Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carlo Covello | -- | 1982 | 2024-01-08 23:14:04 | | | |
| 2 | Rita Xu | -124 word(s) | 1858 | 2024-01-09 07:39:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Del Gaudio, A.; Covello, C.; Di Vincenzo, F.; De Lucia, S.S.; Mezza, T.; Nicoletti, A.; Siciliano, V.; Candelli, M.; Gasbarrini, A.; Nista, E.C. Drug-Induced Acute Pancreatitis in Adults. Encyclopedia. Available online: https://encyclopedia.pub/entry/53575 (accessed on 07 February 2026).
Del Gaudio A, Covello C, Di Vincenzo F, De Lucia SS, Mezza T, Nicoletti A, et al. Drug-Induced Acute Pancreatitis in Adults. Encyclopedia. Available at: https://encyclopedia.pub/entry/53575. Accessed February 07, 2026.
Del Gaudio, Angelo, Carlo Covello, Federica Di Vincenzo, Sara Sofia De Lucia, Teresa Mezza, Alberto Nicoletti, Valentina Siciliano, Marcello Candelli, Antonio Gasbarrini, Enrico Celestino Nista. "Drug-Induced Acute Pancreatitis in Adults" Encyclopedia, https://encyclopedia.pub/entry/53575 (accessed February 07, 2026).
Del Gaudio, A., Covello, C., Di Vincenzo, F., De Lucia, S.S., Mezza, T., Nicoletti, A., Siciliano, V., Candelli, M., Gasbarrini, A., & Nista, E.C. (2024, January 08). Drug-Induced Acute Pancreatitis in Adults. In Encyclopedia. https://encyclopedia.pub/entry/53575
Del Gaudio, Angelo, et al. "Drug-Induced Acute Pancreatitis in Adults." Encyclopedia. Web. 08 January, 2024.
Copy Citation
Acute pancreatitis (AP) is an acute inflammation of the pancreas caused by the activation of digestive enzymes in the pancreatic tissue. The main causes of AP are cholelithiasis and alcohol abuse; less commonly, it can be caused by drugs, with a prevalence of up to 5%. Causal associations between drugs and pancreatitis are largely based on case reports or case series with limited evidence.
acute pancreatitis
drug-induced acute pancreatitis
antimicrobial drugs
antiviral drugs
1. Introduction
Acute pancreatitis (AP) is an acute inflammation of the pancreas resulting from intrapancreatic activation of digestive enzymes. This condition can lead to pancreatic necrosis, organ failure, and multiple organ dysfunction, with a mortality rate of 1–5% [1][2]. In addition, acute pancreatitis may be associated with significant short- and long-term morbidity, with recurrent symptoms and, in severe cases, exocrine and/or endocrine pancreatic failure [3]. The diagnosis of AP requires the identification of two of the following criteria: (1) abdominal pain suggestive of pancreatitis, (2) serum amylase or lipase levels at least three times the upper normal limit, and (3) imaging findings consistent with pancreatitis on computed tomography (CT) or magnetic resonance imaging (MRI) [1][4][5]. It is clear that the first two criteria alone are sufficient for diagnosis; thus, imaging can be performed later to better identify complications of acute pancreatitis. AP can be classified into mild, moderate, and severe forms according to the Atlanta criteria [1].
Mild acute pancreatitis is not associated with local or systemic complications and often resolves within the first week. Usually, mild AP does not result in organ failure. It is the most common form. Moderate AP is associated with local complications or exacerbation of concomitant diseases; transient organ failure may occur. In contrast, severe acute pancreatitis is defined by persistent organ failure (i.e., organ failure > 48 h). Local complications include pancreatic and peripancreatic necrosis (sterile or infected), peripancreatic fluid collections, pseudocysts, and parietal necrosis (sterile or infected) [1]. The main causes of AP are cholelithiasis and alcohol abuse, which account for more than 80% of cases [6][7]. Less commonly, it can also be caused by medications, with a prevalence ranging from 2 to 5.3% [3][7].
2. Drug-Induced Pancreatitis
According to the Agency for Drugs and Medical Devices Adverse Drug Event Reporting Database, drug-induced acute pancreatitis (DIAP) accounted for 0.17% of all adverse drug reactions (ADRs) [8]. Pharmacologic agents potentially responsible for acute pancreatitis include nearly 200 drugs [8][9]. However, causal relationships are largely based on case reports or case series with limited evidence. Consequently, an important indication of a causal relationship between a drug and AP is the recurrence of AP after reintroduction of the drug after discontinuation and recovery from a previous episode [10]. However, for ethical reasons, drugs in which rechallenge episodes are performed are quite rare. Another key concept in determining the association between a particular drug and an adverse event is latency; latency is the time between the assumption of the drug and the occurrence of AP. Several systematic reviews use latency categories (e.g., <24 h, 1–30 days, >30 days), and if >75% of case reports for a drug fell into the same latency category, this was considered good evidence of an association (adequate latency) [9][11][12]. Currently, only three drugs have been associated with the development of AP in a randomized controlled trial (6-mercaptopurine, azathioprine, and didanosine) [9][11][12][13].
In 2007, based on case reports in the literature, Badalov et al. classified drugs potentially responsible for the development of AP into probability classes based on case reports from the literature (Table 1) [14]. This classification was revised in 2019 by C.R. Simons-Linares et al. The first class includes drugs with a positive rechallenge. This class is further divided into two subclasses, a and b, depending on whether other possible causes were excluded or not. Class II includes drugs for which at least four case reports show similar latency. Class III includes drugs for which there are at least two case reports, but which do not meet the criteria of the above classes. The final class (IV) includes the drugs with the weakest evidence, for which only one case report was published [12]. This classification was updated by Wolfe et al. in 2020 [11].
Table 1. The classification of drug-induced pancreatitis, according to Badalov, updated by Simons-Linares et al. [13].
| Class | Description |
|---|---|
| Ia | At least 1 case report with positive rechallenge, with exclusion of all other causes. |
| Ib | At least 1 case report with positive rechallenge, failing to document exclusion of other causes or other possible etiologies were available. |
| II | At least 4 cases in the literature with consistent latency. |
| III | At least 2 cases in the literature with no consistent latency among cases and no rechallenge. |
| IV | Drugs not fitting into the earlier described classes. |
However, there are some differences between these two classifications; methodologically, Wolfe et al. developed the rules of their classification a priori, before performing the literature search, whereas the system of C.R. Simons-Linares et al. is based on their review data. In addition, Wolfe et al. applied more stringent criteria in selecting case reports by including only cases of AP diagnosed according to currently accepted diagnostic criteria and excluding cases in which AP was associated with drug combinations (Table 2). In this classification, the classes differ from the previous one; class I was further divided into three subclasses (a, b, c). Class Ic includes drugs for which there is at least one case report with no positive rechallenge, excluding other causes. Drugs for which there are at least two case reports without evidence of rechallenge but with and without exclusion of other causes are classified as class II and class III, respectively. With adequate latency in class II and insufficient latency in class III [11].
Table 2. The classification of drug-induced pancreatitis, according to Wolfe et al. [11].
| Class | Description |
|---|---|
| Ia | At least 1 case report with positive rechallenge, with exclusion of all other causes. |
| Ib | At least 1 case report with positive rechallenge, failing to document exclusion of other causes or other possible etiologies were available. |
| Ic | At least 1 case report in humans, without a positive rechallenge, other causes are ruled out. |
| II | At least 2 cases in humans reported in the literature, without a positive rechallenge, with consistent latency, and other causes, were not ruled out. |
| III | At least 2 cases in humans reported in the literature, without a positive rechallenge, with inconsistent latency, and other causes, were not ruled out. |
| IV | Drugs not fitting into the earlier described classes. |
More recently, Saini et al. have presented another classification based on an evidence-based approach that includes a comprehensive review of randomized controlled trials (RCTs), cohort studies, pharmacoepidemiologic analyses, and case reports and differs from previous classifications. Table 3 provides an overview of this classification system. They divided drugs considered to trigger AP into four groups according to the quality of the evidence. Thus, class I included RCTs (high evidence), whereas case–control studies and pharmacoepidemiologic studies were classified as class II. Classes III (divided into a, b, and c) and IV included case reports according to quality, presence of rechallenge, adequate latency, and number of reports [9].
Table 3. The classification of drug-induced pancreatitis, according to Saini et al. [9].
| Class | Description |
|---|---|
| I | High quality of evidence for causation of acute pancreatitis: randomized controlled clinical trials. |
| II | Moderate quality of evidence for causation of acute pancreatitis: case–control studies and/or pharmacoepidemiology studies. |
| IIIa | Case reports showing “rechallenge and consistent latency”. |
| IIIb | Case report showing rechallenge only. |
| IIIc | Case report showing consistent latency only. |
| IV | Case Reports with no rechallenge or consistent latency. |
Main Mechanisms of Drug-Induced Pancreatitis
Most of the mechanisms underlying drug-induced pancreatic injury have not been fully elucidated or demonstrated. One of the most common is probably an idiosyncratic delayed immunologic or T-cell-mediated response rather than intrinsic toxicity [13][15]. This has been demonstrated for azathioprine, 6-MP, and sulfasalazine in both in vitro trials and population-based studies [10][16]. Idiosyncratic reactions do not appear to be directly related to dose, and symptoms differ from the pharmacologic effect of the drug. The proposed mechanism for idiosyncratic reactions to drugs is not certain. However, it may be a reactive metabolite of the drug that binds to specific proteins and causes an immune system response (haptenes hypothesis). This response could be triggered by injury or cellular stress (hazard hypothesis) and stimulate the activation of an immune response by the co-stimulation of T lymphocytes [15].
Although the exact mechanism of AP, triggered by thiopurines and antibiotics, remains unknown, there is increasing evidence that certain human leukocyte antigen (HLA) alleles may be predisposed to this type of hypersensitive reaction [17].
Other proposed mechanisms include the hypothesis of a toxic effect on the pancreatic cell membrane (as in the case of valproate) or the formation of edema in the pancreatic ducts, which may occur with angiotensin-converting enzyme inhibitors via the kallikrein/kinin pathway [18]. In addition, some drug therapies have been associated with the occurrence of hypertriglyceridemia pancreatitis [19]. According to some studies, most antiviral protease inhibitors (PI) are associated with a significant increase in plasma triglyceride concentrations; in particular, hypertriglyceridemia is observed more frequently after combination therapy with ritonavir or lopinavir/ritonavir than with other PI -based combinations. As described below, ritonavir and lopinavir/ritonavir-based regimens have been associated with an increased risk of hyperlipidemic pancreatitis [19][20].
Another possible causative mechanism is mitochondrial toxicity [19][21]. This appears to be associated with the use of antiviral drugs such as nuclear reverse transcriptase inhibitors to treat human immunodeficiency virus (HIV) infections [10][22]. This toxicity appears to be due to the inhibition of mitochondrial DNA replication by suppression of the activity of key enzymes involved in mitochondrial DNA replication. Although acute pancreatitis is a well-described complication of HIV infection, an increased incidence of this condition has been reported after the introduction of reverse transcriptase inhibitors in HIV patients [21][23].
Considering antibiotics, it is postulated that tetracyclines may cause DIAP either through a toxin-mediated action of an unknown metabolite or a direct toxic effect on the pancreas due to the supratherapeutic bile concentration of tetracyclines [18]. Pharmacological experiments with tetracyclines have shown that the biliary concentration of minocycline is ten times higher than the serum concentration, and similar results have been observed with tigecycline [24][25].
Another speculative mechanism of metronidazole-induced pancreatitis is the production of redox cycling and hydrogen peroxide, superoxide, and other free radicals under aerobic conditions. These redox-active compounds are toxic to pancreatic β-cells. Oxygen free radicals have been associated with the initiation of pancreatitis [26]. Finally, in the case of opiates, the proposed mechanism is a stimulation of μ-receptors causing hypercontraction of the Oddi sphincter that increases the basal pressure and amplitude of contraction and induces a reflux of pancreatic enzymes into the pancreatic duct [27]. Figure 1 summarizes these mechanisms.
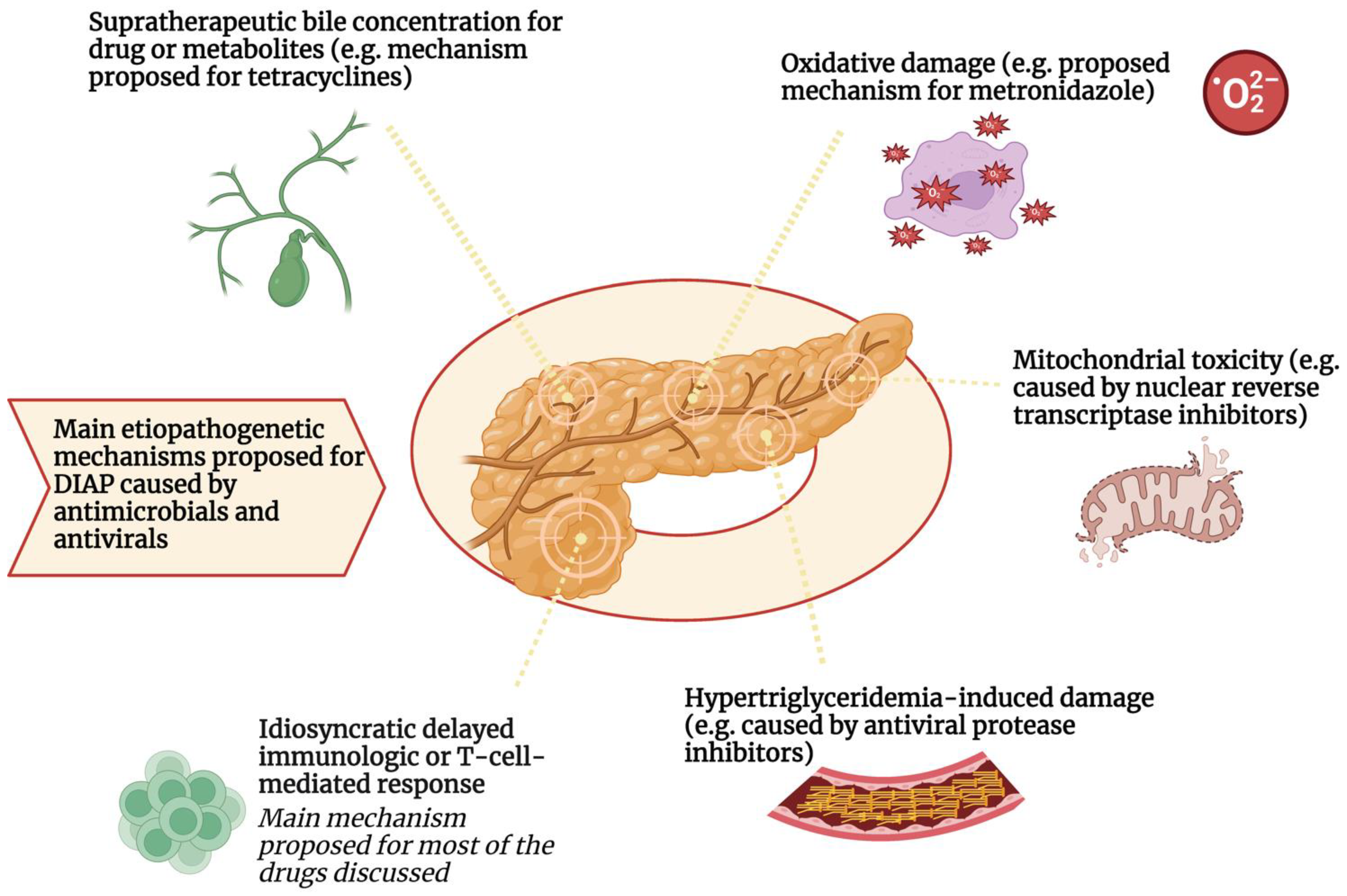
Figure 1. Main etiopathogenetic mechanisms proposed for DIAP caused by antimicrobials and antivirals.
DIAP: Drug-induced acute pancreatitis
The etiopathogenetic mechanism most involved in the development of DIAP from antimicrobials and antivirals appears to be the delayed idiosyncratic or lymphocyte-T-mediated mechanism. Pancreatic damage caused by the immune system also seems to be associated with certain human leukocyte antigen (HLA) alleles which may be predisposed to this type of hypersensitive reaction. Other pathogenetic mechanisms have been postulated for these drug classes. For example, hypertriglyceridemia induced by protease inhibitor antivirals may increase the risk of developing DIAP. Furthermore, mitochondrial toxicity, oxidative damage, and biliary supersaturation by metabolites or the drug itself have been suggested as additional mechanisms involved in DIAP by reverse transcriptase inhibitors, metronidazole, and tetracyclines, respectively.
References
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of Acute Pancreatitis—2012: Revision of the Atlanta Classification and Definitions by International Consensus. Gut 2013, 62, 102–111.
- Andersson, B.; Appelgren, B.; Sjödin, V.; Ansari, D.; Nilsson, J.; Persson, U.; Tingstedt, B.; Andersson, R. Acute Pancreatitis—Costs for Healthcare and Loss of Production. Scand. J. Gastroenterol. 2013, 48, 1459–1465.
- Szatmary, P.; Grammatikopoulos, T.; Cai, W.; Huang, W.; Mukherjee, R.; Halloran, C.; Beyer, G.; Sutton, R. Acute Pancreatitis: Diagnosis and Treatment. Drugs 2022, 82, 1251–1276.
- Rompianesi, G.; Hann, A.; Komolafe, O.; Pereira, S.P.; Davidson, B.R.; Gurusamy, K.S. Serum Amylase and Lipase and Urinary Trypsinogen and Amylase for Diagnosis of Acute Pancreatitis. Cochrane Database Syst. Rev. 2017, 4, CD012010.
- Kiriyama, S.; Gabata, T.; Takada, T.; Hirata, K.; Yoshida, M.; Mayumi, T.; Hirota, M.; Kadoya, M.; Yamanouchi, E.; Hattori, T.; et al. New Diagnostic Criteria of Acute Pancreatitis. J. Hepatobiliary Pancreat. Sci. 2010, 17, 24–36.
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Coward, S.; Forbes, N.; Heitman, S.J.; Shaheen, A.-A.; et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology 2022, 162, 122–134.
- Petrov, M.S.; Yadav, D. Global Epidemiology and Holistic Prevention of Pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 175–184.
- Niinomi, I.; Hosohata, K.; Oyama, S.; Inada, A.; Wakabayashi, T.; Iwanaga, K. Pharmacovigilance Assessment of Drug-Induced Acute Pancreatitis Using a Spontaneous Reporting Database. Int. J. Toxicol. 2019, 38, 487–492.
- Saini, J.; Marino, D.; Badalov, N.; Vugelman, M.; Tenner, S. Drug-Induced Acute Pancreatitis: An Evidence-Based Classification (Revised). Clin. Transl. Gastroenterol. 2023, 14, e00621.
- Sosnowski, K.; Nehring, P.; Przybyłkowski, A. Pancreas and Adverse Drug Reactions: A Literature Review. Drug Saf. 2022, 45, 929–939.
- Wolfe, D.; Kanji, S.; Yazdi, F.; Barbeau, P.; Rice, D.; Beck, A.; Butler, C.; Esmaeilisaraji, L.; Skidmore, B.; Moher, D.; et al. Drug Induced Pancreatitis: A Systematic Review of Case Reports to Determine Potential Drug Associations. PLoS ONE 2020, 15, e0231883.
- Roberto Simons-Linares, C.; Elkhouly, M.A.; Salazar, M.J. Drug-Induced Acute Pancreatitis in Adults: An Update. Pancreas 2019, 48, 1263–1273.
- Trivedi, C.D.; Pitchumoni, C.S. Drug-Induced Pancreatitis. J. Clin. Gastroenterol. 2005, 39, 709–716.
- Badalov, N.; Baradarian, R.; Iswara, K.; Li, J.; Steinberg, W.; Tenner, S. Drug-Induced Acute Pancreatitis: An Evidence-Based Review. Clin. Gastroenterol. Hepatol. 2007, 5, 648–661.e3.
- Dhir, R.; Brown, D.K.; Olden, K.W. Drug-Induced Pancreatitis: A Practical Review. Drugs Today 2007, 43, 499.
- Teich, N.; Mohl, W.; Bokemeyer, B.; Bündgens, B.; Büning, J.; Miehlke, S.; Hüppe, D.; Maaser, C.; Klugmann, T.; Kruis, W.; et al. Azathioprine-Induced Acute Pancreatitis in Patients with Inflammatory Bowel Diseases—A Prospective Study on Incidence and Severity. J. Crohns Colitis 2016, 10, 61–68.
- Pavlos, R.; Mallal, S.; Phillips, E. HLA and Pharmacogenetics of Drug Hypersensitivity. Pharmacogenomics 2012, 13, 1285–1306.
- Gerstner, T.; Büsing, D.; Bell, N.; Longin, E.; Kasper, J.-M.; Klostermann, W.; Hebing, B.; Hanefeld, F.; Eckel, U.; Hoffmann, R.; et al. Valproic Acid-Induced Pancreatitis: 16 New Cases and a Review of the Literature. J. Gastroenterol. 2007, 42, 39–48.
- Cappell, M.S.; Marks, M. Acute Pancreatitis in HIV-Seropositive Patients: A Case Control Study of 44 Patients. Am. J. Med. 1995, 98, 243–248.
- Qin, W.; Zhao, B.; Shang, Y.; Zhang, L. Clinical Profile of Acute Pancreatitis Following Treatment with Protease Inhibitors: A Real-World Analysis of Post-Marketing Surveillance Data. Expert Opin. Drug Saf. 2021, 20, 1109–1115.
- Li, C.; Jiang, M.; Pan, C.; Li, J.; Xu, L. The Global, Regional, and National Burden of Acute Pancreatitis in 204 Countries and Territories, 1990–2019. BMC Gastroenterol. 2021, 21, 332.
- Foli, A.; Benvenuto, F.; Piccinini, G.; Bareggi, A.; Cossarizza, A.; Lisziewicz, J.; Lori, F. Direct Analysis of Mitochondrial Toxicity of Antiretroviral Drugs. AIDS 2001, 15, 1687–1694.
- Manfredi, R.; Calza, L. HIV Infection and the Pancreas: Risk Factors and Potential Management Guidelines. Int. J. STD AIDS 2008, 19, 99–105.
- Tariq, S.; Rizvi, S.F.A.; Anwar, U. Tetracycline: Classification, Structure Activity Relationship and Mechanism of Action as a Theranostic Agent for Infectious Lesions—A Mini Review. Biomed. J. Sci. Tech. Res. 2018, 7, 5787–5796.
- Gilson, M.; Moachon, L.; Jeanne, L.; Dumaine, V.; Eyrolle, L.; Morand, P.; Ben M’rad, M.; Salmon, D. Acute Pancreatitis Related to Tigecycline: Case Report and Review of the Literature. Scand. J. Infect. Dis. 2008, 40, 681–683.
- Sura, M.E.; Heinrich, K.A.; Suseno, M. Metronidazole-Associated Pancreatitis. Ann. Pharmacother. 2000, 34, 1152–1155.
- Wu, S.-D.; Zhang, Z.-H.; Jin, J.-Z.; Kong, J.; Wang, W.; Zhang, Q.; Li, D.-Y.; Wang, M.-F. Effects of Narcotic Analgesic Drugs on Human Oddi’s Sphincter Motility. World J. Gastroenterol. 2004, 10, 2901–2904.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
Gastrointestinal Disease
Revisions:
2 times
(View History)
Update Date:
09 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




