
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marco Cerrano | -- | 4144 | 2024-01-07 20:00:57 | | | |
| 2 | Lindsay Dong | + 4 word(s) | 4148 | 2024-01-08 01:42:10 | | |
Video Upload Options
Anti-CD19 chimeric antigen receptor (CAR)-T cell therapy has led to a treatment paradigm shift for B-cell non-Hodgkin lymphomas, first with the approval for relapsed/refractory (R/R) large B-cell lymphomas and subsequently for R/R mantle cell and follicular lymphoma. Many efforts are continuously being made to extend the therapeutic setting in the lymphoma field. Several reports are supporting the safety and efficacy of CAR-T cells in patients with central nervous system disease involvement. Anti-CD30 CAR-T cells for the treatment of Hodgkin lymphoma are in development and early studies looking for the optimal target for T-cell malignancies are ongoing. Anti-CD19/CD20 and CD19/CD22 dual targeting CAR-T cells are under investigation in order to increase anti-lymphoma activity and overcome tumor immune escape. Allogeneic CAR product engineering is on the way, representing a rapidly accessible ‘off-the-shelf’ and potentially more fit product.
1. Introduction
2. Upcoming Settings
2.1. Follicular Lymphoma
2.2. Other Indolent Non-Hodgkin Lymphomas
3. Extending the Setting for Large B-Cell Lymphoma
3.1. CAR-T Cells for Lymphomas with Central Nervous System Involvement
3.2. Frontline Adoption of CAR-T Cells
4. New Targets for B-NHLs: Multispecific CAR-T Cells
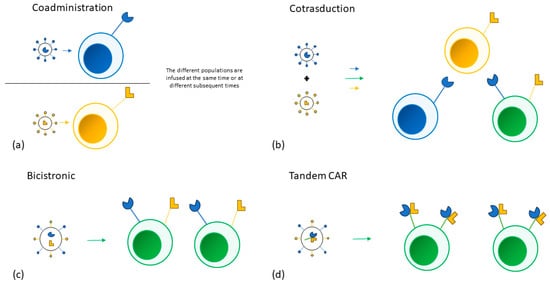
5. New Lymphoma Settings
5.1. Hodgkin Lymphoma
5.2. T-Cell Lymphomas
6. ‘Off-the-Shelf’ Products for NHLs: Allogeneic CAR-T and CAR-NK Cells
6.1. Allogeneic CAR-T Cells
6.2. CAR-NK Cells
6.3. Clinical Experiences with NHLs
7. Other Strategies for CAR-T Cell Manufacturing Improvement
8. Conclusions
References
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. New Engl. J. Med. 2018, 378, 439–448.
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy Bb2121 in Relapsed or Refractory Multiple Myeloma. New Engl. J. Med. 2019, 380, 1726–1737.
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma (CARTITUDE-1): A Phase 1b/2 Open-Label Study. Lancet 2021, 398, 314–324.
- Coscia, M.; Vitale, C.; Cerrano, M.; Maffini, E.; Giaccone, L.; Boccadoro, M.; Bruno, B. Adoptive Immunotherapy with CAR Modified T Cells in Cancer: Current Landscape and Future Perspectives. Front. Biosci.-Landmark 2019, 24, 1284–1315.
- Poletto, S.; Novo, M.; Paruzzo, L.; Frascione, P.M.M.; Vitolo, U. Treatment Strategies for Patients with Diffuse Large B-Cell Lymphoma. Cancer Treat Rev. 2022, 110, 102443.
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New Engl. J. Med. 2017, 377, 2531–2544.
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New Engl. J. Med. 2019, 380, 45–56.
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene Maraleucel for Patients with Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study. Lancet 2020, 396, 839–852.
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1-2 Trial. Lancet Oncol. 2019, 20, 31–42.
- YESCARTA (Axicabtagene Ciloleucel)|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel (accessed on 10 October 2023).
- Yescarta | European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/yescarta (accessed on 10 October 2023).
- BREYANZI (Lisocabtagene Maraleucel)|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/breyanzi-lisocabtagene-maraleucel (accessed on 10 October 2023).
- Breyanzi | European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/breyanzi (accessed on 10 October 2023).
- KYMRIAH (Tisagenlecleucel)|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel (accessed on 10 October 2023).
- Kymriah | European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah (accessed on 10 October 2023).
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. New Engl. J. Med. 2020, 382, 1331–1342.
- TECARTUS (Brexucabtagene Autoleucel)|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/tecartus-brexucabtagene-autoleucel (accessed on 10 October 2023).
- Tecartus|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tecartus (accessed on 10 October 2023).
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. New Engl. J. Med. 2022, 386, 640–654.
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene Maraleucel versus Standard of Care with Salvage Chemotherapy Followed by Autologous Stem Cell Transplantation as Second-Line Treatment in Patients with Relapsed or Refractory Large B-Cell Lymphoma (TRANSFORM): Results from an Interim Analysis of an Open-Label, Randomised, Phase 3 Trial. Lancet 2022, 399, 2294–2308.
- Bishop, M.R.; Dickinson, M.; Purtill, D.; Barba, P.; Santoro, A.; Hamad, N.; Kato, K.; Sureda, A.; Greil, R.; Thieblemont, C.; et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. New Engl. J. Med. 2022, 386, 629–639.
- Cerrano, M.; Ruella, M.; Perales, M.A.; Vitale, C.; Faraci, D.G.; Giaccone, L.; Coscia, M.; Maloy, M.; Sanchez-Escamilla, M.; Elsabah, H.; et al. The Advent of CAR T-Cell Therapy for Lymphoproliferative Neoplasms: Integrating Research into Clinical Practice. Front. Immunol. 2020, 11, 888.
- Plaks, V.; Rossi, J.M.; Chou, J.; Wang, L.; Poddar, S.; Han, G.; Wang, Z.; Kuang, S.Q.; Chu, F.; Davis, R.E.; et al. CD19 Target Evasion as a Mechanism of Relapse in Large B-Cell Lymphoma Treated with Axicabtagene Ciloleucel. Blood 2021, 138, 1081–1085.
- Jain, M.D.; Zhao, H.; Wang, X.; Atkins, R.; Menges, M.; Reid, K.; Spitler, K.; Faramand, R.; Bachmeier, C.; Dean, E.A.; et al. Tumor Interferon Signaling and Suppressive Myeloid Cells Are Associated with CAR T-Cell Failure in Large B-Cell Lymphoma. Blood 2021, 137, 2621–2633.
- Allen, E.S.; Stroncek, D.F.; Ren, J.; Eder, A.F.; West, K.A.; Fry, T.J.; Lee, D.W.; Mackall, C.L.; Conry-Cantilena, C. Autologous Lymphapheresis for the Production of Chimeric Antigen Receptor T Cells. Transfusion 2017, 57, 1133–1141.
- Jo, T.; Yoshihara, S.; Okuyama, Y.; Fujii, K.; Henzan, T.; Kahata, K.; Yamazaki, R.; Takeda, W.; Umezawa, Y.; Fukushima, K.; et al. Risk Factors for CAR-T Cell Manufacturing Failure among DLBCL Patients: A Nationwide Survey in Japan. Br. J. Haematol. 2023, 202, 256–266.
- Jacobson, C.; Chavez, J.C.; Sehgal, A.R.; William, B.M.; Munoz, J.; Salles, G.; Munshi, P.N.; Casulo, C.; Maloney, D.; de Vos, S.; et al. Primary Analysis of Zuma-5: A Phase 2 Study of Axicabtagene Ciloleucel (Axi-Cel) in Patients with Relapsed/Refractory (R/R) Indolent Non-Hodgkin Lymphoma (INHL). Blood 2020, 136, 40–41.
- Neelapu, S.S.; Chavez, J.; Sehgal, A.R.; Epperla, N.; Ulrickson, M.; Bachy, E.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. 3-Year Follow-up Analysis of ZUMA-5: A Phase 2 Study of Axicabtagene Ciloleucel (Axi-Cel) in Patients with Relapsed/Refractory (R/R) Indolent Non-Hodgkin Lymphoma (INHL). Blood 2022, 140, 10380–10383.
- Palomba, M.L.; Ghione, P.; Patel, A.R.; Nahas, M.; Beygi, S.; Hatswell, A.J.; Kanters, S.; Limbrick-Oldfield, E.H.; Wade, S.W.; Ray, M.D.; et al. A 24-Month Updated Analysis of the Comparative Effectiveness of ZUMA-5 (Axi-Cel) vs. SCHOLAR-5 External Control in Relapsed/Refractory Follicular Lymphoma. Expert Rev. Anticancer Ther. 2023, 23, 199–206.
- Dreyling, M.; Dickinson, M.; Martinez Lopez, J.; Kolstad, A.; Butler, J.P.; Ghosh, M.; Popplewell, L.L.; Chavez, J.; Bachy, E.; Kato, K.; et al. Long-Term Clinical Outcomes and Correlative Efficacy Analyses in Patients (Pts) with Relapsed/Refractory Follicular Lymphoma (r/r FL) Treated with Tisagenlecleucel in the Elara Trial. Blood 2022, 140, 1459–1463.
- Salles, G.; Schuster, S.J.; Dreyling, M.; Fischer, L.; Kuruvilla, J.; Patten, P.E.M.; von Tresckow, B.; Smith, S.M.; Jiménez-Ubieto, A.; Davis, K.L.; et al. Efficacy Comparison of Tisagenlecleucel vs Usual Care in Patients with Relapsed or Refractory Follicular Lymphoma. Blood Adv. 2022, 6, 5835–5843.
- Holtzman, N.G.; Shah, N.N. CAR T-Cell Therapy for Indolent Lymphoma: A New Treatment Paradigm? Lancet Oncol. 2022, 23, 6–8.
- Lia Palomba, M.; Qualls, D.; Monette, S.; Sethi, S.; Dogan, A.; Roshal, M.; Senechal, B.; Wang, X.; Rivière, I.; Sadelain, M.; et al. CD19-Directed Chimeric Antigen Receptor T Cell Therapy in Waldenström Macroglobulinemia: A Preclinical Model and Initial Clinical Experience. J. Immunother Cancer 2022, 10, e004128.
- Shadman, M.; Yeung, C.; Redman, M.W.; Lee, S.Y.; Lee, D.H.; Ra, S.; Qian, D.H.; Dezube, B.; Chapuis, A.; Green, D.; et al. P1097: CD20 CAR-T THERAPY WITH MB-106 FOR BTK INHIBITOR-REFRACTORY WALDENSTRÖM MACROGLOBULINEMIA (WM)/LYMPHOPLASMACYTIC LYMPHOMA (LPL)—SINGLE INSTITUTION STUDY. Hemasphere 2023, 7, e68877ca.
- Jahnke, K.; Thiel, E.; Martus, P.; Herrlinger, U.; Weller, M.; Fischer, L.; Korfel, A. Relapse of Primary Central Nervous System Lymphoma: Clinical Features, Outcome and Prognostic Factors. J. Neurooncol. 2006, 80, 159–165.
- Zinzani, P.L.; Magagnoli, M.; Frezza, G.; Prologo, G.; Gherlinzoni, F.; Bendandi, M.; Albertini, P.; Babini, L.; D’Alessandro, R.; Tura, S. Isolated Central Nervous System Relapse in Aggressive Non-Hodgkin’s Lymphoma: The Bologna Experience. Leuk. Lymphoma 1999, 32, 571–576.
- Chihara, D.; Dunleavy, K. Primary Central Nervous System Lymphoma: Evolving Biologic Insights and Recent Therapeutic Advances. Clin. Lymphoma Myeloma Leuk. 2021, 21, 73–79.
- Parker, K.R.; Migliorini, D.; Perkey, E.; Yost, K.E.; Bhaduri, A.; Bagga, P.; Haris, M.; Wilson, N.E.; Liu, F.; Gabunia, K.; et al. Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies. Cell 2020, 183, 126–142.e17.
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric Antigen Receptor–Modified T Cells for Acute Lymphoid Leukemia. New Engl. J. Med. 2013, 368, 1509–1518.
- Keu, K.V.; Witney, T.H.; Yaghoubi, S.; Rosenberg, J.; Kurien, A.; Magnusson, R.; Williams, J.; Habte, F.; Wagner, J.R.; Forman, S.; et al. Reporter Gene Imaging of Targeted T Cell Immunotherapy in Recurrent Glioma. Sci. Transl. Med. 2017, 9, eaag2196.
- Abramson, J.S.; McGree, B.; Noyes, S.; Plummer, S.; Wong, C.; Chen, Y.-B.; Palmer, E.; Albertson, T.; Ferry, J.A.; Arrillaga-Romany, I.C. Anti-CD19 CAR T Cells in CNS Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 783–784.
- Novo, M.; Ruff, M.W.; Skrabek, P.J.; Lin, Y. Axicabtagene Ciloleucel Chimeric Antigen Receptor T Cell Therapy in Lymphoma with Secondary Central Nervous System Involvement. Mayo Clin. Proc. 2019, 94, 2361–2364.
- Frigault, M.J.; Maus, M.V.; Dietrich, J.; Martinez-Lage, M.; Leick, M.; Choi, B.D.; DeFilipp, Z.; Chen, Y.-B.; Abramson, J.; Crombie, J.; et al. Tisagenlecleucel CAR T-Cell Therapy in Secondary CNS Lymphoma. Blood 2019, 134, 860–866.
- Ghafouri, S.; Timmerman, J.; Larson, S.; Mead, M.D. Axicabtagene Ciloleucel CAR T-Cell Therapy for Relapsed/Refractory Secondary CNS Non-Hodgkin Lymphoma: Comparable Outcomes and Toxicities, but Shorter Remissions May Warrant Alternative Consolidative Strategies? Bone Marrow Transpl. 2021, 56, 974–977.
- Ahmed, G.; Hamadani, M.; Shah, N.N. CAR T-Cell Therapy for Secondary CNS DLBCL. Blood Adv. 2021, 5, 5626–5630.
- Bennani, N.N.; Maurer, M.J.; Nastoupil, L.J.; Jain, M.D.; Chavez, J.C.; Cashen, A.F.; Dahiya, S.; Lekakis, L.J.; Reagan, P.M.; Oluwole, O.O.; et al. Experience with Axicabtagene Ciloleucel (Axi-Cel) in Patients with Secondary CNS Involvement: Results from the US Lymphoma CAR T Consortium. Blood 2019, 134, 763.
- Cook, M.R.; Dorris, C.S.; Makambi, K.H.; Luo, Y.; Munshi, P.N.; Donato, M.; Rowley, S.; Saad, A.; Goy, A.; Dunleavy, K.; et al. Toxicity and Efficacy of CAR T-Cell Therapy in Primary and Secondary CNS Lymphoma: A Meta-Analysis of 128 Patients. Blood Adv. 2023, 7, 32–39.
- Ayuk, F.; Gagelmann, N.; von Tresckow, B.; Wulf, G.; Rejeski, K.; Stelljes, M.; Penack, O.; Baldus, C.D.; Kröger, N.; Bethge, W.; et al. Real-World Results of CAR T-Cell Therapy for Large B-Cell Lymphoma with CNS Involvement: A GLA/DRST Study. Blood Adv. 2023, 7, 5316–5319.
- Neelapu, S.S.; Dickinson, M.; Munoz, J.; Ulrickson, M.L.; Thieblemont, C.; Oluwole, O.O.; Herrera, A.F.; Ujjani, C.S.; Lin, Y.; Riedell, P.A.; et al. Axicabtagene Ciloleucel as First-Line Therapy in High-Risk Large B-Cell Lymphoma: The Phase 2 ZUMA-12 Trial. Nat. Med. 2022, 28, 735–742.
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058.
- Nastoupil, L.J. Will CAR T-Cell Therapy Be the Preferred Modality in Frontline Treatment of Large B-Cell Lymphoma? Hematologist 2023, 20, doi.
- Westin, J.R.; Kersten, M.J.; Salles, G.; Abramson, J.S.; Schuster, S.J.; Locke, F.L.; Andreadis, C. Efficacy and Safety of CD19-Directed CAR-T Cell Therapies in Patients with Relapsed/Refractory Aggressive B-Cell Lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND Trials. Am. J. Hematol. 2021, 96, 1295–1312.
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554.
- Zah, E.; Lin, M.Y.; Anne, S.B.; Jensen, M.C.; Chen, Y.Y. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol. Res. 2016, 4, 498–508.
- Furqan, F.; Shah, N.N. Multispecific CAR T Cells Deprive Lymphomas of Escape via Antigen Loss. Annu. Rev. Med. 2023, 74, 279–291.
- Shah, N.N.; Maatman, T.; Hari, P.; Johnson, B. Multi Targeted CAR-T Cell Therapies for B-Cell Malignancies. Front. Oncol. 2019, 9, 146.
- Pavlasova, G.; Mraz, M. The Regulation and Function of CD20: An “Enigma” of B-Cell Biology and Targeted Therapy. Haematologica 2020, 105, 1494–1506.
- Zhang, Y.; Wang, Y.; Liu, Y.; Tong, C.; Wang, C.; Guo, Y.; Ti, D.; Yang, Q.; Qiao, S.; Wu, Z.; et al. Long-Term Activity of Tandem CD19/CD20 CAR Therapy in Refractory/Relapsed B-Cell Lymphoma: A Single-Arm, Phase 1-2 Trial. Leukemia 2022, 36, 189–196.
- Meng, Y.; Deng, B.; Rong, L.; Li, C.; Song, W.; Ling, Z.; Xu, J.; Duan, J.; Wang, Z.; Chang, A.H.; et al. Short-Interval Sequential CAR-T Cell Infusion May Enhance Prior CAR-T Cell Expansion to Augment Anti-Lymphoma Response in B-NHL. Front. Oncol. 2021, 11, 640166.
- Schneider, D.; Xiong, Y.; Wu, D.; Hu, P.; Alabanza, L.; Steimle, B.; Mahmud, H.; Anthony-Gonda, K.; Krueger, W.; Zhu, Z.; et al. Trispecific CD19-CD20-CD22-Targeting DuoCAR-T Cells Eliminate Antigen-Heterogeneous B Cell Tumors in Preclinical Models. Sci. Transl. Med. 2021, 13, eabc6401.
- Roddie, C.; Lekakis, L.J.; Marzolini, M.A.V.; Ramakrishnan, A.; Zhang, Y.; Hu, Y.; Peddareddigari, V.G.R.; Khokhar, N.Z.; Chen, R.W.; Basilico, S.; et al. Dual Targeting of CD19 and CD22 with Bicistronic CAR-T Cells in Patients with Relapsed/Refractory Large B-Cell Lymphoma. Blood 2023, 141, 2470–2482.
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illés, Á.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 378, 331–344.
- Ansell, S.M.; Radford, J.; Connors, J.M.; Długosz-Danecka, M.; Kim, W.-S.; Gallamini, A.; Ramchandren, R.; Friedberg, J.W.; Advani, R.; Hutchings, M.; et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2022, 387, 310–320.
- Herrera, A.F.; LeBlanc, M.L.; Castellino, S.M.; Li, H.; Rutherford, S.C.; Evens, A.M.; Davison, K.; Punnett, A.; Hodgson, D.C.; Parsons, S.K.; et al. SWOG S1826, a Randomized Study of Nivolumab(N)-AVD versus Brentuximab Vedotin(BV)-AVD in Advanced Stage (AS) Classic Hodgkin Lymphoma (HL). J. Clin. Oncol. 2023, 41, LBA4.
- Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Rahbarizadeh, F. CAR-T Cell Therapy in T-Cell Malignancies: Is Success a Low-Hanging Fruit? Stem Cell Res. Ther. 2021, 12, 527.
- Leonard, W.J. Cytokines and Immunodeficiency Diseases. Nat. Rev. Immunol. 2001, 1, 200–208.
- Alcantara, M.; Tesio, M.; June, C.H.; Houot, R. CAR T-Cells for T-Cell Malignancies: Challenges in Distinguishing between Therapeutic, Normal, and Neoplastic T-Cells. Leukemia 2018, 32, 2307–2315.
- Cooper, M.L.; Choi, J.; Staser, K.; Ritchey, J.K.; Devenport, J.M.; Eckardt, K.; Rettig, M.P.; Wang, B.; Eissenberg, L.G.; Ghobadi, A.; et al. An “off-the-Shelf” Fratricide-Resistant CAR-T for the Treatment of T Cell Hematologic Malignancies. Leukemia 2018, 32, 1970–1983.
- Ruella, M.; Xu, J.; Barrett, D.M.; Fraietta, J.A.; Reich, T.J.; Ambrose, D.E.; Klichinsky, M.; Shestova, O.; Patel, P.R.; Kulikovskaya, I.; et al. Induction of Resistance to Chimeric Antigen Receptor T Cell Therapy by Transduction of a Single Leukemic B Cell. Nat. Med. 2018, 24, 1499–1503.
- Jeyakumar, N.; Smith, M. Custom CARs: Leveraging the Adaptability of Allogeneic CAR Therapies to Address Current Challenges in Relapsed/Refractory DLBCL. Front. Immunol. 2022, 13, 887866.
- Brudno, J.N.; Somerville, R.P.T.; Shi, V.; Rose, J.J.; Halverson, D.C.; Fowler, D.H.; Gea-Banacloche, J.C.; Pavletic, S.Z.; Hickstein, D.D.; Lu, T.L.; et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J. Clin. Oncol. 2016, 34, 1112–1121.
- Chen, Y.J.; Abila, B.; Mostafa Kamel, Y. CAR-T: What Is Next? Cancers 2023, 15, 663.
- Sheikh, S.; Migliorini, D.; Lang, N. CAR T-Based Therapies in Lymphoma: A Review of Current Practice and Perspectives. Biomedicines 2022, 10, 1960.
- Khurana, A.; Lin, Y. Allogeneic Chimeric Antigen Receptor Therapy in Lymphoma. Curr. Treat Options Oncol. 2022, 23, 171–187.
- Leen, A.M.; Bollard, C.M.; Mendizabal, A.M.; Shpall, E.J.; Szabolcs, P.; Antin, J.H.; Kapoor, N.; Pai, S.Y.; Rowley, S.D.; Kebriaei, P.; et al. Multicenter Study of Banked Third-Party Virus-Specific T Cells to Treat Severe Viral Infections after Hematopoietic Stem Cell Transplantation. Blood 2013, 121, 5113–5123.
- Cruz, C.R.Y.; Micklethwaite, K.P.; Savoldo, B.; Ramos, C.A.; Lam, S.; Ku, S.; Diouf, O.; Liu, E.; Barrett, A.J.; Ito, S.; et al. Infusion of Donor-Derived CD19-Redirected Virus-Specific T Cells for B-Cell Malignancies Relapsed after Allogeneic Stem Cell Transplant: A Phase 1 Study. Blood 2013, 122, 2956–2973.
- Qasim, W.; Zhan, H.; Samarasinghe, S.; Adams, S.; Amrolia, P.; Stafford, S.; Butler, K.; Rivat, C.; Wright, G.; Somana, K.; et al. Molecular Remission of Infant B-ALL after Infusion of Universal TALEN Gene-Edited CAR T Cells. Sci. Transl. Med. 2017, 9, eaaj2013.
- Chiesa, R.; Georgiadis, C.; Syed, F.; Zhan, H.; Etuk, A.; Gkazi, S.A.; Preece, R.; Ottaviano, G.; Braybrook, T.; Chu, J.; et al. Base-Edited CAR7 T Cells for Relapsed T-Cell Acute Lymphoblastic Leukemia. New Engl. J. Med. 2023, 389, 899–910.
- Mehta, R.S.; Rezvani, K. Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer. Front. Immunol. 2018, 9, 283.
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553.
- Neelapu, S.S.; Nath, R.; Munoz, J.; Tees, M.; Miklos, D.B.; Frank, M.J.; Malik, S.A.; Stevens, D.; Shin, C.R.; Balakumaran, A.; et al. ALPHA Study: ALLO-501 Produced Deep and Durable Responses in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma Comparable to Autologous CAR T. Blood 2021, 138, 3878.
- Gumber, D.; Wang, L.D. Improving CAR-T Immunotherapy: Overcoming the Challenges of T Cell Exhaustion. EBioMedicine 2022, 77, 103941.
- Irving, M.; Lanitis, E.; Migliorini, D.; Ivics, Z.; Guedan, S. Choosing the Right Tool for Genetic Engineering: Clinical Lessons from Chimeric Antigen Receptor-T Cells. Hum. Gene Ther. 2021, 32, 1044–1058.
- Reichenbach, P.; Giordano Attianese, G.M.P.; Ouchen, K.; Cribioli, E.; Triboulet, M.; Ash, S.; Saillard, M.; Vuillefroy de Silly, R.; Coukos, G.; Irving, M. A Lentiviral Vector for the Production of T Cells with an Inducible Transgene and a Constitutively Expressed Tumour-Targeting Receptor. Nat. Biomed. Eng. 2023, 7, 1063–1080.
- Prinzing, B.; Zebley, C.C.; Petersen, C.T.; Fan, Y.; Anido, A.A.; Yi, Z.; Nguyen, P.; Houke, H.; Bell, M.; Haydar, D.; et al. Deleting DNMT3A in CAR T Cells Prevents Exhaustion and Enhances Antitumor Activity. Sci. Transl. Med. 2021, 13, eabh0272.
- Fraietta, J.A.; Nobles, C.L.; Sammons, M.A.; Lundh, S.; Carty, S.A.; Reich, T.J.; Cogdill, A.P.; Morrissette, J.J.D.; DeNizio, J.E.; Reddy, S.; et al. Disruption of TET2 Promotes the Therapeutic Efficacy of CD19-Targeted T Cells. Nature 2018, 558, 307–312.
- Labanieh, L.; Mackall, C.L. CAR Immune Cells: Design Principles, Resistance and the next Generation. Nature 2023, 614, 635–648.




