Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Isabel da Silva Santos | -- | 3585 | 2024-01-08 13:28:16 | | | |
| 2 | Jason Zhu | -20 word(s) | 3565 | 2024-01-15 04:15:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Da Silva Santos, M.I.; Grácio, M.; Silva, M.C.; Pedroso, L.; Lima, A. Minimally Processed Fruits and Vegetables and One Health. Encyclopedia. Available online: https://encyclopedia.pub/entry/53555 (accessed on 07 February 2026).
Da Silva Santos MI, Grácio M, Silva MC, Pedroso L, Lima A. Minimally Processed Fruits and Vegetables and One Health. Encyclopedia. Available at: https://encyclopedia.pub/entry/53555. Accessed February 07, 2026.
Da Silva Santos, Maria Isabel, Madalena Grácio, Mariana Camoesas Silva, Laurentina Pedroso, Ana Lima. "Minimally Processed Fruits and Vegetables and One Health" Encyclopedia, https://encyclopedia.pub/entry/53555 (accessed February 07, 2026).
Da Silva Santos, M.I., Grácio, M., Silva, M.C., Pedroso, L., & Lima, A. (2024, January 08). Minimally Processed Fruits and Vegetables and One Health. In Encyclopedia. https://encyclopedia.pub/entry/53555
Da Silva Santos, Maria Isabel, et al. "Minimally Processed Fruits and Vegetables and One Health." Encyclopedia. Web. 08 January, 2024.
Copy Citation
While food markets and food production chains are experiencing exponential growth, global attention to food safety is steadily increasing. This is particularly crucial for ready-to-eat products such as fresh-cut salads and fruits, as these items are consumed raw without prior heat treatment, making the presence of pathogenic microorganisms quite frequent. Moreover, many studies on foodborne illnesses associated with these foods often overlook the transmission links from the initial contamination source.
minimally processed foods
foodborne pathogens
One Health
fruits and vegetables food chain production
1. Minimally Processed Fruit and Vegetable Contamination
Minimally processed fruits and vegetables (MPFV) are not sterile products; instead, they undergo only a moderate decrease in the microbiota present during processing. As vegetables are raw agricultural products, MPFV will likely contain potentially pathogenic microorganisms [1][2][3][4][5][6][7][8][9][10] that can arise from different steps in the food chain production. It is, therefore, unsurprising that some of the most nutritionally recommended foods pose significant challenges in terms of food preservation and safety. In recent years, foodborne outbreaks associated with the consumption of raw fruits and vegetables have been on the rise, prompting increased attention from researchers and health authorities to investigate food safety aspects related to microbial contamination of fresh produce [8][10][11][12][13][14][15][16].
2. Main Sources of Microbiological Contamination throughout the Food Chain Production
2.1. Soil
Pathogenic microorganisms of enteric origin can persist for extended periods in human and animal feces, posing a risk of contaminating land and crops [17][18][19][20][21][22][23]. Notably, Escherichia coli O157:H7 can endure for over seven months in soils exposed to rainy winter conditions. Furthermore, the widespread use of inadequately composted manure or feces from domestic or wild animals to fertilize and enhance soil structure has contributed to the spread of these microorganisms in the environment [21][24][25]. Crops, especially those growing closer to the ground, such as lettuce, are particularly vulnerable, as they may encounter soil during cultivation, irrigation, or heavy rains [23][26][27]. To mitigate the risk of introducing pathogens through organic fertilizer, it is recommended to allow a minimum of 90–120 days to pass (depending on whether the edible portions are in contact with the soil or not) between manure application and plant harvesting. The risk of pathogen presence decreases as the time between manure application and produce harvest increases [25][27][28].
2.2. Irrigation Water
Another significant contamination source is the use of contaminated water for irrigation or the application of pesticides. Foodborne outbreak investigations by the Centers for Disease Control and Prevention (CDC) have linked irrigation water to pathogen contamination of produce [29][30][31]. Studies by Dobhal et al. [32] have shown that strains of Salmonella Typhimurium and E. coli O157:H7 can survive in pesticide solutions. This situation is more prevalent in regions with water scarcity or where effluents are utilized for irrigation.
As natural reservoirs of E. coli and Salmonella include cattle, goats, and sheep, the intensification of animal production contributes to increased environmental and water contamination through runoff from production areas. The unrestricted access of farm or wild animals to cultivated fields or irrigation water is another important factor, as they can carry strains of Shiga toxin-producing E. coli (STEC) and other pathogenic microorganisms [9][25][26][33]. Several foodborne outbreaks resulting from produce contamination through irrigation water have been reported. For instance, in Sweden in 2015, an E. coli O157 outbreak was linked to contaminated water from a river used for irrigation of lettuce. Similarly, in the United States in 2008, a large outbreak caused by the consumption of serrano and jalapeño peppers was associated with contaminated irrigation water. Another incident occurred in 2010 when alfalfa sprouts were contaminated by Salmonella, and water runoff revealed the presence of the outbreak strain. In 2015, a multistate outbreak occurred due to the consumption of tomatoes irrigated with contaminated water [34]. Machado-Moreira et al. [15] reported E. coli and L. innocua contamination in lettuce due to spray irrigation with contaminated water, while Coleman et al. [35] demonstrated the contamination of hydroponic tomato plants grown with nutrient solution contaminated with Salmonella enterica.
The situation is particularly concerning due to variations in water regimes observed in recent years, including seasonal floods leading to fecal contamination and subsequent crop contamination. Conversely, dry summers have resulted in an increased reliance on wastewater, derived from effluent treatment on farms, to irrigate vegetable crops. As E. coli and Salmonella spp. can survive well in sediments, seasonal flooding during rainy seasons further contributes to increased contamination [36][37]. The use of untreated human sewage can also be a source of various pathogens, including Shigella spp., Salmonella enterica, different E. coli pathotypes, and enteric viruses [29][37][38]. Additionally, natural disasters such as fires and seasonal floods can lead to fecal contamination and subsequent produce contamination [39].
2.3. Insects
Insects are also a source of contamination for crops [9][17]. Flies are attracted to manure and can carry and transmit pathogenic microorganisms [40]. Experiments with the fruit fly (Ceratitis capitata) contaminated with E. coli strain labeled with a fluorescent protein have demonstrated that this insect can transmit pathogenic bacteria to intact fruits [41]. In addition to transmitting pathogenic microorganisms, insects can damage plant tissues by destroying the waxy cuticle, which is the first defense barrier, making them more vulnerable to pathogen penetration [40].
2.4. Human Manipulation
During harvesting, the contamination of products is exacerbated by poor hygiene practices among rural workers and the lack of sanitation facilities [3][22][24][27][37]. In the post-harvest phase, various factors can contribute to contamination. These include the use of contaminated ice or water, inadequate hygiene practices by handling staff or consumers, damage to plant tissues, issues with transport equipment, the presence of animals or pests in the environment, water quality during production and processing, use of contaminated equipment, cross-contamination, and improper storage conditions [9][22][42][43].
Concerning ready-to-eat vegetables, the primary source of contamination during the processing of lettuce and other minimally processed (MP) vegetables is the cutting stage. This operation has drawbacks, as it is during this stage that vegetables are most susceptible to mechanical damage. Moreover, an increase in the specific surface area makes tissues less effective barriers to the penetration of microorganisms. This results in a loss of cellular integrity, leading to physiological changes when the substrates encounter endogenous enzymes, rapid enzymatic catalysis reactions, and the growth of harmful bacteria [3][11][22][44][45][46][47].
3. Main Types of Contamination
3.1. Microbial Quality Indicators
Being simple tests to perform, the detection and counting of aerobic mesophilic or psychotropic microorganisms and of Enterobacteriaceae and coliforms have been the most widely used by the minimally processed fruits and vegetables (MPFV) industry as hygiene and quality indicators. This is done to compare the mesophilic or psychotropic microorganisms’ counts in MPFV at the time they are processed with those that are present in the natural product [37][44]. MPFV are referred to as more susceptible to microbial multiplication than unprocessed products due to the presence of cutting surfaces, increased nutrients available, plant tissue respiration, and confinement within the package. Additionally, there are no treatments to ensure microbiological stability [48][49]. Several studies report that the mesophilic or psychotropic levels present in lettuce or packaged salads vary widely between 3.0 and 9.40 log cfu.g−1.
Most of the bacteria identified in raw vegetables belong to the group of rod-shaped Gram-negative bacteria (80 to 90%) and include Pseudomonas spp., Flavobacterium spp., Enterobacter spp., Alcaligenes spp., Xantomonas spp., Klebsiella spp., Serratia spp., and Chromobacterium spp. The family Enterobacteriaceae, which includes the coliform group, constitutes about 10% of the microorganisms found in the total enumerations [49][50]. Poubol and Izumi [51], in mango cubes preserved in a CO2 atmosphere, reported that the predominant microbiota was Gram-negative rod-shaped bacteria, of which 60% were Enterobacteriaceae. Thus, high levels of these microorganisms are habitual in minimally processed fruits and vegetables (MPFV) and are not indicative of fecal contamination; however, they may compromise their sensorial and nutritional quality [52].
3.2. Pathogenic Microorganisms and Foodborne Disease-Related Cases
As previously mentioned, in addition to plant deteriorating microorganisms, it is crucial to consider pathogenic microorganisms transmitted by the produce as well. Over the last thirty years, the epidemiology of infectious diseases originating from food has undergone a significant shift, with plant products emerging as new vehicles for the transmission of zoonotic agents [15][29][53][54]. The scientific literature documents numerous outbreaks of this nature, some resulting in the tragic loss of hundreds of lives [12][17][29][44][49][54][55]. Salmonella spp., E. coli O157:H7, and L. monocytogenes are identified as the primary pathogenic microorganisms causing the most concern in such outbreaks [12][29][54][56]. A study conducted in Norway did not detect Salmonella spp. in any of the 118 samples [57].
Regarding outbreaks in 2021, vegetables, fruit juices, and related food products were responsible for 9.6% (n = 34) of confirmed outbreaks with strong evidence, while fruits, berries, and juices and their products accounted for 0.60% (n = 2) of such outbreaks, representing a significant increase, more than twice compared to 2020. It is noteworthy that a diverse range of causative agents were involved, including several Salmonella serovars implicated in 11 outbreaks, Shiga toxin-producing E. coli (STEC), Enteroinvasive E. coli (EIEC), Enterotoxigenic E. coli (ETEC), Yersinia enterocolitica, bacterial toxins such as Staphylococcus aureus, Clostridium botulinum, Bacillus cereus, unspecified bacterial toxins, viruses including Norovirus, and Cryptosporidium parvum. A total of 1715 individuals fell ill, with 131 hospitalizations, although there were no reported deaths. In addition to the three mentioned pathogens, other less frequent agents were also observed, impacting the safety of plant products and consequently the health of consumers. It is important to note that the average size of outbreaks attributed to this type of food (50 cases/outbreak) was significantly higher than those occurring due to the consumption of animal-origin foods (11 cases/outbreak) [58]. Among these occurrences, a notable outbreak was linked to Galia from Honduras. The implicated pathogen was Salmonella Braenderup sequence type 22, responsible for 348 illnesses and 68 hospitalizations between March and July 2021 in 12 European countries, including the United Kingdom.
It is worth mentioning that since 2012, Hepatitis A virus (HAV) outbreaks have been a recurrent problem in Europe, associated with frozen berries’ consumption. In June 2018, in Sweden, an HAV outbreak occurred linked to frozen strawberries imported from Poland. In October of the same year, in Austria, an HAV outbreak with a strain with the same genotype as the Sweden strain was also reported. The study has also concluded that the strawberries were acquired from the same producer from Poland [59]. Later, in Germany, from October 2018 until January 2020, the same HAV strain was responsible for 65 cases of the illness in 2 peaks (August to December 2018 and June to September 2019). The epidemiological research allowed people to conclude that frozen strawberry cakes were the implicated food vehicle in both outbreak waves. The traceback investigations and phylogenetic analyses have demonstrated the strain (the same identified in Sweden and Austria outbreaks and the Polish producer) was also found in berries, sewage, and stools in Egypt, raising the hypothesis that the contamination occurred in this country. As the producer from Poland has received strawberries from Egypt through a German distributor, a unique contaminated batch may have caused all referred outbreaks [60].
Another major outbreak occurred between May and July 2011, marking a significant historical reference due to an unusually high number of cases and the challenges in finding the source of infection. This outbreak took place in Germany, resulting in 3816 cases, of which 845 developed hemolytic uremic syndrome (HUS) and 54 fatalities were reported. Notably, a majority (88%) of HUS cases were observed in adults, contrasting with typical infections by VTEC strains that usually affect children. Moreover, females, particularly those aged between 30 and 34 years, were the most affected, constituting 68% of HUS cases and 58% of gastroenteritis cases. The epidemic strain was identified as E. coli O104:H4 enteroaggregative, which had acquired the stx2a conversion bacteriophage. This outbreak gained international attention, with cases reported in 15 other countries in Europe and the USA. In France, eight cases were reported in individuals who attended a community event, and the isolated strain in these patients was genetically compatible with the epidemic strain from Germany. The investigations traced the outbreak back to the consumption of fenugreek sprouts, with the seeds of the implicated batch imported from Egypt in 2009 [61][62][63].
As illustrated in Figure 1, data from the USA estimate that between 1998 and 2008, fruits and vegetables were responsible for 46% of outbreaks, mostly caused by norovirus, Salmonella spp., and E. coli O157:H7, with leafy vegetables being the most frequent vehicle. Leafy vegetables were responsible for 2.2 million cases per year, representing 22% of all cases and constituting the food product responsible for the largest number of patients. Approximately 24,000 people (41%) are hospitalized each year due to the consumption of plant-origin products, with 38% attributed to fruits and vegetables and 16% to leafy vegetables, making dairy products the leading cause of hospitalizations. In terms of the number of deaths, fruits and vegetables account for 333 cases per year (23%), considerably lower than the 43% attributed to the consumption of animal products (terrestrial). In summary, leafy vegetables account for the largest number of patients (22%), making them the second most frequent cause of hospitalizations (14%) and the fifth cause of death (6%) [12].
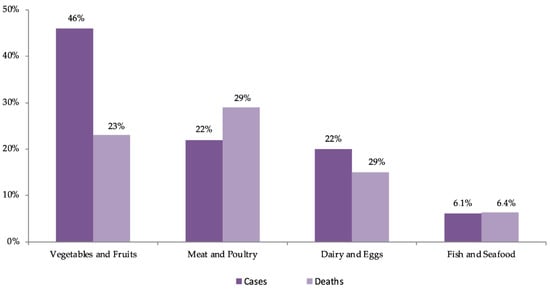
However, in the USA, between September 2013 and May 2016, an outbreak caused by L. monocytogenes, associated with frozen vegetables consumption, occurred, which affected only nine persons (all were hospitalized), and three of them have died, which corresponds to 33.3%, and this percentage is superior to that reported in the literature [65]. The outbreaks occurred, particularly, in 2011 caused by E. coli O104:H4 in vegetable sprouts, in 2006 by contamination of spinach MP with E. coli O157:H7, in 1996 also with E. coli O157:H7 in lettuce, and Salmonella spp. in tomato, juice, fruits, and sprouts reinforce the concern with products that are consumed raw and calls attention to the need to increase preventive strategies [55][63][66][67].
A study conducted by the Interagency Food Safety Analytics Collaboration, utilizing data from 1998 through 2020, estimated the percentage of illnesses caused by three priority pathogens—Salmonella spp., E. coli O157, and L. monocytogenes—in the year 2020. Unfortunately, no results were provided for the fourth priority pathogen, Campylobacter. The study, based on 1287 outbreaks, indicated that 960 were caused by or suspected to be caused by Salmonella spp., 272 by E. coli O157, and 55 by L. monocytogenes.
Yet another pathogenic microorganism that has raised some questions about its possible transmission through the consumption of produce is Clostridioides difficile (previously known as Clostridium difficile) [68]. This is an anaerobic spore-forming pathogenic bacteria that acts negatively in the gastrointestinal tract, causing a serious illness, particularly in hospitalized persons, subject to prolonged treatment with antibiotics. However, in the last two decades the incidence of C. difficile infection (CDI) has increased in the community, oftentimes in people with no history of hospitalization or antibiotic treatment [69][70][71][72]. The rapid expansion of community-acquired CDI raised the hypothesis that C. difficile present in the environment, animals, and retail foods causes this infection in humans [70][72][73][74]. In addition, several studies demonstrate the presence of C. difficile in several foods, including meat, ready-to-eat salads, and raw vegetables (such as cucumber, onions, carrots, etc.) [75][76][77][78]. Given that C. difficile is present in water, animal feces, and livestock manure compost, it could easily be transferred to vegetables.
4. Major Decontamination Methodologies: Related Problems and Possible Solutions
It is crucial to emphasize that MPFV do not undergo any stage ensuring the elimination of the risk related to their consumption, as they lack heat treatment to eliminate pathogens, spores, and toxins at safety levels. Therefore, the vegetable sanitation/disinfection stage, especially in MPFV, becomes critical for food safety. MPFV plants are cut, and the damage to cells makes them more susceptible to microbiological multiplication and biochemical alterations, intensifying respiratory rates and enzymatic activity [1][6][9][49][79][80][81][82].
While washing and disinfection steps of plant products are moderately effective, they are by no means efficient when dealing with internalized pathogenic microorganisms. Pathogens can penetrate plant tissues either in the pre-harvest phase through internalization or in the post-harvest phase through infiltration, complicating the situation. Infiltration, or the suction effect, can occur when a product at room temperature is submerged in colder water, creating a vacuum that sucks water and, if present, pathogenic microorganisms into the tissues through pores, channels, or fissures [33][82][83][84][85][86][87]. Studies have shown that tomatoes submerged in an E. coli O157:H7 suspension exhibited contamination, and Salmonella Typhimurium was found to infiltrate baby spinach during washing operations, dependent on humidity, temperature, and illumination conditions [88][89]. Internalization may also occur during flowering, with pathogenic microorganisms entering through flowers carried by water or insects, through roots from contaminated soil or water, through wounds or cracks, or by entrapment in the waxy film [90]. Laboratory studies have demonstrated the entry of pathogens into plant tissues through natural openings such as stomata, root, and flower junctions, or through tissue damage. After binding to plant tissues, microorganisms have the capability to form biofilms, thereby improving their capacity to endure within the plant structure [33][91][92]. In essence, the internalization of pathogenic microorganisms can take place at any point in the plant’s life cycle, progressing to subsequent phases such as seed, germination, mature plant, flower, and fruit [16].
4.1. Chlorine Disinfection
Currently, the predominant disinfection methods involve the application of chlorine-based disinfectants [6][11]. However, these disinfectants pose risks to human health by generating carcinogenic compounds [44] and are not highly effective, as their disinfecting impact diminishes rapidly, allowing surviving bacterial populations to multiply more rapidly than those in non-disinfected products [6][9]. Chlorinated water is commonly utilized for disinfecting MPFV due to its cost-effectiveness and ease of use [6]. The efficacy of decontamination is assessed not only by the reduction achieved but, more crucially, by the ability to sustain this reduction over the product’s shelf life. Nevertheless, the use of active chlorine raises health concerns due to the formation of toxic by-products such as trihalomethanes and chloramines. This concern has led to restrictions on chlorine’s usage in several European countries, including the Netherlands, Sweden, Germany, Switzerland, Denmark, and Belgium [44][49]. A study by Coroneo et al. [93] investigated the presence of these derivatives during the disinfection process, concluding that toxic or carcinogenic compounds, specifically trihalomethanes, are formed and persist in the final product.
4.2. Other Chemical Methods of Disinfection
Recent advancements have introduced various methodologies relying on chemical disinfectants, including chlorine dioxide [7][11][44], organic acids [6][11][44], hydrogen peroxide [3][7][44], electrolyzed water [6][7][11][44], ozonated water [6][7][11][44], and calcium-based solutions [44][94]. These methods have demonstrated ease of application and a potent bactericidal effect. However, most of them come with certain drawbacks. For instance, the use of chlorine dioxide, while effective in reducing bacterial populations, has been found to impact some organoleptic characteristics. Another consideration is the significant reduction of the native microbial population, which, by decreasing competition for space and nutrients, may potentially result in a subsequent increase in the development of pathogenic microorganisms [49].
2.4.3. Physical Methods of Disinfection
Recent developments have introduced physical treatments such as ionizing radiation [3][11][44], ultraviolet [6][11][44][95], infrared [11][44][96], modified atmosphere packaging [3][44], or combinations such as ultrasound with ε-polylysine [97], aimed at preserving these types of products. Modified atmosphere packaging is a technique currently employed in the industry. These methods may be either bacteriostatic or bactericidal, demonstrating high efficiency in inhibiting microbial contaminations [46]. However, they come with certain challenges; for example, irradiation cannot be used as an isolated step of continuous washing, as it alone does not remove chemical residues or soil [7][44].
5. Possible Future Solutions: The Use of Natural Disinfectants and Smart Packaging as an Alternative for Decontamination of Minimally Processed Fruits and Vegetables
As consumer preferences shift towards natural and minimally processed products with fewer chemical additives and extended shelf life, the use of synthetic antimicrobials is becoming more restricted due to potential toxicity concerns. Consequently, there is a growing need to identify alternative antibacterial substances, preferably of biological origin, that are both effective and harmless to human health and the environment. Natural antibacterial compounds have emerged as a promising alternative, gaining increased interest in their potential to eliminate pathogenic microorganisms, especially considering their resistance to antibiotics [98][99][100][101][102].
Numerous studies have explored new disinfection methods with the dual purpose of eliminating pathogens and preventing the degradation of vegetable products [7][101][103]. It is crucial to investigate techniques that not only decontaminate the product but also maintain low levels of microbiota over its shelf life. These compounds are derived from various sources, including plants (essential oils), microorganisms (such as lactic acid bacteria producing both lactic acid and antimicrobial polypeptides), and animals (for example, lysozyme) [99][103][104]. Antibacterial bioactive compounds are biological substances produced as defense against other organisms, and since these natural products and their components are generally recognized as safe (GRAS), concerns about their safety in preventing the development of pathogenic microorganisms or product alteration are minimal.
In recent decades, alternative compounds with the potential for food disinfection have emerged, including acetic acid, ascorbic acid, lactic acid, essential oils, and cheese whey, among others [98][104], all of which have less reported secondary effects and are more biodegradable. Smart packaging, an emerging technology in the food packaging industry, integrates active and intelligent features to enhance food safety and quality [105][106]. Over the past decades, innovative applications have surfaced, including the use of bioactive compounds such as essential oils (EO) in various packaging forms such as coating, nanoencapsulation, and synergistic pairings with other antibacterial agents [91]. Additionally, using materials with smart packaging properties, such as being impermeable to oxygen, light, moisture, and certain gases, contributes to minimizing spoilage by reducing microbial activity, with nanocomposite materials providing added resistance [105][106].
To optimize smart packaging development, it is crucial to consider cultural, social, and cognitive factors influencing consumer acceptance [107]. Tailoring these technologies to meet consumer preferences and needs, along with effective communication addressing consumer concerns and educating them on the benefits, will be key for successful implementation. However, despite these advancements, there are still limited natural disinfectants proposed in scientific studies that have reached the market. Traditional chemical methods, such as chlorinated compounds, continue to be used, emphasizing the importance of developing natural disinfectant products that can effectively replace chlorine-based products without compromising safety, environmental impact, or the organoleptic characteristics of the product.
References
- Aiyedun, S.O.; Onarinde, B.A.; Swainson, M.; Dixon, R.A. Foodborne outbreaks of microbial infection from fresh produce in Europe and North America: A systematic review of data from this millennium. Int. J. Food Sci. Technol. 2021, 56, 2215–2223.
- De Corato, U.; Cancellara, F.A. Measures, technologies, and incentives for cleaning the minimally processed fruits and vegetables supply chain in the Italian food industry. J. Clean. Prod. 2019, 237, 117735.
- Gil, M.I.; Selma, M.V.; Suslow, T.; Jacxsens, L.; Uyttendaele, M.; Allende, A. Pre- and Postharvest Preventive Measures and Intervention Strategies to Control Microbial Food Safety Hazards of Fresh Leafy Vegetables. Crit. Rev. Food Sci. Nutr. 2014, 55, 453–468.
- Muthukkannan, N.; Kalidas, K. Minimally processed fresh-cut-vegetables for healthy consumption. Adv. Res. J. Crop Improv. 2019, 10, 9–14.
- Perera, C.O. Minimal Processing of Fruit and Vegetables. In Handbook of Food Preservation; Rahman, M.S., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 191–205.
- Raffo, A.; Paoletti, F. Fresh-Cut Vegetables Processing: Environmental Sustainability and Food Safety Issues in a Comprehensive Perspective. Front. Sustain. Food Syst. 2022, 5, 681459.
- Goodburn, C.; Wallace, C.A. The microbiological efficacy of decontamination methodologies for fresh produce: A review. Food Control 2013, 32, 418–427.
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated with Fresh Produce From 2010 to 2017. Front. Microbiol. 2019, 10, 2667.
- Balali, G.I.; Yar, D.D.; Dela, V.G.A.; Adjei-Kusi, P. Microbial Contamination, an Increasing Threat to the Consumption of Fresh Fruits and Vegetables in Today’s World. Int. J. Microbiol. 2020, 2020, 3029295.
- Banach, J.; Van Bokhorst-van de Veen, H.; van Overbeek, L.; van der Zouwen, P.; van der Fels-Klerx, H.; Groot, M.N. The efficacy of chemical sanitizers on the reduction of Salmonella Typhimurium and Escherichia coli affected by bacterial cell history and water quality. Food Control 2017, 81, 137–146.
- Deng, L.-Z.; Mujumdar, A.S.; Pan, Z.; Vidyarthi, S.K.; Xu, J.; Zielinska, M.; Xiao, H.-W. Emerging chemical and physical disinfection technologies of fruits and vegetables: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2481–2508.
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of Foodborne Illnesses, Hospitalizations, and Deaths to Food Commodities by using Outbreak Data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415.
- Elias, S.d.O.; Decol, L.T.; Tondo, E.C. Foodborne outbreaks in Brazil associated with fruits and vegetables: 2008 through 2014. Food Qual. Saf. 2018, 2, 173–181.
- Johnson, R. Foodborne Illnesses and Outbreaks from Fresh Produce. Congressional Research Service, In Foco, IF11092. 2019. Available online: https://sgp.fas.org/crs/misc/IF11092.pdf (accessed on 14 November 2022).
- Machado-Moreira, B.; Richards, K.; Abram, F.; Brennan, F.; Gaffney, M.; Burgess, C.M. Survival of Escherichia coli and Listeria innocua on Lettuce after Irrigation with Contaminated Water in a Temperate Climate. Foods 2021, 10, 2072.
- Brandl, M.T. Fitness of human enteric pathogens on plants and implications for food safety. Annu. Rev. Phytopathol. 2006, 44, 367–392.
- European Food Safety Authority. Scientific Opinion on the risk posed by pathogens in food of non-animal origin. Part 1 (outbreak data analysis and risk ranking of food/pathogen combinations). EFSA J. 2013, 11, 3025.
- Barrera, M.J.; Blenkinsop, R.; Warriner, K. The effect of different processing parameters on the efficacy of commercial post-harvest washing of minimally processed spinach and shredded lettuce. Food Control 2013, 25, 745–751.
- Fongaro, G.; García-González, M.C.; Hernández, M.; Kunz, A.; Barardi, C.R.M.; Rodríguez-Lázaro, D. Different Behavior of Enteric Bacteria and Viruses in Clay and Sandy Soils after Biofertilization with Swine Digestate. Front. Microbiol. 2017, 8, 74.
- Guo, X.; Hu, H.; Meng, H.; Liu, L.; Xu, X.; Zhao, T. Vertical distribution and affecting factors of Escherichia coli over a 0–400 cm soil profile irrigated with sewage effluents in northern China. Ecotoxicol. Environ. Saf. 2020, 205, 111357.
- Black, Z.; Balta, I.; Black, L.; Naughton, P.J.; Dooley, J.S.G.; Corcionivoschi, N. The Fate of Foodborne Pathogens in Manure Treated Soil. Front. Microbiol. 2021, 12, 781357.
- Alegbeleye, O.O.; Sant’ana, A.S. Manure-borne pathogens as an important source of water contamination: An update on the dynamics of pathogen survival/transport as well as practical risk mitigation strategies. Int. J. Hyg. Environ. Health 2020, 227, 113524.
- Millner, P.D. Manure management. In The Produce Contamination Problem Causes and Solutions, 2nd ed.; Matthews, K.R., Sapers, G.M., Gerba, C.P., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 85–106.
- Jung, Y.; Jang, H.; Matthews, K.R. Effect of the food production chain from farm practices to vegetable processing on outbreak incidence. Microb. Biotechnol. 2014, 7, 517–527.
- Alegbeleye, O.O.; Singleton, I.; Sant’ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208.
- Mügler, C.; Ribolzi, O.; Viguier, M.; Janeau, J.-L.; Jardé, E.; Latsachack, K.; Henry-Des-Tureaux, T.; Thammahacksa, C.; Valentin, C.; Sengtaheuanghoung, O.; et al. Experimental and modelling evidence of splash effects on manure borne Escherichia coli washoff. Environ. Sci. Pollut. Res. 2021, 28, 33009–33020.
- Ramos, T.D.M.; Jay-Russell, M.T.; Millner, P.D.; Baron, J.N.; Stover, J.; Pagliari, P.; Hutchinson, M.; Lilley, J.; Rowley, N.; Haghani, V.; et al. Survival and Persistence of Foodborne Pathogens in Manure-Amended Soils and Prevalence on Fresh Produce in Certified Organic Farms: A Multi-Regional Baseline Analysis. Front. Sustain. Food Syst. 2021, 5, 674767.
- Blaiotta, G.; Di Cerbo, A.; Murru, N.; Coppola, R.; Aponte, M. Persistence of bacterial indicators and zoonotic pathogens in contaminated cattle wastes. BMC Microbiol. 2016, 16, 87.
- Rao, A.P.; Patel, J.; Pradhan, A.K. Application of alternative sources of water in agricultural food production—Current trends and future prospects. Curr. Opin. Food Sci. 2022, 47, 100877.
- Liu, H.; Whitehouse, C.A.; Li, B. Presence and Persistence of Salmonella in Water: The Impact on Microbial Quality of Water and Food Safety. Front. Public Health 2018, 6, 159.
- Toro, M.; Weller, D.; Ramos, R.; Diaz, L.; Alvarez, F.P.; Reyes-Jara, A.; Moreno-Switt, A.I.; Meng, J.; Adell, A.D. Environmental and anthropogenic factors associated with the likelihood of detecting Salmonella in agricultural watersheds. Environ. Pollut. 2022, 306, 119298.
- Dobhal, S.; Zhang, G.; Royer, T.; Damicone, J.; Ma, L.M. Survival and growth of foodborne pathogens in pesticide solutions routinely used in leafy green vegetables and tomato production. J. Sci. Food Agric. 2014, 94, 2958–2964.
- Xia, X.; Luo, Y.; Yang, Y.; Vinyard, B.; Schneider, K.; Meng, J. Effects of Tomato Variety, Temperature Differential, and Post–Stem Removal Time on Internalization of Salmonella enterica Serovar Thompson in Tomatoes. J. Food Prot. 2012, 75, 297–303.
- Rodrigues, C.; da Silva, A.L.B.R.; Dunn, L.L. Factors Impacting the Prevalence of Foodborne Pathogens in Agricultural Water Sources in the Southeastern United States. Water 2019, 12, 51.
- Coleman, S.M.; Bisha, B.; Newman, S.E.; Bunning, M.; Goodridge, L.D. Transmission and Persistence of Salmonella enterica in Nutrient Solution of Hydroponic Greenhouse Grown Tomatoes. HortScience 2017, 52, 713–718.
- Andrade, L.; O’Dwyer, J.; O’Neill, E.; Hynds, P. Surface water flooding, groundwater contamination, and enteric disease in developed countries: A scoping review of connections and consequences. Environ. Pollut. 2018, 236, 540–549.
- Food and Agricultural Organization of the United Nations; World Health Organization. Microbiological Hazards in Fresh Leafy Vegetables and Herbs: Meeting Report. Microbiological Risk Assessment Series, No. 14. Rome, Italy. 2008. Available online: https://apps.who.int/iris/handle/10665/44031 (accessed on 14 November 2022).
- Faour-Klingbeil, D.; Murtada, M.; Kuri, V.; Todd, E.C. Understanding the routes of contamination of ready-to-eat vegetables in the Middle East. Food Control 2016, 62, 125–133.
- Garcia, S.N.; Osburn, B.I.; Jay-Russell, M.T. One Health for Food Safety, Food Security, and Sustainable Food Pro-duction. Front. Sustain. Food Syst. 2020, 4, 1.
- Berry, E.D.; Wells, J.E.; Durso, L.M.; Friesen, K.M.; Bono, J.L.; Suslow, T.V. Occurrence of Escherichia coli O157:H7 in Pest Flies Captured in Leafy Greens Plots Grown Near a Beef Cattle Feedlot. J. Food Prot. 2019, 82, 1300–1307.
- Sela, S.; Nestel, D.; Pinto, R.; Nemny-Lavy, E.; Bar-Joseph, M. Mediterranean fruit fly as a potential vector of bacterial pathogens. Appl. Environ. Microbiol. 2005, 71, 4052–4056.
- Gil, M.I.; Allende, A. Water and wastewater use in the fresh produce industry: Food safety and environmental im-plications. In Quantitative Methods for Food Safety and Quality in the Vegetable Industry; Pérez-Rodríguez, F., Skandamis, P., Valdramidis, V., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 59–76.
- European Food Safety Authority. Urgent advice on the public health risk of Shiga-toxin producing Escherichia coli in fresh vegetables. EFSA J. 2011, 9, 2274.
- De Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975.
- Ali, A.; Yeoh, W.K.; Forney, C.; Siddiqui, M.W. Advances in postharvest technologies to extend the storage life of minimally processed fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 58, 2632–2649.
- Perumal, A.B.; Huang, L.; Nambiar, R.B.; He, Y.; Li, X.; Sellamuthu, P.S. Application of essential oils in packaging films for the preservation of fruits and vegetables: A review. Food Chem. 2022, 375, 131810.
- Wang, L.; Teplitski, M. Microbiological food safety considerations in shelf-life extension of fresh fruits and vegetables. Curr. Opin. Biotechnol. 2023, 80, 102895.
- Iturralde-García, R.D.; Cinco-Moroyoqui, F.J.; Martínez-Cruz, O.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Cornejo-Ramírez, Y.I.; Bernal-Mercado, A.T.; Del-Toro-Sánchez, C.L. Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage. Horticulturae 2022, 8, 731.
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apples and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84.
- Anthony, A.A.; Divine-Anthony, O. Bacteria Associated with the Spoilage of Salad, their resistotyping and Potential Public Health Implications. Int. J. Eng. Res. Sci. 2015, 1, 55–59.
- Poubol, J.; Izumi, H. Shelf life and microbial quality of fresh-cut mango cubes stored in high CO2 atmospheres. J. Food Sci. 2005, 70, M69–M74.
- Santos, M.; Cavaco, A.; Gouveia, J.; Novais, M.; Nogueira, P.; Pedroso, L.; Ferreira, M.A.S.S. Evaluation of minimally processed salads commercialized in Portugal. Food Control 2012, 23, 275–281.
- European Commission. Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union—Legis. 2005, 338, 1–26. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32005R2073&qid=1672315192734 (accessed on 12 November 2022).
- Gurtler, J.B.; E Gibson, K. Irrigation water and contamination of fresh produce with bacterial foodborne pathogens. Curr. Opin. Food Sci. 2022, 47, 100889.
- Esmael, A.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Filimban, A.A.R.; Alseghayer, M.S.; Almaneea, A.M.; Alhadlaq, M.A.; Ayubu, J.; Teklemariam, A.D. Fresh Produce as a Potential Vector and Reservoir for Human Bacterial Pathogens: Revealing the Ambiguity of Interaction and Transmission. Microorganisms 2023, 11, 753.
- Ramos, B.; Miller, F.A.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15.
- Veterinærinstituttet. Escherichia coli og Salmonella i Friske Spiseklare Vegetabiler 2021. Report 29/2022. 2022. Available online: https://www.mattilsynet.no/mat_og_vann/smitte_fra_mat_og_drikke/bakterier_i_mat_og_drikke/rapport_smittestoffer_i_sukker-erter_og_vasket_salat_2021.48288/binary/Rapport:%20Smittestoffer%20i%20sukkererter%20og%20vasket%20salat%202021 (accessed on 18 November 2022).
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666.
- Enkirch, T.; Eriksson, R.; Persson, S.; Schmid, D.; Aberle, S.W.; Löf, E.; Wittesjö, B.; Holmgren, B.; Johnzon, C.; Gustafsson, E.X.; et al. Hepatitis A outbreak linked to imported frozen strawberries by sequencing, Sweden and Austria, June to September 2018. Eurosurveillance 2018, 23, 1800528.
- Ruscher, C.; Faber, M.; Werber, D.; Stark, K.; Bitzegeio, J.; Michaelis, K.; Sagebiel, D.; Wenzel, J.J.; Enkelmann, J. Resurgence of an international hepatitis A outbreak linked to imported frozen strawberries, Germany, 2018 to 2020. Eurosurveillance 2020, 25, 1900670.
- Gossner, C.M.; de Jong, B.; Hoebe, C.J.; Kornschober, C.; Schmid, D.; Quoilin, S.; Parmakova, K.; Petrov, P.; Koliou, M.; Kralova, R.; et al. Event-based surveillance of food- and waterborne diseases in Europe: Urgent inquiries (outbreak alerts) during 2008 to 2013. EuroSurveillance 2015, 20, 21166. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21166 (accessed on 14 November 2023).
- King, L.A.; Nogareda, F.; Weill, F.-X.; Mariani-Kurkdjian, P.; Loukiadis, E.; Gault, G.; Jourdan-DaSilva, N.; Bingen, E.; Macé, M.; Thevenot, D.; et al. Outbreak of shiga toxin-producing Escherichia coli O104:H4 associated with organic fenugreek sprouts, France, June 2011. Clin. Infect. Dis. 2012, 54, 1588–1594.
- World Health Organization. Public Health Review of the Enterohaemorrhagic Escherichia coli Outbreak in Germany. 2011. Available online: https://apps.who.int/iris/bitstream/handle/10665/349978/WHO-EURO-2011-4324-44087-62183-eng.pdf?sequence=1&isAllowed=y (accessed on 29 June 2023).
- Centers for Disease Control. Estimates of Foodborne Illnessin the United States. 2014. Available online: http://www.cdc.gov/foodborneburden/attribution/ (accessed on 28 September 2022).
- Centers for Disease Control. List of Selected Multistate Foodborne Outbreak Investigation. 2016. Available online: https://www.cdc.gov/listeria/outbreaks/frozen-vegetables-05-16/index.html (accessed on 14 July 2022).
- Bennett, S.D.; Sodha, S.V.; Ayers, T.L.; Lynch, M.F.; Gould, L.H.; Tauxe, R.V. Produce-associated foodborne disease outbreaks, USA, 1998–2013. Epidemiol. Infect. 2018, 146, 1397–1406.
- Luna-Guevara, J.J.; Arenas-Hernandez, M.M.P.; de la Peña, C.M.; Silva, J.L.; Luna-Guevara, M.L. The Role of Pathogenic E. coli in Fresh Vegetables: Behavior, Contamination Factors, and Preventive Measures. Int. J. Microbiol. 2019, 2019, 2894328.
- Diseases, T.L.I. C difficile—A rose by any other name. Lancet Infect. Dis. 2019, 19, 449.
- Metcalf, D.S.; Costa, M.C.; Dew WM, V.; Weese, J.S. Clostridium difficile in vegetables, Canada. Lett. Appl. Microbiol. 2010, 51, 600–602.
- Candel-Pérez, C.; Ros-Berruezo, G.; Martínez-Graciá, C. A review of Clostridioides difficile occurrence through the food chain. Food Microbiol. 2019, 77, 118–129.
- Lim, S.C.; Foster, N.F.; Elliott, B.; Riley, T.V. High prevalence of Clostridium difficile on retail root vegetables, Western Australia. J. Appl. Microbiol. 2017, 124, 585–590.
- Usui, M.; Maruko, A.; Harada, M.; Kawabata, F.; Sudo, T.; Noto, S.; Sato, T.; Shinagawa, M.; Takahashi, S.; Tamura, Y. Prevalence and characterization of Clostridioides difficile isolates from retail food products (vegetables and meats) in Japan. Anaerobe 2019, 61, 102132.
- Barbosa, J.; Campos, A.; Teixeira, P. Methods currently applied to study the prevalence of Clostridioides difficile in foods. AIMS Agric. Food 2020, 5, 102–128.
- Pal, M.; Bulcha, M.R. Clostridium difficile as an emerging foodborne pathogen of public health significance. Acta Sci. Microbiol. 2021, 4, 46–49.
- Eckert, C.; Burghoffer, B.; Barbut, F. Contamination of ready-to-eat raw vegetables with Clostridium difficile in France. J. Med. Microbiol. 2013, 62, 1435–1438.
- Lund, B.M.; Peck, M.W. A Possible Route for Foodborne Transmission of Clostridium difficile? Foodborne Pathog. Dis. 2015, 12, 177–182.
- Ünlü, G. The Prevalence, Transmission, and Control of C. difficile in Food. Food Technol. Mag. 2020, 74, 72–77. Available online: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2020/february/columns/the-prevalence-transmission--and-control-of-c-difficile-in-food (accessed on 11 December 2022).
- Mirlohi, M.; Yamoudy, M.; Isfahani, B.N.; Jalali, M.; Esfandiari, Z.; Hosseini, N.S. Isolation of toxigenic Clostridium difficile from ready-to-eat salads by multiplex polymerase chain reaction in Isfahan, Iran. Adv. Biomed. Res. 2015, 4, 87.
- Bhilwadikar, T.; Pounraj, S.; Manivannan, S.; Rastogi, N.K.; Negi, P.S. Decontamination of Microorganisms and Pesticides from Fresh Fruits and Vegetables: A Comprehensive Review from Common Household Processes to Modern Techniques. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1003–1038.
- Botondi, R.; Barone, M.; Grasso, C. A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables. Foods 2021, 10, 748.
- Finnegan, E.; O’beirne, D. Characterising Deterioration Patterns in Fresh-Cut Fruit Using Principal Component Analysis. II: Effects of Ripeness Stage, Seasonality, Processing and Packaging. Postharvest Biol. Technol. 2015, 100, 91–98.
- Warriner, K.; Namvar, A. Recent advances in fresh produce post-harvest decontamination technologies to enhance microbiological safety. Stewart Postharvest Rev. 2013, 9, 1–8.
- Food and Drug Administration. Guide to Minimize Food Safety Hazards of Fresh-Cut Produce: Draft Guidance for Industry. 2018. Available online: https://www.fda.gov/media/117526/download (accessed on 18 June 2022).
- Allende, A.; Ölmez, H. Strategies to Combat Microbial Hazards Associated with Fresh Produce. Cult. Thermoscientific 2015, 35. Available online: https://assets.thermofisher.com/TFS-Assets/MBD/Vector-Information/Culture-35-2-Microbial-Hazards-Fresh-Produce-LT2174A-EN.pdf (accessed on 28 June 2023).
- Bartz, J.A.; Yuk, H.-G.; Mahovic, M.J.; Warren, B.R.; Sreedharan, A.; Schneider, K.R. Internalization of Salmonella enterica by tomato fruit. Food Control 2015, 55, 141–150.
- Macarisin, D.; Wooten, A.; De Jesus, A.; Hur, M.; Bae, S.; Patel, J.; Evans, P.; Brown, E.; Hammack, T.; Chen, Y. Internalization of Listeria monocytogenes in cantaloupes during dump tank washing and hydrocooling. Int. J. Food Microbiol. 2017, 257, 165–175.
- Zhou, B.; Luo, Y.; Bauchan, G.R.; Feng, H.; Stommel, J.R. Visualizing Pathogen Internalization Pathways in Fresh Tomatoes Using MicroCT and Confocal Laser Scanning Microscopy. Food Control 2018, 85, 276–282.
- Gómez-López, V.M.; Marín, A.; Allende, A.; Beuchat, L.R.; Gil, M.I. Postharvest handling conditions affect internalization of salmonella in baby spinach during washing. J. Food Prot. 2013, 76, 1145–1151.
- Riggio, G.; Jones, S.; Gibson, K. Risk of Human Pathogen Internalization in Leafy Vegetables During Lab-Scale Hydroponic Cultivation. Horticulturae 2019, 5, 25.
- Santos, M.I.S.; Marques, C.; Mota, J.; Pedroso, L.; Lima, A. Applications of Essential Oils as Antibacterial Agents in Minimally Processed Fruits and Vegetables—A Review. Microorganisms 2022, 10, 760.
- Yaron, S.; Römling, U. Biofilm formation by enteric pathogens and its role in plant colonization and persistence. Microb. Biotechnol. 2014, 7, 496–516.
- Coroneo, V.; Carraro, V.; Marras, B.; Marrucci, A.; Succa, S.; Meloni, B.; Pinna, A.; Angioni, A.; Sanna, A.; Schintu, M. Presence of Trihalomethanes in ready-to-eat vegetables disinfected with chlorine. Food Addit. Contam. Part A 2017, 34, 2111–2117.
- Matsuzaki, S.; Azuma, K.; Lin, X.; Kuragano, M.; Uwai, K.; Yamanaka, S.; Tokuraku, K. Farm use of calcium hydroxide as an effective barrier against pathogens. Sci. Rep. 2021, 11, 7941.
- Birmpa, A.; Sfika, V.; Vantarakis, A. Ultraviolet light and Ultrasound as non-thermal treatments for the inactivation of microorganisms in fresh ready-to-eat foods. Int. J. Food Microbiol. 2013, 167, 96–102.
- Mahendran, R.; Ramanan, K.R.; Barba, F.J.; Lorenzo, J.M.; López-Fernández, O.; Munekata, P.E.; Roohinejad, S.; Sant’Ana, A.S.; Tiwari, B.K. Recent advances in the application of pulsed light processing for improving food safety and increasing shelf life. Trends Food Sci. Technol. 2019, 88, 67–79.
- Fan, K.; Zhang, M.; Bhandari, B.; Jiang, F. A combination treatment of ultrasound and ε-polylysine to improve microorganisms and storage quality of fresh-cut lettuce. LWT 2019, 113, 108315.
- Carvalho, F.; Coimbra, A.T.; Silva, L.; Duarte, A.P.; Ferreira, S. Melissa officinalis essential oil as an antimicrobial agent against Listeria monocytogenes in watermelon juice. Food Microbiol. 2023, 109, 104105.
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429.
- Hayek, S.A.; Gyawali, R.; Ibrahim, S.A. Antimicrobial natural products. In Microbial Pathogens and Strategies for Combating Them: Science, Technology, and Education; Mendéz-Villas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 910–921. Available online: https://tarjomefa.com/wp-content/uploads/2016/11/5690-English.pdf (accessed on 2 December 2022).
- Santos, M.; Martins, S.; Pedroso, L.; Sousa, I.; Ferreira, M.A.S.S. Potential bio-activity of whey fermented extract as sanitizer of organic grown lettuce. Food Control 2015, 50, 477–481.
- Santos, M.I.; Lima, A.I.; Monteiro, S.A.; Ferreira, R.M.; Pedroso, L.; Sousa, I.; Ferreira, M.A.S.S. Preliminary Study on the Effect of Fermented Cheese Whey on Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella Goldcoast Populations Inoculated onto Fresh Organic Lettuce. Foodborne Pathog. Dis. 2016, 13, 423–427.
- Meireles, A.; Giaouris, E.; Simões, M. Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res. Int. 2016, 82, 71–85.
- Santos, M.I.S.; Fradinho, P.; Martins, S.; Lima, A.I.G.; Ferreira, R.M.S.B.; Pedroso, L.; Ferreira, M.A.S.S.; Sousa, I. A Novel Way for Whey: Cheese Whey Fermentation Produces an Effective and Environmentally-Safe Alternative to Chlorine. Appl. Sci. 2019, 9, 2800.
- Madhusudan, P.; Chellukuri, N.; Shivakumar, N. Smart packaging of food for the 21st century—A review with futuristic trends, their feasibility and economics. Mater. Today Proc. 2018, 5, 21018–21022.
- Young, E.; Mirosa, M.; Bremer, P. A Systematic Review of Consumer Perceptions of Smart Packaging Technologies for Food. Front. Sustain. Food Syst. 2020, 4, 63.
- Bánáti, D. European perspectives of food safety. J. Sci. Food Agric. 2014, 94, 1941–1946.
- Gizaw, Z. Public health risks related to food safety issues in the food market: A systematic literature review. Environ. Health Prev. Med. 2019, 24, 68.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
15 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




