dorzagliatin. The study also investigated the appropriate dose for diabetes control in patients with diabetic kidney disease (DKD). Patients with ESRD were matched with healthy volunteers. The study concluded that systemic exposure to dorzagliatin is not clinically affected by ESRD, and no dose-adjustment was required for patients with DKD with various levels of RI. Dorzagliatin (25 mg QD) was well-tolerated in both groups, i.e., non-dialysis ESRD patients and healthy volunteers. The TEAEs reported were mild, with only one case of mild hypoglycemia reported in a healthy volunteer. In the non-dialysis ESRD group, patients treated with dorzagliatin experienced headaches and dry mouths and showed an increase in blood alkaline phosphatase [11].
A recent double-blind, randomized, crossover study investigated the effects of 75 mg dorzagliatin on insulin secretion rates (ISRs) and β-cell glucose sensitivity (βCGS) in participants with GCK-MODY and recent-onset T2DM. The study found that dorzagliatin lowered basal blood glucose levels in participants with GCK-MODY but had no effect on steady-state blood glucose levels in either group. In patients with T2DM, dorzagliatin increased ISRs compared to those of the placebo. Meanwhile, in patients with GCK-MODY, dorzagliatin increased βCGS compared to that of the placebo. In vitro studies on patients with GCK-MODY demonstrated that dorzagliatin restores glucose sensing in wild-type and mutant GK enzyme activity. Altogether, dorzagliatin was well-tolerated in both groups [12].
The DREAM study was a longitudinal follow-up of the SEED clinical trial that was conducted to investigate the long-term effects of dorzagliatin in newly diagnosed patients with T2DM who had previously achieved stable glycemic control and sustained disease remission. In this study, T2DM remission was achieved with a notable improvement in β-cell function, insulin sensitivity, and time in range (TIR). Additionally, the Kaplan–Meier and the American Diabetes Association (ADA) remission probabilities were 65.2% (at week 52) and 52.0% (at week 12), respectively. Dorzagliatin emerged as an effective and safe treatment for newly diagnosed T2DM patients. In conclusion, dorzagliatin treatment demonstrated the potential to achieve sustained glycemic control and drug-free diabetes remission in these patients [13].
A recent phase I clinical trial was conducted to evaluate the safety, pharmacokinetics, and pharmacodynamics of dorzagliatin when co-administered with sitagliptin in patients with obesity and T2DM. The trial found that there were no clinically meaningful pharmacokinetic interactions between dorzagliatin and sitagliptin. The combination treatment was well-tolerated and did not increase the incidence of AEs. Additionally, the combination treatment demonstrated improved glycemic control compared to that of either monotherapy. These findings suggest that the co-administration of dorzagliatin and sitagliptin may represent a promising treatment option for patients with T2DM and obesity [14].
References
- Liu, W.; Yao, C.; Shang, Q.; Liu, Y.; Liu, C.; Meng, F. Insights into the binding of dorzagliatin with glucokinase: A molecular dynamics simulation. J. Theor. Comput. Chem. 2020, 19, 2050027.
- Wang, P.; Liu, H.; Chen, L.; Duan, Y.; Chen, Q.; Xi, S. Effects of a Novel Glucokinase Activator, HMS5552, on Glucose Metabolism in a Rat Model of Type 2 Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 5812607.
- Kumari, V.; Li, C. Comparative docking assessment of glucokinase interactions with its allosteric activators. Curr. Chem. Genom. 2008, 2, 76–89.
- Xu, H.; Sheng, L.; Chen, W.; Yuan, F.; Yang, M.; Li, H.; Li, X.; Choi, J.; Zhao, G.; Hu, T.; et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of novel glucokinase activator HMS5552: Results from a first-in-human single ascending dose study. Drug Des. Dev. Ther. 2016, 10, 1619–1626.
- Zhu, X.X.; Zhu, D.L.; Li, X.Y.; Li, Y.L.; Jin, X.W.; Hu, T.X.; Zhao, Y.; Li, Y.G.; Zhao, G.Y.; Ren, S.; et al. Dorzagliatin (HMS5552), a novel dual-acting glucokinase activator, improves glycaemic control and pancreatic beta-cell function in patients with type 2 diabetes: A 28-day treatment study using biomarker-guided patient selection. Diabetes Obes. Metab. 2018, 20, 2113–2120.
- Zhu, D.; Gan, S.; Liu, Y.; Ma, J.; Dong, X.; Song, W.; Zeng, J.; Wang, G.; Zhao, W.; Zhang, Q.; et al. Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: A dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Diabetes Endocrinol. 2018, 6, 627–636.
- Zhu, D.; Zhao, Y.; Tang, C., Sr.; Tianxin, H.; Li, Y.-G.; Zhao, G.; Hou, X., Sr.; Zhang, Y.; Chen, L.; HMM0201 Study Group. Pharmacodynamics Post-Hoc Analysis of Glucose Kinase Activator Dorzagliatin (HMS5552)—Twelve Weeks Treatment in T2D Patients in China. Diabetes 2018, 67 (Suppl. S1), 1201-P.
- Zhu, D.; Zhang, Y.; Chen, L. 182-OR: A novel dual-acting glucokinase activator (GKA) dorzagliatin (HMS5552) achieved primary efficacy endpoint with good safety profiles in T2DM patients after 24 weeks of treatment in a phase III monotherapy trial. Diabetes 2020, 69 (Suppl. S1), 182-OR.
- Zhu, D.; Li, X.; Ma, J.; Zeng, J.; Gan, S.; Dong, X.; Yang, J.; Lin, X.; Cai, H.; Song, W.; et al. Dorzagliatin in drug-naive patients with type 2 diabetes: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2022, 28, 965–973.
- Yang, W.; Zhu, D.; Gan, S.; Dong, X.; Su, J.; Li, W.; Jiang, H.; Zhao, W.; Yao, M.; Song, W.; et al. Dorzagliatin add-on therapy to metformin in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2022, 28, 974–981.
- Miao, J.; Fu, P.; Ren, S.; Hu, C.; Wang, Y.; Jiao, C.; Li, P.; Zhao, Y.; Tang, C.; Qian, Y.; et al. Effect of renal impairment on the pharmacokinetics and safety of dorzagliatin, a novel dual-acting glucokinase activator. Clin. Transl. Sci. 2022, 15, 548–557.
- Chow, E.; Wang, K.; Lim, C.K.P.; Tsoi, S.T.F.; Fan, B.; Poon, E.; Luk, A.O.Y.; Ma, R.C.W.; Ferrannini, E.; Mari, A.; et al. Dorzagliatin, a Dual-Acting Glucokinase Activator, Increases Insulin Secretion and Glucose Sensitivity in Glucokinase Maturity-Onset Diabetes of the Young and Recent-Onset Type 2 Diabetes. Diabetes 2023, 72, 299–308.
- Zeng, J.; Gan, S.; Mi, N.; Liu, Y.; Su, X.; Zhang, W.; Zhang, J.; Yu, F.; Dong, X.; Han, M.; et al. Diabetes remission in drug-naïve patients with type 2 diabetes after dorzagliatin treatment: A prospective cohort study. Diabetes Obes. Metab. 2023, 25, 2878–2887.
- Chen, L.; Zhang, J.; Sun, Y.; Zhao, Y.; Liu, X.; Fang, Z.; Feng, L.; He, B.; Zou, Q.; Tracey, G.J. A phase I open-label clinical trial to study drug-drug interactions of Dorzagliatin and Sitagliptin in patients with type 2 diabetes and obesity. Nat. Commun. 2023, 14, 1405.
More
Information
Subjects:
Medicine, General & Internal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
09 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




