Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhou, J.; Huang, D.; Liu, C.; Hu, Z.; Li, H.; Lou, S. Heterologous Production of Crocins in Different Species. Encyclopedia. Available online: https://encyclopedia.pub/entry/53507 (accessed on 11 January 2026).
Zhou J, Huang D, Liu C, Hu Z, Li H, Lou S. Heterologous Production of Crocins in Different Species. Encyclopedia. Available at: https://encyclopedia.pub/entry/53507. Accessed January 11, 2026.
Zhou, Junjie, Danqiong Huang, Chenglong Liu, Zhangli Hu, Hui Li, Sulin Lou. "Heterologous Production of Crocins in Different Species" Encyclopedia, https://encyclopedia.pub/entry/53507 (accessed January 11, 2026).
Zhou, J., Huang, D., Liu, C., Hu, Z., Li, H., & Lou, S. (2024, January 06). Heterologous Production of Crocins in Different Species. In Encyclopedia. https://encyclopedia.pub/entry/53507
Zhou, Junjie, et al. "Heterologous Production of Crocins in Different Species." Encyclopedia. Web. 06 January, 2024.
Copy Citation
Crocin is one of the most valuable components of the Chinese medicinal plant Crocus sativus and is widely used in the food, cosmetics, and pharmaceutical industries. Traditional planting of C. sativus is unable to fulfill the increasing demand for crocin in the global market, however, such that researchers have turned their attention to the heterologous production of crocin in a variety of hosts.
synthetic biology
crocin
heterologous production
microalgae
1. Introduction
As a high-value carotenoid, crocins have great potential in pharmacology. Nowadays, many key enzymes in the crocins synthesis pathway have been widely revealed by transcriptomic and dynamic metabolomics studies, while the traditional cultivation model cannot solve the crocins production problem in a short time. It may be a new direction to use genetic engineering technology to transform the key enzymes of the synthetic pathway of crocetin to produce crocetin in species with a high yield of β-carotene or the potential to synthesize β-carotene or crocetin (Figure 1). According to available reports, many microorganisms have been successfully transformed to synthesize crocetin or crocetin, including E. coli [1], yeast [2], Nicotiana glauca [3], Chlorella vulgaris [4], and Dunaliella salina [5]. Recently, the transient transformation of N. benthamiana to synthesize crocins was reported [6]. The above cases provide solid theoretical support and practical basis for further heterologous production of crocin. Below, these cases are divided into higher plant hosts and microbial hosts, which are divided into E. coli, yeast, and microalgal (Figure 1).
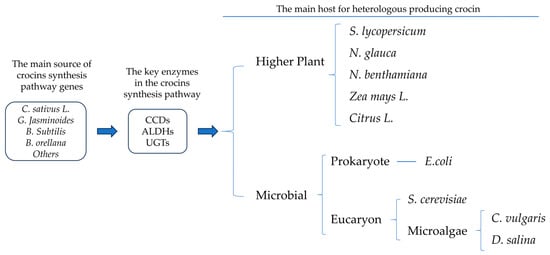
Figure 1. Basic logic of heterologous synthesis of crocin by genetic engineering and the common host.
2. Biosynthesis of Crocins in Higher Plant Hosts
In plants, β-carotene can be converted into other carotenoids to meet particular needs, especially in plants that utilize carotenoids to reduce photooxidative damage [7][8]. Heterologous production of crocins in plants has the advantage of requiring the introduction of only one or two genes since the other genes from the carotenoid pathway are already present [9]. Higher plant cells have abundant β-carotene storage capacity, a complete carotenoid synthesis system, and a complete endomembrane system and thus are ideal host cells for heterologous production of crocin [8]. In addition to C. sativus, crocin is also found in a variety of plants outside the Iridaceae, such as the flowers of B. davidii and the fruit of G. jasminoides [10][11]. However, such sources cannot meet the existing demand [12]. Therefore, researchers are investigating how to produce crocin efficiently in other higher plants.
In 1986, scientists first expressed human growth hormone in tobacco cells and proposed the concept of using plant cells as a platform for recombinant protein production [13]. After more than 30 years of development, plant hosts have become extremely diversified and include whole plants, various plant tissues, suspension cells, and other systems; in addition, there are multiple expression methods within each system [14][15]. Whole-plant cultivation requires special land and climatic conditions and is not suitable for rapid production of specific metabolites [16][17]. However, similarly to E. coli and S. cerevisiae, an isolated single-celled plant callus can be suspended and dispersed in liquid medium for rapid propagation and expression of products [18]. Therefore, suspension culture of plant cells has more prospects than whole-plant cultivation for large-scale industrial applications [19] and the production of high-value-added natural active products [14][20].
N. benthamiana is a plant that does not contain crocin itself, but when it is engineered with the appropriate CCD enzyme, it can overexpress upstream or downstream carotenoid-metabolizing genes, leveraging the crocin synthesis pathway. Zheng et al. [9] used a white citrus callus as host cells with a co-expression system comprising three genes, i.e., Tp-CrtB, Os-BCH, and Gj-CCD4a, and successfully constructed a non-green starch-rich tissue/organ expression platform for effective production of crocin. When the platform was introduced into the leaves of N. benthamiana, up to 1.6 mg/g dry weight crocin was obtained. It was found that Gj-CCD4a had higher substrate specificity and catalytic efficiency in the leaves, demonstrating that a single enzyme, Gj-CCD4a, could drive the synthesis of crocin [9]. Xie et al. [6] combined the strategy of fusion with the 2A polypeptide connection and successfully constructed a multi-gene vector containing four genes to N. benthamiana, which transformed GjCCD4a and two downstream glycosyltransferase genes Gj-UGT74F8 and Gj-UGT94E13, to achieve higher substrate conversion efficiency that solved the problem of the low proportion of the main active components crocin I and crocin II, especially crocin I, as evidenced in previous research for synthesizing crocin in transgenic tobacco, and transformed ALDH introduced into tobacco for the first time.
A related species, N. glauca, contains carotenoid pigments in its petals. Huang et al. [3] expressed the Bd-CCD4.1 enzyme from B. davidii constitutively in its petals and leaves and obtained 321.6 ± 21.3 µg/g and 302.7 ± 25.6 µg/g DCW crocin, respectively. Interestingly, in their transgenic lines expressing CsCCD2L, the difference in the accumulation of crocin between leaves and petals may have been due to the relatively higher accumulation of zeaxanthin or the tissue specificity of CCD in leaves [3]. Martí et al. used tobacco etch virus to drive the expression of Cs-CCD2L and Bd-CCD4.1 in N. benthamiana and found that only Cs-CCD2L could produce 2.18 ± 0.23 mg of crocins and 8.24 ± 2.93 mg of picrocrocin per gram (DCW) over 13 days. The study also found that CCD can intercept the metabolic flux in leaves and reduce the synthesis of lutein, which sharply increases the expression levels of phytoene and drives the carotenoid metabolic pathway in the direction of crocin synthesis [21].
Frusciante et al. introduced CCD2 into zeaxanthin-rich maize endosperm by Agrobacterium-mediated transient expression and found that, unlike in E. coli, where only crocetin dialdehyde could be detected, zeaxanthin was not only converted to crocin dialdehyde but also further oxidized to crocetin. This is likely because maize endosperm possesses an endogenous aldehyde dehydrogenase to facilitate the oxidation step.
Due to the presence of vacuoles, plant cells are large compared with those of E. coli and S. cerevisiae. Accordingly, when using comparable culture volumes, it is difficult to improve production with plant cell cultures by increasing the number of cells. As a result, relatively low yields of recombinant protein product (0.01 to 10 mg/L) are achieved in plant cell systems [14][15]. In addition, not all plant species can be adapted to suspension cell culture in a fermenter due to the presence of exogenous plant enzymes [22].
3. Microbial Biosynthesis of Crocins
Another approach to the production of crocins is combinatorial biosynthesis, which consists of combining enzyme-encoding genes from different species and designing a new set of gene clusters to produce bioactive compounds in a heterologous host. The commonly used microbial hosts for crocin production are E. coli among the prokaryotes and the yeast S. cerevisiae among the eukaryotes [1][2]. It is very important to select a suitable host organism for the optimization of product yield and quality, and there are pros and cons for both bacteria and yeast in this context. Bacterial hosts have a short life cycle, offer easy genetic manipulation and handling, and have a higher growth rate and excellent potential for protein and enzyme overexpression; however, they are not as beneficial for large proteins and proteins requiring post-translational modifications, which may be essential for correct folding and functional activity [19]. The S. cerevisiae is also well characterized and easy to grow and manipulate like E. coli but in addition can express proteins with appropriate post-translational modifications and offers better expression of membrane proteins; moreover, it has food-grade status (generally recognized as safe; GRAS) [23]. However, it results in lower yields than bacteria and can add a large number of mannose residues to recombinant proteins, resulting in protein misfolding and problems with activity [22].
All in all, heterologous production of crocins in microorganisms is highly advantageous since they can grow on inexpensive substrates and, compared to plants, are easier to manipulate and have very rapid production cycles, allowing crocins to be produced faster and possibly in larger amounts [24]. Thus, taking into consideration all the pros and cons, the commercial application of heterologous production of crocins by microorganisms is the more attractive route.
3.1. Biosynthesis of Crocins in E. coli
Wild-type E. coli does not have the ability to synthesize carotenoids itself, but after metabolic engineering, it can successfully synthesize β-carotene and various other carotenoids [25][26][27]. Therefore, E. coli has the potential to synthesize crocin after appropriate pathway modification.
These were the first reports to demonstrate functional expression of a carotene gene cluster in E. coli: Perry et al. [28] and Tuveson et al. [29] introduced a 12.4 kb carotene gene cluster from Erwinia herbicola (reclassified as Pantoea agglomerans) into E. coli and successfully produced yellow pigmentation. Misawa et al. [30] isolated a 6.9 kb yellow-pigment-producing gene cluster fragment from the above genome segment and found six open reading frames: CrtE, CrtI, CrtB, CrtX, CrtY, and CrtZ. It was confirmed that the yellow substance produced using this gene cluster was zeaxanthin and that the recombinant E. coli could also synthesize phytoene, lycopene, β-carotene, zeaxanthin, and basic carotenoids with GGPP as a substrate. In recent years, E. coli has often been used as a host strain for the production of various carotenoids, which thus provides a theoretical basis for the heterologous synthesis of crocin in prokaryotes.
In terms of crocins synthesis, Wang et al. [1] introduced the Cs-CCD2L gene of C. sativus and the glycosyltransferases Gj-UGT94E5 and Gj-UGT75L6 of G. jasminoides into E. coli, which was then capable of producing zeaxanthin and crocetin dialdehyde. This strain was able to produce crocetin after further engineering with the pTrc-ALD8 gene from Neurospora crassa. Finally, the glycosyltransferases of Bs-YjiC, Bs-YdhE, and Bs-YojK were introduced into the expression system to obtain crocin V with a yield of 4.42 mg/L. This was the first time that a heterologous crocetin and crocin synthesis pathway was successfully constructed in E. coli [1]. Ding et al. [31] successfully mined two microbially derived glycosyltransferases with higher heterologous production and catalytic efficiency to improve crocin production. It was found that Bs-GT glycosyltransferase from Bacillus subtilis 168 could achieve a maximum crocetin glycosylation conversion efficiency of 81.9% and a yield of 476.8 mg/L crocin V and crocin III. Bc-GTA showed a much lower conversion efficiency and specificity than Bs-GT [31]. Pu et al. [32] found that G.-jasminoides-derived Gj-UGT74F8 and Gj-UGT94E13 gave whole-cell biotransformation rates as high as 66.1% and 59.6% for 50 mg/L crocin, respectively, which was higher than was achieved using UGTs from microorganisms. By precisely controlling the glycosylation time course, a high concentration of crocin with a specific degree of glycosylation can be obtained. Further optimization of gardenia UGTs may provide a valuable tool for the industrial production of crocin [33]. At the same time, Pu et al. also found that the glucose content in the culture environment is one of the key factors for obtaining crocin. When the endogenous UDPG supply in engineered strains is insufficient for the efficient production of crocin, appropriate supplementation with a certain concentration of glucose can improve the catalytic activity of heterologously expressed UGTs to maintain efficient and sustainable production [32].
The synthesis of crocin in E. coli has been well studied. However, like other prokaryotes, the E. coli does not have a complex internal membrane system, as do eukaryotes. Thus, heterologous production of various eukaryotic enzymes in E. coli may result in differences in folding and functional group modification, which may in turn lead to reduced catalytic efficiency or no enzyme activity. Since C. sativus is a eukaryote, the synthesis of crocin involves the transfer of metabolites between multiple subcellular compartments, e.g., from plastids to vacuoles, and the cooperation of various related enzymes [34]. Lack of these enzymes or use of structurally defective enzymes may affect crocin production or produce toxic byproducts. Zheng et al. [9] obtained crocetin dialdehyde in vitro by incubating β-apo-8′-carotene as a substrate with crude lysates of E. coli cells that expressed Gj-CCD4a, showing that Gj-CCD4a expressed in E. coli has enzyme activity. However, although E. coli itself has no endogenous ALDs, it has been reported that the properties of ALDs from microbial sources expressed in E. coli are better than those of endogenous C. sativus ALDs, while other plant-derived ALDs are expressed at very low levels in E. coli [1]. Further investigation and optimization of candidate ALDs is required.
3.2. Biosynthesis of Crocins in S. cerevisiae
On account of its GRAS status, S. cerevisiae is often used in the field of food processing. Unlike the bacterial model of E. coli, S. cerevisiae is a eukaryotic microorganism and thus has a complete set of intracellular membranes, including nuclear membranes and various organelle membranes, which are similar to those in plant and mammalian cells and provide a complete transcription, translation, and modification environment for foreign genes [35]. The various compartments in the cell interior can also provide transport and storage space for gene expression products and metabolites. Since S. cerevisiae does not have an endogenous biochemical pathway for the synthesis of carotenoids, it is necessary to redesign the enzymes of crocin synthesis that initiate the MVA pathway to increase the levels of substrates to those required by the downstream pathway [36][37]. Shimada et al. redirected the ergosterol biosynthetic pathway in S. cerevisiae by introducing three genes required for lycopene synthesis, namely CrtE, CrtB, and CrtI, and they were thus able to synthesize lycopene with a yield of 1.1 mg/g dry cell weight (DCW). Ergosterol is a type of isoprene that shares a precursor with β-carotene and can provide abundant substrate for the production of crocin [38]. Lv et al. [39] designed a dual-metabolic pathway in S. cerevisiae that simultaneously uses acetyl-CoA in the cytoplasm and mitochondria. In terms of improving the utilization rate of precursors and expanding the production of isoprene, it was shown that this dual-metabolic pathway has advantages over those that only use the mitochondrial pathway or the cytoplasmic pathway in recombinant strains.
When Eu-CrtZ was introduced into S. cerevisiae, along with knock-out of the genes Lpp1 and Dpp1, which are responsible for directing farnesyl pyrophosphate towards ergosterol synthesis, Mei et al. [40] initially found that zeaxanthin production was only increased by a small amount, but a high yield of 96.2 mg/L of zeaxanthin was achieved when three copies of the GAL1 high-strength promoter were used. Improvement of Zeaxanthin Production by Multiple-Copy Integration of Eu-crtZ [41]. Enhancing zeaxanthin production in Y. lipolytica was achieved by integrating the Eu-crtZ gene, in which the gene led to the highest titer and content for producing the target molecule, the expression cassette, into the 26S rDNA region. Xie et al. [41] achieved a 4.02-fold increase in the titer of zeaxanthin and a 721% increase in the content of zeaxanthin than the single copy and achieved a 21.98 ± 1.80 mg/L zeaxanthin titer. This high-yield engineered strain was named SyBE-Sc0123Z020. Chai et al. [2] selected three key enzymes, namely CrtZ, CCD, and ALD, from different species for expression in the S. cerevisiae strain SyBE-SC0014CY06, which was capable of producing β-carotene. The best combination of the three genes was Ps-CrtZ from Pantoea stewartii, Cs-CCD2L from C. sativus, and Syn-ALD from Synechocystis sp. PCC6803, which together produced 0.633 mg/L crocin. Tan et al. [42] designed, optimized, and synthesized a new Cs-ALD enzyme and introduced it into S. cerevisiae SyBE-Sc02070187-189, which was then capable of producing zeaxanthin, obtaining a yield of 62.79 µg/g DCW crocetin dialdehyde. Song et al. [36] knocked out CIT2 and MLS1, two genes that consume acetyl-CoA in the cytoplasm, and increased the production of lycopene by 50%. They then constructed a fusion enzyme composed of Ps-CrtZ and CsCCD2, which increased the concentration of crocin by 44%, yielding 12.43 ± 0.62 mg/L crocin, which was twice as high as that produced by the initial strain SyBE-Sc0123C050 [2]. The above examples suggest that crocin production in S. cerevisiae is feasible, and this could provide a safe and efficient route of crocin production in eukaryotes.
However, S. cerevisiae contains five characterized endogenous ALDH genes and a large number of other endogenous ALDH genes that have not been fully characterized and are difficult to remove. These endogenous ALDH genes will seriously interfere with the expression and function of exogenous ALDH genes, significantly reducing crocin productivity [43]. Amplifying the copy number of exogenous ALDH genes in S. cerevisiae can competitively inhibit the expression and function of the endogenous ALDH genes, improving the expression and specificity of the exogenous ALDH genes, thereby increasing the production of crocetin [43]. When Chai et al. [43] used the multicopy plasmid pRS426 to increase the copy number of Cs-CCD2L and Syn-ALD, the production of crocin was further increased to 1.219 mg/L, which was twice the yield obtained with a single-copy plasmid.
3.3. Biosynthesis of Crocins in Microalgal Hosts
Microalgae are microscopic photosynthetic eukaryotes that live in aquatic environments [44]. As single-celled organisms and the ancestors of land plants originating about 100 million years ago, microalgae nevertheless have a carotenoid synthesis pathway similar to that of higher plants [45][46]. Thus, homologs of CCD1, CCD7, CCD8, and NCED are present in microalgae such that heterologous synthesis of crocin from β-carotene is possible [45][47]. Indeed, the complex carotenoid metabolism system in microalgae can synthesize a variety of carotenoids that are found in land plants, such as lutein, astaxanthin, fucoxanthin, and β-carotene [48]. Based on the background of Chlamydomonas β-carotene synthesis pathway, it can greatly reduce the building line of the crocin synthesis pathway module and workload.
Microalgae are characterized by a fast growth rate, relatively easy modification of endogenous metabolic pathways, and a complement of silent genes or genes expressed at low levels; this simplifies their metabolic engineering for use as a crocin bioreactor [49]. Carotenoids from microalgae have already been used for commercial purposes. For example, C. vulgaris can use lycopene as a precursor for the synthesis of β-carotene, zeaxanthin, astaxanthin, and other substances under different culture conditions [49]. D. salina, which can survive in extremely high-salt environments, produces β-carotene naturally. One benefit of the high-salinity culture environment is that it can effectively inhibit contamination by other microorganisms, thereby reducing culture costs [50].
In the 1960s, C. vulgaris became the first single-celled green alga to be exploited on a large scale because of its simple structure, fast growth, and low maintenance costs [51]. C. vulgaris has been used as a cell factory and can synthesize various nutrients through photosynthesis; it is also capable of synthesizing proteins, carbohydrates, carotenoids, and lipids. Its protein content can be as high as 68%, and it is widely used in human health foods and additives as well as for animal feed in aquaculture [52][53][54][55]. However, unbalanced cellular metabolic fluxes and competition between intermediate and precursor metabolites are challenges for the heterologous expression of crocin in microalgae. Lycopene ε-cyclase (LCYE) is a crucial enzyme that cyclizes lycopene to α-carotene and provides a large pool of substrate for the synthesis of lutein [56]. The enzyme of LCYE is encoded by the CvLCYE gene, whose nucleotide sequence is highly conserved in a variety of green algae [4]. Overexpression of the CvLCYE gene can greatly improve lutein production in C. vulgaris [4]. By blocking or silencing the expression of CvLCYE gene, more lycopene can flow to β-carotene synthesis, thereby providing more substrate for the synthesis of crocin.
Based on this characteristic of C. vulgaris, Lou et al. [57] used Agrobacterium-mediated transient expression of the CrtRB gene from Haematococcus pluvialis and the ZCD1 gene from the stigma of C. sativus in C. vulgaris and successfully detected the accumulation of crocin. This was the first report to demonstrate crocin production in microalgae. ZCD1 is a 13-amino-acid mutant of Cs-ZCD, which originally lacked the residues and domains necessary for zeaxanthin dioxygenase activity; this modification restores the activity [57][58], which is important for modifying the weak activity of CCD and increasing the production of crocin.
D. salina is a free-moving, single-celled green microalga with flagella but without a rigid cell wall [59]. The intracellular glycerol content of D. salina is more than 50% its weight, which allows it to regulate the osmotic pressure by changing the intracellular glycerol concentration [60]. Therefore, it can survive in salt solutions of 0.5% to 35%, i.e., up to nearly saturated solutions [60]. It is one of the most salt-tolerant eukaryotes known [61]. The optimal-growth salt concentration range for D. salina is 1.0–2.0 M NaCl [62]. Under high salt-stress conditions, i.e., 3.0–4.0 M NaCl, the synthesis of chlorophyll and cell growth are inhibited [63]. However, when operating at optimal salt concentrations, contamination by most non-halotolerant bacteria or protists is minimal, thus reducing production costs and helping to maintain an axenic environment [64]. Compared with higher plants, microalgae grow fast. Most higher plants depend on photosynthesis for their growth and reproduction [60][65]. On the other hand, D. salina has the highest known content of β-carotene in the plant kingdom [66][67]. It is rich in lutein, zeaxanthin, and β-carotene, the latter of which accounts for 14% of DCW [68]. D. salina is one of the most widely used algal species for the commercial production of β-carotene, and it also has strong potential for crocin synthesis [59][69][70][71]. Due to their versatility in adapting to a variety of growing conditions and climates (e.g., glacial to tropical and freshwater to highly saline) and different pH values, microalgae show distinct advantages over higher plants, reducing the need for sophisticated culture equipment and thereby reducing costs. Microalgae generally have higher carotenoid contents than higher plants. The major carotenoids in D. salina include 9- or 9′-cis-β-carotene and all-trans-β-carotene, which is preferentially absorbed compared to the 9-cis-β-isomer [66]. Nevertheless, the 9-cis-β-isomer has a higher antioxidant activity due to the higher reactivity of the cis bond compared to the trans bond [66]. Among all natural sources studied to date, D. salina possesses the highest content of 9-cis-β-carotene, reaching levels of up to 100 g/kg of DCW [66][67]. This would provide a large substrate pool for the production of crocin by D. salina [72]. The relative carotenoid content (% of total carotenoids) of octahydro-lycopene increased more than 48-fold in D. salina after treatment with mitogenic inhibitors (propyzamide and chlorpropham) for 10 h [72]. The production of lycopene and β-carotene was also significantly increased after exposure to red light. This is due to the accumulation of the more readily degraded 9-cis β-carotene under high-intensity red-light conditions; such conditions are associated with high rates of photooxidation, which in turn increases the activity of β-carotene isomerases, the gene transcripts of which are induced by light stress [73]. These characteristics of D. salina provide some conditions for the synthesis of crocin by transgenic technology.
By mining the transcriptome and genome of D. salina using deep sequencing, Lou et al. [74] found that, under high-light and high-salinity stress, D. salina activates an endogenous miRNA, m0533-3p, which in response to the stress signals inhibits malate dehydrogenase. This is likely to lead to a reduced flow of acetyl-CoA into the tricarboxylic acid cycle and instead greater participation of acetyl-CoA in the synthesis of GGPP, with a concomitant increase in β-carotene levels. However, as salt concentration increases, D. salina is more inclined to divert β-carotene to α-ionone and β-ionone synthesis to improve stress resistance, resulting in a decrease in β-carotene reserves, thus affecting the conversion efficiency of crocin [75][76][77]. Therefore, to balance these two opposing fluxes, the optimal salt concentration for D. salina should be 1.5 M NaCl [75][76]. Hou [5] introduced CrtRB, Cs-ZCD, and CCD2 as target genes into D. salina by the glass-bead method and successfully detected trace amounts of crocetin dialdehyde.
D. salina has many applications in the pharmaceutical, nutraceutical, and cosmeceutical industries. However, although there are thorough and comprehensive research methods for using microalgae to produce other carotenoid products, they are still in the initial stages as hosts for the production of crocin; still, they have great potential for this application [78][79]. Nevertheless, it will not be enough to identify and modify the key enzymes in engineered pathways; there will also be a requirement for increased investment in the optimization of algal strains and for further investigation and optimization of culture conditions, methods of exogenous gene transformation, and the selection of transcription and translation-related factors [80].
References
- Wang, W.; He, P.; Zhao, D.; Ye, L.; Dai, L.; Zhang, X.; Sun, Y.; Zheng, J.; Bi, C. Construction of Escherichia coli cell factories for crocin biosynthesis. Microb. Cell Factories 2019, 18, 120.
- Chai, F.; Wang, Y.; Mei, X.; Yao, M.; Chen, Y.; Liu, H.; Xiao, W.; Yuan, Y. Heterologous biosynthesis and manipulation of crocetin in Saccharomyces cerevisiae. Microb. Cell Factories 2017, 16, 54.
- Huang, X.; Morote, L.; Zhu, C.; Ahrazem, O.; Capell, T.; Christou, P.; Gómez-Gómez, L. The Biosynthesis of Non-Endogenous Apocarotenoids in Transgenic Nicotiana glauca. Metabolites 2022, 12, 575.
- Lou, S.; Lin, X.; Liu, C.; Anwar, M.; Li, H.; Hu, Z. Molecular cloning and functional characterization of CvLCYE, a key enzyme in lutein synthesis pathway in Chlorella vulgaris. Algal Res. 2021, 55, 102246.
- Hou, K. Construction and Identification of ZCD Transgenic Dunaliella salina. Master’s Thesis, Shenzhen University, Shenzhen, China, 2020.
- Xie, L.; Luo, Z.; Jia, X.; Mo, C.; Huang, X.; Suo, Y.; Cui, S.; Zang, Y.; Liao, J.; Ma, X. Synthesis of Crocin I and Crocin II by Multigene Stacking in Nicotiana benthamiana. Int. J. Mol. Sci. 2023, 24, 14139.
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93.
- Ahrazem, O.; Diretto, G.; Rambla, J.L.; Rubio-Moraga, Á.; Lobato-Gómez, M.; Frusciante, S.; Argandoña, J.; Presa, S.; Granell, A.; Gómez-Gómez, L. Engineering high levels of saffron apocarotenoids in tomato. Hortic. Res. 2022, 9, uhac074.
- Zheng, X.; Mi, J.; Balakrishna, A.; Liew, K.X.; Ablazov, A.; Sougrat, R.; Al-Babili, S. Gardenia carotenoid cleavage dioxygenase 4a is an efficient tool for biotechnological production of crocins in green and non-green plant tissues. Plant Biotechnol. J. 2022, 20, 2202–2216.
- Guo, Z.; Li, M.; Li, X.; Wang, P.; Wang, W.; Du, W.; Yang, Z.; Chen, S.; Wu, D.; Tian, X. Crocetin: A Systematic Review. Front. Pharmacol. 2022, 12, 745683.
- Ahrazem, O.; Zhu, C.; Huang, X.; Rubio-Moraga, A.; Capell, T.; Christou, P.; Gómez-Gómez, L. Metabolic Engineering of Crocin Biosynthesis in Nicotiana Species. Front. Plant Sci. 2022, 13, 526.
- Diretto, G.; López-Jiménez, A.J.; Ahrazem, O.; Frusciante, S.; Song, J.; Rubio-Moraga, Á.; Gómez-Gómez, L. Identification and characterization of apocarotenoid modifiers and carotenogenic enzymes for biosynthesis of crocins in Buddleja davidii flowers. J. Exp. Bot. 2021, 72, 3200–3218.
- Tae Hyug, J.; Keunho, J. Overexpression and Characterization of Lycopene Cyclase (CrtY) from Marine Bacterium Paracoccus haeundaensis. J. Microbiol. Biotechnol. 2013, 23, 144–148.
- Schillberg, S.; Raven, N.; Spiegel, H.; Rasche, S.; Buntru, M. Critical Analysis of the Commercial Potential of Plants for the Production of Recombinant Proteins. Front. Plant Sci. 2019, 10, 720.
- Karki, U.; Fang, H.; Guo, W.; Unnold-Cofre, C.; Xu, J. Cellular engineering of plant cells for improved therapeutic protein production. Plant Cell Rep. 2021, 40, 1087–1099.
- Melis, A. Photosynthesis-to-fuels: From sunlight to hydrogen, isoprene, and botryococcene production. Energy Environ. Sci. 2012, 5, 5531–5539.
- Lopes da Silva, T.; Moniz, P.; Silva, C.; Reis, A. The Dark Side of Microalgae Biotechnology: A Heterotrophic Biorefinery Platform Directed to ω-3 Rich Lipid Production. Microorganisms 2019, 7, 670.
- Georgiev, M.I.; Weber, J.; Maciuk, A. Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl. Microbiol. Biotechnol. 2009, 83, 809–823.
- Xu, J.; Zhang, N. On the way to commercializing plant cell culture platform for biopharmaceuticals: Present status and prospect. Pharm. Bioprocess. 2014, 2, 499–518.
- Tiwari, S.; Verma, P.C.; Singh, P.K.; Tuli, R. Plants as bioreactors for the production of vaccine antigens. Biotechnol. Adv. 2009, 27, 449–467.
- Martí, M.; Diretto, G.; Aragonés, V.; Frusciante, S.; Ahrazem, O.; Gómez-Gómez, L.; Daròs, J.-A. Efficient production of saffron crocins and picrocrocin in Nicotiana benthamiana using a virus-driven system. Metab. Eng. 2020, 61, 238–250.
- Jareonsin, S.; Pumas, C. Advantages of Heterotrophic Microalgae as a Host for Phytochemicals Production. Front. Bioeng. Biotechnol. 2021, 9, 628597.
- Ye, M.; Ye, Y.; Du, Z.; Chen, G. Cell-surface engineering of yeasts for whole-cell biocatalysts. Bioprocess Biosyst. Eng. 2021, 44, 1003–1019.
- Rodrigues, J.L.; Prather, K.L.; Kluskens, L.D.; Rodrigues, L.R. Heterologous production of curcuminoids. Microbiol. Mol. Biol. Rev. 2015, 79, 39–60.
- Misawa, N. When Carotenoid Biosynthesis Genes Met Escherichia coli: The Early Days and These Days. In Carotenoids: Biosynthetic and Biofunctional Approaches; Misawa, N., Ed.; Springer: Singapore, 2021; pp. 183–189.
- Sandmann, G.; Misawa, N. Carotenoid Production in Escherichia coli: Case of Acyclic Carotenoids. Adv. Exp. Med. Biol. 2021, 1261, 201–208.
- Misawa, N.; Maoka, T.; Takemura, M. Chapter Three—Carotenoids: Carotenoid and apocarotenoid analysis—Use of E. coli to produce carotenoid standards. In Methods in Enzymology; Wurtzel, E.T., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 670, pp. 87–137.
- Perry, K.L.; Simonitch, T.A.; Harrison-Lavoie, K.J.; Liu, S.T. Cloning and regulation of Erwinia herbicola pigment genes. J. Bacteriol. 1986, 168, 607–612.
- Tuveson, R.W.; Larson, R.A.; Kagan, J. Role of cloned carotenoid genes expressed in Escherichia coli in protecting against inactivation by near-UV light and specific phototoxic molecules. J. Bacteriol. 1988, 170, 4675–4680.
- Misawa, N.; Nakagawa, M.; Kobayashi, K.; Yamano, S.; Izawa, Y.; Nakamura, K.; Harashima, K. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J. Bacteriol. 1990, 172, 6704–6712.
- Ding, F.; Liu, F.; Shao, W.; Chu, J.; Wu, B.; He, B. Efficient Synthesis of Crocins from Crocetin by a Microbial Glycosyltransferase from Bacillus subtilis 168. J. Agric. Food Chem. 2018, 66, 11701–11708.
- Pu, X.; He, C.; Yang, Y.; Wang, W.; Hu, K.; Xu, Z.; Song, J. In Vivo Production of Five Crocins in the Engineered Escherichia coli. ACS Synth. Biol. 2020, 9, 1160–1168.
- Chyau, C.-C.; Chiu, C.-Y.; Hsieh, H.-L.; Hsieh, D.W.-C.; Hsieh, C.-R.; Chang, C.-H.; Peng, R.Y. High-Purity Preparation of Enzyme Transformed Trans-Crocetin Reclaimed from Gardenia Fruit Waste. Plants 2022, 11, 281.
- Demurtas, O.C.; Frusciante, S.; Ferrante, P.; Diretto, G.; Azad, N.H.; Pietrella, M.; Aprea, G.; Taddei, A.R.; Romano, E.; Mi, J.; et al. Candidate Enzymes for Saffron Crocin Biosynthesis Are Localized in Multiple Cellular Compartments. Plant Physiol. 2018, 177, 990–1006.
- Chu, L.L.; Huy, N.Q.; Tung, N.H. Microorganisms for Ginsenosides Biosynthesis: Recent Progress, Challenges, and Perspectives. Molecules 2023, 28, 1437.
- Song, T.Q.; Wu, N.; Wang, C.; Wang, Y.; Chai, F.H.; Ding, M.Z.; Li, X.; Yao, M.D.; Xiao, W.H.; Yuan, Y.J. Crocetin Overproduction in Engineered Saccharomyces cerevisiaevia Tuning Key Enzymes Coupled with Precursor Engineering. Front. Bioeng. Biotechnol. 2020, 8, 578005.
- Shimada, H.; Kondo, K.; Fraser Paul, D.; Miura, Y.; Saito, T.; Misawa, N. Increased Carotenoid Production by the Food YeastCandida utilis through Metabolic Engineering of the Isoprenoid Pathway. Appl. Environ. Microbiol. 1998, 64, 2676–2680.
- Miura, Y.; Kondo, K.; Shimada, H.; Saito, T.; Nakamura, K.; Misawa, N. Production of lycopene by the food yeast, Candida utilis that does not naturally synthesize carotenoid. Biotechnol. Bioeng. 1998, 58, 306–308.
- Lv, X.; Wang, F.; Zhou, P.; Ye, L.; Xie, W.; Xu, H.; Yu, H. Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization for improved isoprene production in Saccharomyces cerevisiae. Nat. Commun. 2016, 7, 12851.
- Mei, X.; Chen, Y.; Wang, R.; Xiao, W.; Wang, Y.; Li, X.; Yuan, Y. Engineered Yeast Cell for Producing Zeaxanthin. Chin. Biotechnol. 2016, 36, 64–72.
- Xie, Y.; Chen, S.; Xiong, X. Metabolic Engineering of Non-carotenoid-Producing Yeast Yarrowia lipolytica for the Biosynthesis of Zeaxanthin. Front. Microbiol. 2021, 12, 699235.
- Koulakiotis, N.S.; Gikas, E.; Iatrou, G.; Lamari, F.N.; Tsarbopoulos, A. Quantitation of Crocins and Picrocrocin in Saffron by HPLC: Application to Quality Control and Phytochemical Differentiation from Other Crocus Taxa. Planta Medica 2015, 81, 606–612.
- Tan, H.X.; Chen, X.H.; Liang, N.; Chen, R.B.; Chen, J.F.; Hu, C.Y.; Li, Q.; Li, Q.; Pei, W.Z.; Xiao, W.H.; et al. Transcriptome analysis reveals novel enzymes for apo-carotenoid biosynthesis in saffron and allows construction of a pathway for crocetin synthesis in yeast. J. Exp. Bot. 2019, 70, 4819–4833.
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232.
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412.
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250.
- Liang, M.H.; He, Y.J.; Liu, D.M.; Jiang, J.G. Regulation of carotenoid degradation and production of apocarotenoids in natural and engineered organisms. Crit. Rev. Biotechnol. 2021, 41, 513–534.
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640.
- Lin, J.; Lee, D.; Chang, J. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428.
- Chuka-ogwude, D.; Nafisi, M.; Taher, H.; Ogbonna, J.C.; Moheimani, N.R. Food waste digestate as a source of nitrogen for the cultivation of Dunaliella salina: Influence on growth and carotenogenesis under hyper osmotic stress. J. Appl. Phycol. 2022, 34, 101–112.
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96.
- Liu, J.; Chen, F. Biology and Industrial Applications of Chlorella: Advances and Prospects. In Microalgae Biotechnology; Posten, C., Feng Chen, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–35.
- Dantas, D.M.M.; Cahú, T.B.; Oliveira, C.Y.B.; Abadie-Guedes, R.; Roberto, N.A.; Santana, W.M.; Gálvez, A.O.; Guedes, R.C.A.; Bezerra, R.S. Chlorella vulgaris functional alcoholic beverage: Effect on propagation of cortical spreading depression and functional properties. PLoS ONE 2021, 16, e0255996.
- An, B.K.; Jeon, J.Y.; Kang, C.W.; Kim, J.M.; Hwang, J.K. The Tissue Distribution of Lutein in Laying Hens Fed Lutein Fortified Chlorella and Production of Chicken Eggs Enriched with Lutein. Korean J. Food Sci. Anim. Resour. 2014, 34, 172–177.
- Saadaoui, I.; Rasheed, R.; Aguilar, A.; Cherif, M.; Al Jabri, H.; Sayadi, S.; Manning, S.R. Microalgal-based feed: Promising alternative feedstocks for livestock and poultry production. J. Anim. Sci. Biotechnol. 2021, 12, 76.
- Ting, L.; Chunlei, S.; Zhibing, G.; Xianming, S. Cloning and analysis of the gene encoding lycopene epsilon cyclase in Chlorella protothecoides CS-41. Acta Microbiol. Sin. 2009, 49, 1180–1189.
- Lou, S.; Wang, L.; He, L.; Wang, Z.; Wang, G.; Lin, X. Production of crocetin in transgenic Chlorella vulgaris expressing genes crtRB and ZCD1. J. Appl. Phycol. 2016, 28, 1657–1665.
- Rubio, A.; Rambla, J.L.; Santaella, M.; Gómez, M.D.; Orzaez, D.; Granell, A.; Gómez-Gómez, L. Cytosolic and plastoglobule-targeted carotenoid dioxygenases from Crocus sativus are both involved in beta-ionone release. J. Biol. Chem. 2008, 283, 24816–24825.
- Pourkarimi, S.; Hallajisani, A.; Alizadehdakhel, A.; Nouralishahi, A.; Golzary, A. Factors affecting production of beta-carotene from Dunaliella salina microalgae. Biocatal. Agric. Biotechnol. 2020, 29, 101771.
- Hosseini Tafreshi, A.; Shariati, M. Dunaliella biotechnology: Methods and applications. J. Appl. Microbiol. 2009, 107, 14–35.
- Chen, H.H.; Liang, M.H.; Ye, Z.W.; Zhu, Y.H.; Jiang, J.G. Engineering the β-Carotene Metabolic Pathway of Microalgae Dunaliella to Confirm Its Carotenoid Synthesis Pattern in Comparison to Bacteria and Plants. Microbiol. Spectr. 2023, 11, e04361-22.
- García-González, M.; Moreno, J.; Manzano, J.C.; Florencio, F.J.; Guerrero, M.G. Production of Dunaliella salina biomass rich in 9-cis-β-carotene and lutein in a closed tubular photobioreactor. J. Biotechnol. 2005, 115, 81–90.
- Liang, M.; Qv, X.; Chen, H.; Wang, Q.; Jiang, J. Effects of Salt Concentrations and Nitrogen and Phosphorus Starvations on Neutral Lipid Contents in the Green Microalga Dunaliella tertiolecta. J. Agric. Food Chem. 2017, 65, 3190–3197.
- Borowitzka, L.; Borowitzka, M. Commercial Production of β-Carotene by Dunaliella Salina in Open Ponds. Bull. Mar. Sci. 1990, 47, 244–252.
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36.
- Hsu, Y.; Tsai, C.; Chang, W.; Ho, Y.; Chen, W.; Lu, F. Protective effects of Dunaliella salina—A carotenoids-rich alga, against carbon tetrachloride-induced hepatotoxicity in mice. Food Chem. Toxicol. 2008, 46, 3311–3317.
- Wolf, L.; Cummings, T.; Müller, K.; Reppke, M.; Volkmar, M.; Weuster-Botz, D. Production of β-carotene with Dunaliella salina CCAP19/18 at physically simulated outdoor conditions. Eng. Life Sci. 2021, 21, 115–125.
- Sui, Y.; Muys, M.; Van de Waal, D.B.; D’Adamo, S.; Vermeir, P.; Fernandes, T.V.; Vlaeminck, S.E. Enhancement of co-production of nutritional protein and carotenoids in Dunaliella salina using a two-phase cultivation assisted by nitrogen level and light intensity. Bioresour. Technol. 2019, 287, 121398.
- Han, S.; Kim, S.; Lee, C.; Choi, Y. Blue-Red LED wavelength shifting strategy for enhancing beta-carotene production from halotolerant microalga, Dunaliella salina. J. Microbiol. 2019, 57, 101–106.
- Ye, Z.; Jiang, J. Analysis of an Essential Carotenogenic Enzyme: Zeta-Carotene Desaturase from Unicellular Alga Dunaliella saline. J. Agric. Food Chem. 2010, 58, 11477–11482.
- Xu, Y.A.; Ibrahim, I.M.; Wosu, C.I.; Ben-Amotz, A.; Harvey, P.J. Potential of New Isolates of Dunaliella salina for Natural beta-Carotene Production. Biology 2018, 7, 14.
- Xu, Y.; Harvey, P.J. Mitosis Inhibitors Induce Massive Accumulation of Phytoene in the Microalga Dunaliella salina. Mar. Drugs 2021, 19, 595.
- Xu, Y.; Harvey, P.J. Red Light Control of β-Carotene Isomerisation to 9-cis β-Carotene and Carotenoid Accumulation in Dunaliella salina. Antioxidants 2019, 8, 148.
- Lou, S.; Zhu, X.; Zeng, Z.; Wang, H.; Jia, B.; Li, H.; Hu, Z. Identification of microRNAs response to high light and salinity that involved in beta-carotene accumulation in microalga Dunaliella salina. Algal Res. 2020, 48, 101925.
- Liang, M.; Wu, F.; Liang, Z.; Chen, H.; Jiang, J. Induction of carotenoid cleavage by salt stress and the effect of their products on cell growth and pigment accumulation in Dunaliella sp. FACHB-847. Algal Res. 2020, 48, 101901.
- Elleuch, F.; Hlima, H.B.; Barkallah, M.; Baril, P.; Abdelkafi, S.; Pichon, C.; Fendri, I. Carotenoids Overproduction in Dunaliella sp.: Transcriptional Changes and New Insights through Lycopene β Cyclase Regulation. Appl. Sci. 2019, 9, 5389.
- Ramel, F.; Mialoundama, A.S.; Havaux, M. Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J. Exp. Bot. 2012, 64, 799–805.
- Wang, Y.; Han, T.; Zhang, X.-G.; Zheng, C.-J.; Rahman, K.; Qin, L.-P. LC Fingerprint and Hierarchical Cluster Analysis of Crocus sativus L. from Different Locations in China. Chroma 2009, 70, 143–149.
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536.
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Attalage, R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: Astaxanthin from H. pluvialis and β-carotene from D. salina. Food Chem. 2019, 277, 128–134.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
08 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




