Achieving glycemic control and sustaining functional pancreatic β-cell activity remains an unmet medical need in the treatment of type 2 diabetes mellitus (T2DM). Glucokinase activators (GKAs) constitute a class of anti-diabetic drugs designed to regulate blood sugar levels and enhance β-cell function in patients with diabetes. A significant progression in GKA development is underway to address the limitations of earlier generations. Dorzagliatin, a dual-acting GKA, targets both the liver and pancreas and has successfully completed two phase III trials, demonstrating favorable results in diabetes treatment.
- dorzagliatin
- TTP399
- glucokinase activator
- type 2 diabetes
1. Dorzagliatin: Mechanisms of Action
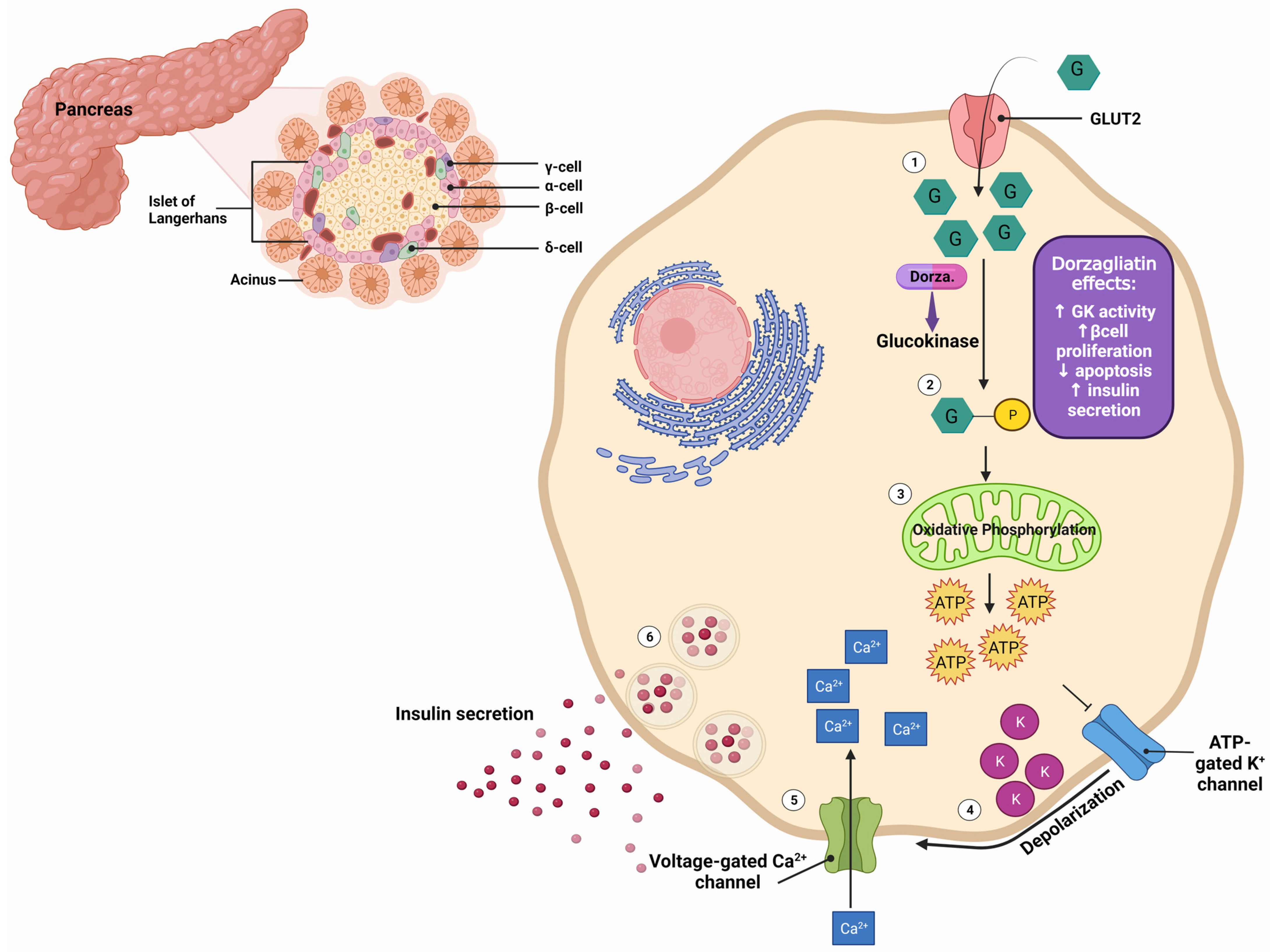
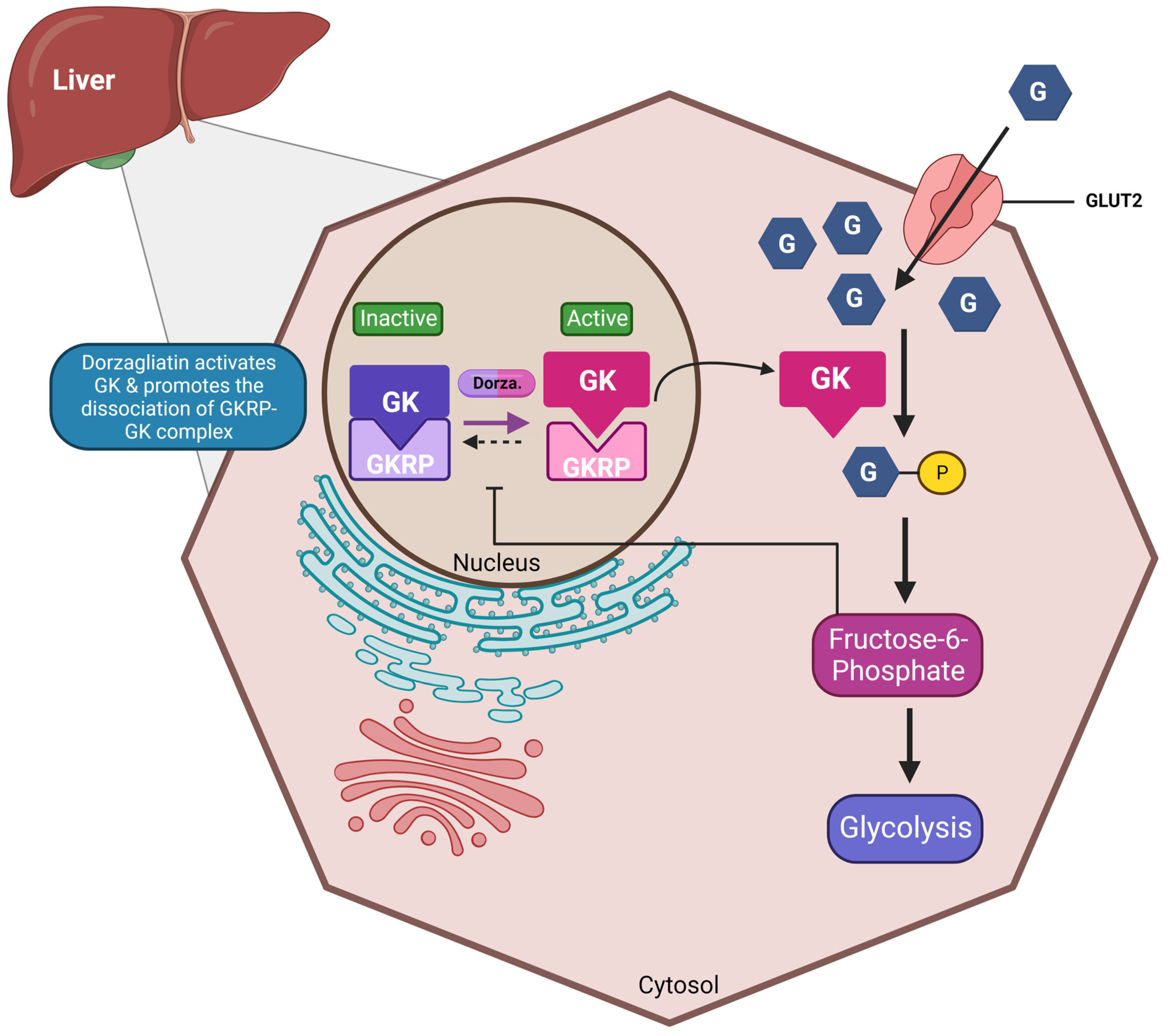
| Preclinical Studies | |||||
|---|---|---|---|---|---|
| Reference | Animal | Duration (Weeks) | Interventions | Primary Findings | |
| 2017, Wang et al. [2] | Male Sprague–Dawley (SD) rats with T2DM | 4 | Control, diabetic, 10 mg/kg, 30 mg/kg | Reduction in FPG by ∼18% (10 mg/kg) and 23% (30 mg/kg) Reduction in FINS: 28.40 mU/L (10 mg/kg) and 18.74 mU/L (30 mg/kg) Levels of TC and TG unchanged Increase in FG: 43 pg/mL (10 mg/kg) and 51 pg/mL (30 mg/kg) Reduction in OGTT: 9 mmol/L (10 mg/kg) and 7 mmol/L (30 mg/kg) compared to diabetic rats Increased expression of GK-immunopositive cells and insulin (Western blot) |
|
| Clinical Trials | |||||
| Reference | Total Participants (N) | Duration (weeks) | Study design | Interventions | Primary findings |
| 2016, Xu et al. [4] | 60 (31 M, 29 F) | XXX | Phase Ia: randomized, double-blind, placebo-controlled, parallel-group, administered to healthy subjects | Six dose cohorts (5, 10, 15, 25, 35, and 50 mg), 10 randomized subjects (8 receiving HMS5552 and 2 receiving placebo) | HMS5552 at doses up to 50 mg in healthy subjects is safe and well-tolerated Dose-related glucose-lowering effects and post-prandial insulin secretion AEs: belching, dizziness, palpitation, cold sweat, and proteinuria No hypoglycemia |
| 2018, Zhu et al. [5] | 24 (17 M, 7 F) | 4 | T2DM patients randomized at 1:1 ratio to receive two concentrations of dorzagliatin | 75 mg QD, 75 mg BID | Overall, QD treatment was better than BID Decrease in HBA1c, FPG and PPG Increase in C-peptide Reduction in AUC Increase in HOMA2 parameter %B Increase in the dynamic state parameter ΔC30/ΔG30 Hypoglycemia: 17% |
| 2018, Zhu et al. [6][7] | 258 (154 M, 104 F) | 12 | Phase II: multicenter, randomized, double-blind, placebo-controlled T2DM patients were on a diet and exercise regimen; drug-naïve or previously treated with metformin or α-glucosidase inhibitor monotherapy |
75 or 100 mg QD, 50 or 75 mg BID, placebo | Decrease in HbA1c, FPG, and PPG, particularly in patients who received 100 QD or 75 BID AE: URTi, hyperuricemia, dizziness Hypoglycemia 6% in all studied groups |
| 2020–2022, Zhu et al. (SEED trial) [8][9] | 463 (301 M, 162 F) | 52 | Phase III: multicenter, randomized, double-blind, placebo-controlled (24 weeks), open-label (28 weeks) study T2DM drug-naïve patients |
75 mg BID, placebo | Decrease in HbA1c and FPG at week 24, sustained through week 52 Increase in HOMA2-β AEs: URTi, hyperlipidemia, proteinuria, abnormal hepatic function, hypertension Hypoglycemia 0.3% in all studied groups |
| 2022, Yang et al. (DAWN trial) [10] | 767 (475 M, 292 F) | 52 | Phase III: randomized, double-blind, placebo-controlled (24 weeks), open-label (28 weeks) study T2DM add-on therapy to metformin |
75 mg BID, placebo, add-on metformin 1500 mg | Decrease in HbA1c < 7% in 44.4% patients at week 24 Decrease in FPG and HOMA2-IR Increase in HOMA2-β Hypoglycemia 0.3%, no weight gain in all studied groups |
| 2022, Miao et al. [11] | 17 (7 M, 10 F) | ½ | Open-label, single-dose, sequential two-part, parallel-group study 8 non-dialysis ESRD, including 1 T2DM; 9 healthy volunteers |
25 mg QD | End-stage renal insufficiency does not affect dorzagliatin efficacy Dorzagliatin absorption is rapid peak plasma concentration (Cmax) 1.25–2.5 h post-dose Dorzagliatin elimination half-life (t1/2) for dorzagliatin is 4.5–8.6 h |
| 2023, Chow et al. [12] | 18 (6 M, 12 F) | 2 | Phase II: randomized, double-blind, cross-over study 8 GCK-MODY; 10 T2DM |
Single dose First group: 75 mg dorzagliatin (first visit), placebo (second visit) Second group: Placebo (first visit), 75 mg dorzagliatin (second visit) |
GCK-MODY, dorzagliatin significantly increased absolute and incremental second-phase ISRs Dorzagliatin improves β-cell glucose sensitivity in GCK-MODY Dorzagliatin increases basal prehepatic insulin secretion rates in T2DM Dorzagliatin restores GK enzymatic activity |
| 2023, Zeng et al. [13] (DREAM, longitudinal SEED study) | 69 (48 M, 21 F) | 52 | Phase III: randomized, double-blind, placebo-controlled (24 weeks), open-label (28 weeks) study Patients who completed the SEED trial (56), who achieved stable glycemic control with potential to sustain drug-free remission Glycemic control status was assessed at weeks 0, 12, 26, 39, and 52. |
T2DM remission, glycemic control status was assessed at weeks 0, 12, 26, 39, and 52. | Dorzagliatin leads to stable glycemic control and drug-free remission in drug-naïve patients with T2DM Dorzagliatin sustains HbA1c and FPG levels and glucose homeostasis Dorzagliatin maintains the steady-state β-cell function and insulin resistance |
| 2023, Chen et al. [14] | 15 | 2 | Phase I: open-label, single-sequence, multiple-dose, single-center Dorzagliatin + sitagliptin in obese T2DM patients |
Day 1–5: sitagliptin 100 mg QD on Day 1–5 Day 6–10: sitagliptin 100 mg QD and dorzagliatin 75 mg BID Day 11–15: dorzagliatin 75 mg BID alone |
Combination treatment did not increase AEs and was well-tolerated in T2DM No pharmacokinetic interactions between dorzagliatin and sitagliptin Improvement of glycemic control under combination conditions |
2. Dorzagliatin: Preclinical Studies
3. Dorzagliatin: Clinical Studies
This entry is adapted from the peer-reviewed paper 10.3390/ijms25010571
References
- Liu, W.; Yao, C.; Shang, Q.; Liu, Y.; Liu, C.; Meng, F. Insights into the binding of dorzagliatin with glucokinase: A molecular dynamics simulation. J. Theor. Comput. Chem. 2020, 19, 2050027.
- Wang, P.; Liu, H.; Chen, L.; Duan, Y.; Chen, Q.; Xi, S. Effects of a Novel Glucokinase Activator, HMS5552, on Glucose Metabolism in a Rat Model of Type 2 Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 5812607.
- Kumari, V.; Li, C. Comparative docking assessment of glucokinase interactions with its allosteric activators. Curr. Chem. Genom. 2008, 2, 76–89.
- Xu, H.; Sheng, L.; Chen, W.; Yuan, F.; Yang, M.; Li, H.; Li, X.; Choi, J.; Zhao, G.; Hu, T.; et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of novel glucokinase activator HMS5552: Results from a first-in-human single ascending dose study. Drug Des. Dev. Ther. 2016, 10, 1619–1626.
- Zhu, X.X.; Zhu, D.L.; Li, X.Y.; Li, Y.L.; Jin, X.W.; Hu, T.X.; Zhao, Y.; Li, Y.G.; Zhao, G.Y.; Ren, S.; et al. Dorzagliatin (HMS5552), a novel dual-acting glucokinase activator, improves glycaemic control and pancreatic beta-cell function in patients with type 2 diabetes: A 28-day treatment study using biomarker-guided patient selection. Diabetes Obes. Metab. 2018, 20, 2113–2120.
- Zhu, D.; Gan, S.; Liu, Y.; Ma, J.; Dong, X.; Song, W.; Zeng, J.; Wang, G.; Zhao, W.; Zhang, Q.; et al. Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: A dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Diabetes Endocrinol. 2018, 6, 627–636.
- Zhu, D.; Zhao, Y.; Tang, C., Sr.; Tianxin, H.; Li, Y.-G.; Zhao, G.; Hou, X., Sr.; Zhang, Y.; Chen, L.; HMM0201 Study Group. Pharmacodynamics Post-Hoc Analysis of Glucose Kinase Activator Dorzagliatin (HMS5552)—Twelve Weeks Treatment in T2D Patients in China. Diabetes 2018, 67 (Suppl. S1), 1201-P.
- Zhu, D.; Zhang, Y.; Chen, L. 182-OR: A novel dual-acting glucokinase activator (GKA) dorzagliatin (HMS5552) achieved primary efficacy endpoint with good safety profiles in T2DM patients after 24 weeks of treatment in a phase III monotherapy trial. Diabetes 2020, 69 (Suppl. S1), 182-OR.
- Zhu, D.; Li, X.; Ma, J.; Zeng, J.; Gan, S.; Dong, X.; Yang, J.; Lin, X.; Cai, H.; Song, W.; et al. Dorzagliatin in drug-naive patients with type 2 diabetes: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2022, 28, 965–973.
- Yang, W.; Zhu, D.; Gan, S.; Dong, X.; Su, J.; Li, W.; Jiang, H.; Zhao, W.; Yao, M.; Song, W.; et al. Dorzagliatin add-on therapy to metformin in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2022, 28, 974–981.
- Miao, J.; Fu, P.; Ren, S.; Hu, C.; Wang, Y.; Jiao, C.; Li, P.; Zhao, Y.; Tang, C.; Qian, Y.; et al. Effect of renal impairment on the pharmacokinetics and safety of dorzagliatin, a novel dual-acting glucokinase activator. Clin. Transl. Sci. 2022, 15, 548–557.
- Chow, E.; Wang, K.; Lim, C.K.P.; Tsoi, S.T.F.; Fan, B.; Poon, E.; Luk, A.O.Y.; Ma, R.C.W.; Ferrannini, E.; Mari, A.; et al. Dorzagliatin, a Dual-Acting Glucokinase Activator, Increases Insulin Secretion and Glucose Sensitivity in Glucokinase Maturity-Onset Diabetes of the Young and Recent-Onset Type 2 Diabetes. Diabetes 2023, 72, 299–308.
- Zeng, J.; Gan, S.; Mi, N.; Liu, Y.; Su, X.; Zhang, W.; Zhang, J.; Yu, F.; Dong, X.; Han, M.; et al. Diabetes remission in drug-naïve patients with type 2 diabetes after dorzagliatin treatment: A prospective cohort study. Diabetes Obes. Metab. 2023, 25, 2878–2887.
- Chen, L.; Zhang, J.; Sun, Y.; Zhao, Y.; Liu, X.; Fang, Z.; Feng, L.; He, B.; Zou, Q.; Tracey, G.J. A phase I open-label clinical trial to study drug-drug interactions of Dorzagliatin and Sitagliptin in patients with type 2 diabetes and obesity. Nat. Commun. 2023, 14, 1405.
