Anti-CD19 CAR-T cell therapy has led to a treatment paradigm shift for B-cell non-Hodgkin lymphomas, first with the approval for relapsed/refractory (R/R) large B-cell lymphomas and subsequently for R/R mantle cell and follicular lymphoma. Many efforts are continuously being made to extend the therapeutic setting in the lymphoma field. Several reports are supporting the safety and efficacy of CAR-T cells in patients with central nervous system disease involvement. Anti-CD30 CAR-T cells for the treatment of Hodgkin lymphoma are in development and early studies looking for the optimal target for T-cell malignancies are ongoing. Anti-CD19/CD20 and CD19/CD22 dual targeting CAR-T cells are under investigation in order to increase anti-lymphoma activity and overcome tumor immune escape. Allogeneic CAR product engineering is on the way, representing a rapidly accessible ‘off-the-shelf’ and potentially more fit product.
1. Introduction
The therapeutic landscape for lymphomas has been drastically implemented in recent years. Among novel treatment options, chimeric antigen receptor (CAR)-T cells represent the most striking advance for relapsed and refractory (R/R) B-cell non-Hodgkin lymphomas (B-NHLs), along with B-cell acute lymphoblastic leukemia (ALL) and multiple myeloma [
1,
2,
3,
4,
5]. Autologous lymphocytes are collected and engineered with the transduction of a CAR by a replicant-incompetent viral vector, causing T-cell recognition and killing of tumor cells by a specific antigen. CD19-targeting CAR-T cells have been introduced in clinical practice for the treatment of large B-cell lymphomas (LBCLs) since 2017. Three pivotal trials demonstrated high efficacy of three different anti-CD19 CAR-T products, namely, axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel) and lisocabtagene maraleucel (liso-cel), against R/R LBCL after two or more prior lines of therapy [
6,
7,
8]. The three compounds, despite some structural differences that are mainly derived from a distinct costimulatory domain (CD28 for axi-cel, 4-1BB for tisa-cel and liso-cel), demonstrated comparable efficacy as a third line, achieving durable remissions in around 35–40% of patients. Thus, they obtained initial approval from international regulatory agencies for the treatment of R/R LBCL after at least two lines of therapy, with some differences in the disease histological subtypes included in the label (extended to primary mediastinal B-cell lymphomas for axi-cel and liso-cel, and to follicular grade 3B lymphomas for liso-cel) [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15]. Many steps forward have been accomplished thereafter to ameliorate the safety and efficacy of CAR-T cell therapy and to extend its accessibility to other settings. Brexucabtagene ciloleucel (brexu-cel) is an anti-CD19 CAR-T cell product with the same structure as axi-cel but a manufacturing process that includes the removal of circulating CD19+ malignant cells. This compound demonstrated relevant efficacy against R/R mantle cell lymphoma (MCL) and its use was thus approved in this setting first in the US in July 2020 and subsequently in Europe [
16,
17,
18]. More recently, the use of CAR-T cells has been anticipated for the first relapse/progression after frontline treatment to improve the cure rate of R/R LBCL. Three randomized phase III trials compared axi-cel (ZUMA-7 trial), liso-cel (TRANSFORM trial) and tisa-cel (BELINDA trial) with the standard-of-care (SOC) approach consisting of platinum-based salvage chemotherapy regimens plus autologous stem-cell transplantation (ASCT). The first two studies demonstrated a significant advantage for CAR-T cells (axi-cel and liso-cel) both in terms of response rates and relapse-free survival and lead to the approval of axi-cel and liso-cel as second-line treatments for refractory and early relapsed LBCL [
10,
11,
12,
13,
19,
20,
21].
Despite the high efficacy demonstrated for B-NHLs, the rate of treatment failure remains high, with 40 to 60% of the patients being resistant or eventually relapsing after CAR-T cell treatment. Moreover, the accessibility to the treatment is restricted to selected subgroups of patients, with limitations regarding the histological disease subtype, age (in some countries), comorbidities and disease dissemination [e.g., excluding cases with central nervous system (CNS) involvement] [
23]. Some of the limitations of CAR-T cell therapy regard the products themselves. First, all commercially available products for lymphoma are directed against the antigen CD19, making them ineffective in cases of CD19 expression loss [
24]. Moreover, the immunosuppressive microenvironment surrounding lymphoma cells can also limit CAR-T cell efficacy. For instance, interferon signaling boosts the expression of programmed death ligand 1 (PDL-1) on cancer cells, which promotes T-cell exhaustion [
25]. The quality of T-cell leukapheresis for the manufacturing of autologous CAR-T cells is also dependent on patients’ lymphocyte counts; patients’ age, disease status and recent exposure to lymphodepleting drugs can all cause insufficient cell collection and manufacturing failures [
26,
27].
2. Upcoming Settings
2.1. Follicular Lymphoma
Treating follicular lymphoma (FL) patients with anti-CD19 CAR-T cells in clinical practice is already possible, since axi-cel and tisa-cel recently received Food and Drug Administration (FDA) and European Medicine Agency (EMA) approval. In the US, both products are indicated after at least 2 prior lines of therapy, while in Europe, axi-cel requires 3 previous lines [
10,
11,
14,
15].
Axi-cel was authorized based on the data from the pivotal ZUMA-5 trial. Eligible patients had R/R FL after ≥2 lines of therapy, including an anti-CD20 monoclonal antibody plus an alkylating agent. Among 124 treated patients, 36-month PFS and OS rates were 54.4% and 75%, respectively. Grade ≥ 3 cytokine release syndrome (CRS) occurred in 6% of the patients, and grade ≥ 3 immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 15% [
28,
29]. A propensity score matching comparison between patients with R/R FL treated in the ZUMA-5 setting and patients treated with SOC therapies strongly favored axi-cel both in terms of PFS (HR 0.28, 95% CI: 0.17–0.45) and OS (HR 0.52, 95% CI: 0.28–0.95) [
30].
Tisa-cel was approved based on the ELARA trial, which also included patients with R/R FL after ≥2 lines of therapy. Ninety-four patients were treated, and the estimated 24-month PFS and OS were 57.4% and 87.7%, respectively. Toxicities were seemingly lower than those of axi-cel, with no CRS exceeding grade 2 and grade ≥ 3 ICANS of 3% [
31]. Again, a propensity score matching study confirmed the stronger efficacy of CAR-T cell therapy compared to usual care both in terms of PFS and OS [
32].
2.2. Other Indolent Non-Hodgkin Lymphomas
In addition to FL cases, 28 patients with R/R marginal zone lymphoma (MZL) were treated with axi-cel in the ZUMA-5 trial. At a median follow up of 31.8 months, the median PFS was not reached. Grade ≥ 3 CRS occurred in 9% of the patients, while, surprisingly, the grade ≥ 3 ICANS rate was 36%, which was significantly higher than that observed in FL. The reason for this discrepancy is not known, but it could be related to a stronger and more prolonged CAR-T cell expansion in patients with MZL [
28,
29,
35].
Very small reports of CAR-T cell use for Waldenstrom macroglobulinemia (WM)/lymphoplasmacytic lymphoma are available. Three heavily pretreated patients infused with two different anti-CD19 CAR-T cell products attained a clinical response; however, all three had recurrent disease between 3 and 26 months after the initial treatment [
36]. More recently, a promising report of an anti-CD20 CAR-T product infused in four heavily pretreated and Bruton-tyrosine kinase inhibitor (BTKi) refractory patients has been published: the authors reported one death in complete remission 7 months after treatment due to COVID-19 infection and three patients were free from progression at 1.5–19 months, with manageable CAR-T cell-related toxicities [
37].
3. Extending the Setting for Large B-Cell Lymphoma
3.1. CAR-T Cells for Lymphomas with Central Nervous System Involvement
LBCL with CNS involvement, both isolated (primary CNS lymphoma, PCNSL) or concomitant with systemic disease (secondary CNS lymphoma, SCNSL), represents a rare subset with particularly unfavorable outcomes, especially in cases of disease relapse or frontline refractoriness [
70,
71]. The need for agents capable of penetrating the blood–brain barrier (BBB) has led to specific management paradigms for PCNSL and SCNSL, which therefore have been excluded from many LBCL clinical trials with novel agents [
72]. The adoption of CAR-T cells in this setting was limited by the concern of potential severe neurological toxicities. This fear was supported by the identification of a brain mural pericyte cell population with CD19 antigen expression, representing a potential off-tumor target for anti-CD19 CAR-T cells [
73].
The capability of peripheral expansion of CAR-T cells, CNS trafficking and subsequent therapeutic activity were first highlighted in cases of ALL and primary brain solid tumors [
74,
75]. In 2017, Abramson et al. [
76] described for the first time a case of LBCL with synchronous systemic and parenchymal brain disease recurrence treated with anti-CD19 CAR-T cells (liso-cel), obtaining a complete response with no CRS or neurotoxicity observed. Afterwards, a rising number of case series confirmed the efficacy and safety of both axi-cel and tisa-cel for R/R LBCL with CNS recurrence, comparable to non-CNS LBCL [
77,
78,
79,
80,
81]. In the case series reported by Frigault M et al. [
78], six of eight patients presented isolated CNS disease at the time of tisa-cel infusion and three of six obtained a clinical response, confirming the potential capability of CAR-T cells to expand even in the absence of systemic disease. Supported by the early evidence of efficacy against SCNSL, CAR-T cells have been tested against R/R PCNSL. In a retrospective analysis of five patients with R/R PCNSL treated with an academic anti-CD19 CAR-T product at City of Hope, three of five patients achieved CR at day 28 post-infusion.
Given the rarity of the scenario, the available data for CAR-T cell therapy in patients with CNS lymphoma are so far limited to very small case series. Thus, Cook MR et al. [
84] conducted a meta-analysis of patients with PCNSL or SCNSL treated with CAR-T cells both in clinical trials and in the real-life setting. One hundred twenty-eight patients treated with axi-cel, tisa-cel, liso-cel or other investigational anti-CD19 CAR-T compounds were included in the analysis (
n = 30 with PCNSL,
n = 98 with SCNSL) and the authors could confirm the efficacy and tolerability of CAR-T cells in patients with CNS disease. The CRS incidence was superimposable for PCNSL and SCNSL and was observed in around 70% of the patients, with roughly 10% being grade 3–4. ICANS occurred in 50% of the patients and a higher proportion of grade 3–4 cases was reported in SCNSL patients (26%) vs. PCNSL patients (18%), possibly because the presence of a systemic disease might translate into higher systemic inflammation and circulating cytokine levels. Fifty-six percent of PCNSL patients and 47% of SCNSL patients achieved CR as the best response and approximately 37% of the patients in both groups were in ongoing CR 6 months after the infusion. Of note, the authors observed higher toxicities and possibly superior efficacy for CD28 costimulatory domain-based products compared to 4-1BB compounds, similar to LBCL without CNS involvement.
Lastly, the German Lymphoma Group just reported the largest retrospective series of SCNSL treated with anti-CD19 CAR-T cells so far, including 28 consecutive patients, half receiving axi-cel and half receiving tisa-cel. Sixty-four percent of the patients responded, with a CR rate of 32% and a 12-month PFS of 40%, which was significantly better for patients treated with axi-cel than tisa-cel (12-month PFS of 62% vs. 19%, respectively). Of note, comparing the 28 SCNSL patients with 168 consecutive patients without CNS involvement treated with CAR-T cells, no differences in terms of survival or toxicity were observed [
85].
3.2. Frontline Adoption of CAR-T Cells
The anticipation of CAR-T cell therapy from the third to second line demonstrated a significant benefit for refractory or early relapsed patients with LBCL in two of the three randomized phase III trials mentioned above, leading to the approval of axi-cel and liso-cel in this setting [
19,
20,
21]. Following these favorable results, CAR-T cells have been explored as first-line therapy for high-risk LBCL patients. Axi-cel has been first tested as part of the frontline treatment in the phase II single arm ZUMA-12 trial [
88]. In the study, 40 patients with high-risk LBCL, defined by an IPI score ≥ 3 or double/triple-hit histology and with a PET-guided incomplete response after two courses of R-CHOP (based on the Deauville criteria) [
89], were treated with axi-cel. The trial met the primary end-point, reaching high rates of response (ORR 89%), with a 78% CR rate and 73% of the patients in a continuous response after a median follow-up of almost 16 months. Next, the phase III ZUMA-23 trial has been designed to prove the real benefit of axi-cel over the SOC in the frontline setting [
90]. Treatment-naïve patients with very high-risk LBCL, defined by an IPI score ≥ 4, are randomized to receive axi-cel or continue with SOC following a first cycle of chemoimmunotherapy (R-CHOP or rituximab, etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin [R-EPOCH]). The trial is currently ongoing and will hopefully demonstrate whether a frontline CAR-T cell approach may be the best option for selected high-risk LBCL patients.
4. New Targets for B-NHLs: Multispecific CAR-T Cells
Long-term PFS of patients with aggressive NHL treated with anti-CD19 CAR-T cells remains around 30–40% [
91,
92] and about a third of the patients who relapse show CD19 negativity in tumor biopsy samples due to selection of CD19-negative clones and antigen downregulation [
24]. Thus, multi-targeted CAR-T cell constructs are being developed to overcome this issue [
93,
94].
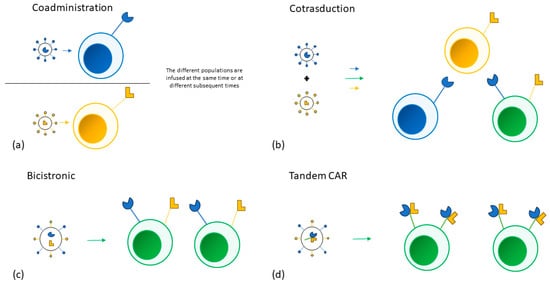
Multiple approaches have been adopted to target more than one tumor antigen. Different single targeting CAR-T cell products can be infused in the same patient at the same time or sequentially. T-cells can also be transduced by different vectors at the same time, creating a population of cells that express different combinations of CARs. A single bicistronic vector encoding two different CARs can be used to create a single T-cell population expressing both CARs. Lastly, a single vector can also be used to encode two CARs on the same receptor (tandem CAR).
No data comparing the different approaches is available [
95]. The different strategies could also be combined in the treatment of the same patient and are represented in
Figure 1.
Figure 1. (a) Two different single targeting CAR-T products are separately manufactured and are then infused in the same patient at the same time or at different subsequent times. (b) T-cells are transduced by two different vectors at the same time, creating three distinct T cell populations. (c) A single bicistronic vector encoding two different CARs is used to transduce a single T-cell population. (d) A single vector encoding two different CARs on the same receptor is used to transduce a single T-cell population.
The most studied approach involves bispecific CAR-T cell constructs: anti-CD19/CD22 and anti-CD19/CD20 CARs have been studied in both preclinical and clinical settings.
CD20 has been a target of immunotherapies since the advent of rituximab. It is expressed almost exclusively in B-cells, and its expression was found to remain stable even in the context of CD19-negative relapses [
24,
96]. Two phase I/II trials reported encouraging results from a total of 103 patients with R/R B-NHL. In the study by Zhang et al. [
39], a CR rate of 70% was obtained and, after a median follow-up of 27.7 months, the median PFS was 27.6 months and median OS was not reached. Nine patients (10%) experienced grade ≥ 3 CRS and two (2%) had grade ≥ 3 ICANS. Three patients died of treatment-related complications. Seven out of nine patients who were previously treated with anti-CD19 CAR-T cells responded to the treatment. Of note, 12 of the patients who relapsed were biopsied: only one demonstrated a double negative CD19-/CD20- disease and no loss of either CD19 or CD20 alone was observed.
Increasing the number of target antigens beyond bispecific CAR-T cells should further reduce the risk of antigen escape and thus of disease relapse. In vitro and in vivo experiments demonstrated increased CAR-T cell expansion and improved antitumor effects through the sequential administration of single targeting anti-CD19, anti-CD20 and anti-CD22 cells. Compassionate treatment of two patients with Burkitt lymphoma and LBCL seems to confirm the observation, showing delayed re-expansion of previously infused cells upon the administration of subsequent products. CAR-T cell-specific toxicities were manageable, consisting of mild to moderate CRS, with no ICANS observed [
102].
No clinical data are available regarding efficacy and safety of trispecific constructs in clinical settings, while preclinical data in mice showed strong activity even against cells negative for one of the three targeted antigens [
103].
Although available data regarding multispecific CAR-T cells show promise in reducing CD19-negative lymphoma relapse, they are still limited to small phase I trials and some early phase II trials. Moreover, treatment with multispecific CAR-T cell constructs does not have an impact on reducing relapse due to reasons other than antigen escape. To address the latter limitation and avoid T-cell exhaustion, the association of bispecific CAR-T cells with anti PD-1 monoclonal antibodies has been explored, with encouraging early results [
104].
5. New Lymphoma Settings
5.1. Hodgkin Lymphoma
Despite the success of frontline therapy, 20–30% of patients with classical Hodgkin lymphoma (cHL) progress or relapse during the course of their disease history. Salvage with high-dose chemotherapy followed by ASCT remains the standard of care in second-line treatment. Targeted therapy with the anti-CD30 antibody-drug conjugate brentuximab vedotin and immunotherapy with anti PD1/PDL1 are currently considered the best options for subsequent relapses and, more recently, their introduction in combination with chemotherapy regimens in the upfront setting further improved outcomes for patients with advanced stage disease [
105,
106,
107]. Nevertheless, a significant fraction of patients needs alternative approaches.
cHL is characterized by a reduced number of malignant Hodgkin and Reed–Sternberg (HRS) cells and an abundance of inflammatory and immune cells, including reactive T- and B-lymphocytes, macrophages, granulocytes and fibroblast-like cells. CD30, a member of the TNF superfamily, is selectively over-expressed in HRS cells with very low expression in normal tissues, and therefore it is considered a promising target for novel treatments.
5.2. T-Cell Lymphomas
Despite their rapidly expanding use against B-cell malignancies, the development of CAR-T cell therapy for T-cell neoplasms is rather challenging and no products are currently available for this subset of lymphomas.
Most antigens tested as CAR-T cell targets for T-cell neoplasms (such as CD3, CD5, and CD7) are also expressed by normal T-cells [
110]. Therefore, the lack of a tumor-specific antigen can cause the eradication of normal T-lymphocytes by CAR-T cell treatment, leading to T-cell aplasia, a possibly life-threatening phenomenon [
111,
112].
Additionally, the expression of their own target antigens by CAR-T cells leads to mutual killing, also known as fratricide, resulting in impaired persistence and efficacy [
113].
Although less common than in ALL, circulating tumor cells might be found in peripheral blood. Therefore, T-cell collection could be contaminated with malignant cells, which can be erroneously transduced, as reported in a patient who relapsed by expressing anti-CD19 CAR-tumor cells [
114].
One of the fundamental challenges in developing effective CAR-T cell therapies for T-cell lymphomas lies in the identification of suitable target antigens. The ideal target should be specific for the malignant cells and not expressed on normal cells, but T-cell lymphomas originate from mature T-cells and lack a clear distinctive cell surface marker.
6. ‘Off-the-Shelf’ Products for NHLs: Allogeneic CAR-T and CAR-NK Cells
6.1. Allogeneic CAR-T Cells
In contrast to autologous CAR-T cells, off-the-shelf products offer several potential advantages. First, T-cells are collected from healthy donors, thus avoiding the potential detrimental effects of cancer or and cytotoxic agents. Additionally, large quantities of allo-CAR-Ts can be derived from a single donor, enabling the creation of readily available batches of preserved CAR-T cells for immediate patient access. The increased availability of the product may result in a reduced need for bridging chemotherapy and lower costs [
128,
129]. Finally, since allo-CAR-Ts can be created from T-cell subsets that may confer properties such as memory or stemness, a better persistence might be obtained [
127,
130].
Despite the potential benefits of allo-CAR-Ts over autologous CAR-T cell products, if immune cells are sourced from MHC-mismatched donors, these products may lead to graft rejection and to the development of graft-versus-host disease (GVHD). Thus, new sources of T-cells for allogeneic approaches have been explored both in preclinical and clinical studies, including virus-specific T-cells, genetically modified conventional T-cells, and non-conventional T-cells [
127,
131].
The adoption of virus-specific T-cells represents a promising way for mitigating the GVHD risk, given their established role in treating post-transplant viral infections [
132]. The safety of this approach was demonstrated in a phase I basket trial involving patients with various B-cell malignancies who received CAR virus-specific T-cells, in which severe GVHD did not occur [
133].
T-cell genetic modification offers another strategy to address GVHD and rejection concerns by removing endogenous molecules such as αβ T-cell receptors (TCR) and MHC. In an early clinical study, two infants with R/R ALL were successfully treated with universal CAR-T cells, a product in which CD52 and αβ TCR were disrupted through a transcription activator-like effector nuclease (TALEN) gene editing technique [
134]. While TCR suppression mitigated the GHVD risk, genetic disruption of CD52 expression allowed the adoption of the anti-CD52 alemtuzumab monoclonal antibody as part of the lymphodepletion without affecting CAR-T cell activity. More recently, a CRISPR/Cas9 base-edited anti-CD7 CAR-T product characterized by a triple CD52/CD7/βTCR gene suppression pattern showed efficacy against R/R T-ALL [
135]. In this case, the gene inactivation of CD52, CD7 and the β chain of the αβ TCR favored the evasion of lymphodepleting therapy, fratricide and GVHD, respectively.
6.2. CAR-NK Cells
Natural killer (NK) cells and macrophages are emerging as highly promising candidates for the development of next-generation off-the-shelf CARs, thanks to their advantageous characteristics. NK cells and macrophages are innate immune system components capable of directly recognizing target cells independent of MHC. Importantly, they do not trigger GVHD and their ability to recognize tumor cells even when MHC molecules are downregulated might prevent antigen escape.
The efficacy of CAR-NK cells has been demonstrated in preclinical models across a range of hematological and solid tumors [
138]. Furthermore, in a phase I/II clinical study involving 11 patients with CD19-positive hematological malignancies, promising antitumor effects without significant toxicities were reported following allogeneic UCB-derived CAR-NK cell administration [
63].
6.3. Clinical Experiences with NHLs
The first clinical trial of allo-CAR-Ts (phase I ALPHA study, NCT03939026) showing the safety and feasibility of this approach included patients with R/R LBCL or FL after at least two lines of therapy. These patients received a single infusion of healthy donor-derived CAR-T cells without prior lymphodepletion chemotherapy. The ALLO-501 CAR-T cells are genetically modified anti-CD19 CAR-T cells with disrupted TCR alpha and CD52 genes, thus reducing the risk of GVHD and allowing the use of a humanized anti-CD52 mAb (ALLO-647) for selective and transitory host lymphodepletion. Forty-six out of forty-seven enrolled patients received ALLO-501, and the treatment was initiated rapidly, with a median time of 5 days from enrollment to therapy start. No GVHD was reported and limited ICANS and CRS were observed. Cytopenias occurred in 82.6% of the patients, and grade ≥ 3 infections were observed in 23.9% of the cases, similar to what is observed with autologous CAR-T cells. The 6-month CR rate for LBCL patients was 36.4% [
57]. The ongoing ALPHA2 study (NCT04416984) is evaluating ALLO-501A (the next-generation product based on ALLO-501 results and lacking the rituximab recognition domains) with either a single or an additional consolidative dose of treatment. In this latter group, patients with ≥SD at day 28 received consolidation with a second ALLO-647 and ALLO-501A cell infusion.
7. Other Strategies for CAR-T Cell Manufacturing Improvement
The vector choice for T-cell transduction can also impact CAR activity and clinical outcomes: traditional “always-on” promoters, such as those carried by retroviral vectors, more commonly lead to tonic signaling and overstimulation of CAR-T cells, which can lead to exhaustion and disease relapse. Enhancing immune cell fitness and limiting or interrupting the interaction between CAR-T cells and target antigens are promising strategies to overcome this limitation [
142,
143,
144].
Both these objectives can be reached thanks to the use of next-generation gene modification strategies, such as the use of lentiviral vectors, which do not rely on cell division and have a higher transduction efficiency, or with nonviral methods, such as CRISPR/Cas9 technology. Disruption of the regulation of DNA methylation in CAR-T cells has resulted in enhanced T-cell proliferation and a more prolonged antitumor response, as was first observed in a clinical trial as a result of casual disruption via lentiviral integration of the gene TET2. This observation was later confirmed in a preclinical model by knocking out the gene DNMT3A [
145,
146]. The downregulation of the activity of specific genes or the overexpression of target transcription factors can lead to a substantial improvement in T-cell potency, expansion and persistency, and many potential targets are being explored [
147].
8. Conclusions
In the last decade, anti-CD19 CAR-T cells reshaped the treatment paradigm for patients with R/R LBCL and MCL, and many steps forward have been made since the first approval in these settings. Axi-cel and liso-cel have already moved to the second line for refractory and early relapsed patients with LBCL and, more recently, CAR-T cell access has been extended to R/R FL. Several reports demonstrated the efficacy and safety of CAR-T cells in patients with CNS lymphoma and could hopefully support the extension of the regulatory approval to this setting. The use of axi-cel as part of frontline treatment for patients with adverse risk LBCL showed promising results in the phase II single arm ZUMA-12 trial, and the ongoing phase III randomized-controlled ZUMA-23 trial will prove whether this approach will be the new SOC for selected patients with very-high-risk LBCL. New strategies are actively being tested to improve CAR-T cell efficacy and accessibility. Dual targeting CD22/CD19- and CD20/CD19-CAR-T cell products showed significant activity against R/R B-NHLs, emerging as a promising strategy to overcome tumor antigen escape mechanisms. Allogeneic CAR products represent an attractive alternative to autologous CAR-T cells thanks to their ‘off-the-shelf’ nature and potential increased antitumor activity, but further data are necessary to thoroughly assess the long-term implications. Finally, early studies demonstrated promising efficacy of CAR-T cells against new disease subtypes, including R/R cHL and, despite the challenges, T-cell lymphoma.
This entry is adapted from the peer-reviewed paper 10.3390/cancers16010046