Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Lou sulin and Version 2 by Jason Zhu.
Crocin is one of the most valuable components of the Chinese medicinal plant Crocus sativus and is widely used in the food, cosmetics, and pharmaceutical industries. Traditional planting of C. sativus is unable to fulfill the increasing demand for crocin in the global market, however, such that researchers have turned their attention to the heterologous production of crocin in a variety of hosts. 藏红花素是中药用植物番红花中最有价值的成分之一,广泛用于食品、化妆品和制药行业。然而,传统的藏红花种植无法满足全球市场对藏红花素日益增长的需求,因此研究人员已将注意力转向藏红花素在多种寄主中的异源生产。目前,有报道称,在大肠杆菌、酿酒酵母、微藻和不自然产生藏红花素的植物中成功异源生产藏红花素。
- synthetic biology
- crocin
- heterologous production
- microalgae
1. Introduction引言
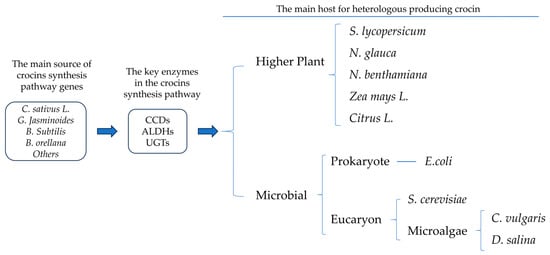
As a high-value carotenoid, crocins have great potential in pharmacology. Nowadays, many key enzymes in the crocins synthesis pathway have been widely revealed by transcriptomic and dynamic metabolomics studies, while the traditional cultivation model cannot solve the crocins production problem in a short time. It may be a new direction to use genetic engineering technology to transform the key enzymes of the synthetic pathway of crocetin to produce crocetin in species with a high yield of 藏红花素作为一种高价值的类胡萝卜素,在药理学上具有巨大的潜力。如今,藏红花素合成途径中的许多关键酶已被转录组学和动态代谢组学研究广泛揭示,而传统的培养模式无法在短时间内解决藏红花素的产生问题。利用基因工程技术改造藏红花素合成途径的关键酶,在β-carotene or the potential to synthesize β-carotene or crocetin (Figure 胡萝卜素产率高或具有合成β-胡萝卜素或藏红花素潜力的物种中产生藏红花素,可能是一个新的方向(图1). According to available reports, many microorganisms have been successfully transformed to synthesize crocetin or crocetin, including E. coli [1], yeast [2], Nicotiana glauca [3], Chlorella vulgaris [4], and Dunaliella salina [5]. Recently, the transient transformation of N. benthamiana to synthesize crocins was reported [6]. The above cases provide solid theoretical support and practical basis for further heterologous production of crocin. Below, these cases are divided into higher plant hosts and microbial hosts, which are divided into E. coli, yeast, and microalgal (Figure )。根据现有报道,许多微生物已被成功转化合成藏红花素或藏红花素,包括大肠杆菌[7]、酵母[62]、青烟[108]、寻常小球藻[109]和杜氏盐藻[104]。最近,有报道称本氏猪笼草瞬时转化合成藏红花素[102]。以上案例为藏红花素的进一步异源生产提供了坚实的理论支持和实践依据。下面,这些病例分为高等植物宿主和微生物宿主,又分为大肠杆菌、酵母和微藻(图1).)。
Figure图 1. Basic logic of heterologous synthesis of crocin by genetic engineering and the common host.
基因工程和共同宿主异源合成藏红花素的基本逻辑。
2. Biosynthesis of Crocins in Higher Plant Hosts藏红花素在高等植物寄主中的生物合成
In plants, 在植物中,β-carotene can be converted into other carotenoids to meet particular needs, especially in plants that utilize carotenoids to reduce photooxidative damage [7][8]. Heterologous production of crocins in plants has the advantage of requiring the introduction of only one or two genes since the other genes from the carotenoid pathway are already present [9]. Higher plant cells have abundant 胡萝卜素可以转化为其他类胡萝卜素以满足特定需求,特别是在利用类胡萝卜素减少光氧化损伤的植物中[14,107]。藏红花素在植物中的异源生产的优点是只需要引入一个或两个基因,因为类胡萝卜素途径中的其他基因已经存在[98]。高等植物细胞具有丰富的β-carotene storage capacity, a complete carotenoid synthesis system, and a complete endomembrane system and thus are ideal host cells for heterologous production of crocin [8]. In addition to C. sativus, crocin is also found in a variety of plants outside the Iridaceae, such as the flowers of B. davidii and the fruit of G. jasminoides [10][11]. However, such sources cannot meet the existing demand [12]. Therefore, researchers are investigating how to produce crocin efficiently in other higher plants.胡萝卜素储存能力、完整的类胡萝卜素合成系统和完整的内膜系统,是异源生产藏红花素的理想宿主细胞[107]。除苜蓿外,藏红花素还存在于鸢尾科以外的多种植物中,如大菖蒲的花和茉莉花的果实[5,12]。然而,这些来源无法满足现有需求[49]。因此,研究人员正在研究如何在其他高等植物中有效地生产藏红花素。
In 1986, scientists first expressed human growth hormone in tobacco cells and proposed the concept of using plant cells as a platform for recombinant protein production [13]. After more than 年,科学家首次在烟草细胞中表达人类生长激素,并提出了利用植物细胞作为重组蛋白生产平台的概念[88]。经过30 years of development, plant hosts have become extremely diversified and include whole plants, various plant tissues, suspension cells, and other systems多年的发展,植物宿主已经变得极其多样化,包括整株植物、各种植物组织、悬浮细胞等系统; in addition, there are multiple expression methods within each system [14][15]. Whole-plant cultivation requires special land and climatic conditions and is not suitable for rapid production of specific metabolites [16][17]. However, similarly to E. coli and S. cerevisiae, an isolated single-celled plant callus can be suspended and dispersed in liquid medium for rapid propagation and expression of products [18]. Therefore, suspension culture of plant cells has more prospects than whole-plant cultivation for large-scale industrial applications [19] and the production of high-value-added natural active products [14][20].此外,每个系统内有多种表达方法[110,111]。全株栽培需要特殊的土地和气候条件,不适合快速生产特定的代谢产物[112,113]。然而,与大肠杆菌和酿酒酵母类似,分离的单细胞植物愈伤组织可以悬浮并分散在液体培养基中,以实现产物的快速繁殖和表达[114]。因此,植物细胞悬浮培养比全株培养更具大规模工业化应用前景[115]和生产高附加值的天然活性产物[110,116]。
N. benthamiana is a本氏猪笼草是一种本身不含藏红花素的植物,但当它用适当的 plant that does not contain crocin itself, but when it is engineered with the appropriate CCD enzyme, it can overexpress upstream or downstream carotenoid-metabolizing genes, leveraging the crocin synthesis pathway. Zheng et al. [9] used a white citrus callus as host cells with a co-expression system comprising three genes, i.e., 酶进行改造时,它可以利用藏红红花素合成途径过表达上游或下游类胡萝卜素代谢基因。Zheng等[98]以白色柑橘愈伤组织为宿主细胞,其共表达系统由Tp-CrtB, 、Os-BCH, and 和Gj-CCD4a, and successfully constructed a non-green starch-rich tissue三个基因组成,成功构建了非绿色富含淀粉的组织/organ expression platform for effective production of crocin. When the platform was introduced into the leaves of N. benthamiana, up to 器官表达平台,有效生产藏红花素。当将平台引入本氏猪笼草的叶片中时,可获得高达1.6 mg/g dry weight crocin was obtained. It was found that 干重的藏红花素。研究发现,Gj-CCD4a had higher substrate specificity and catalytic efficiency in the leaves, demonstrating that a single enzyme, 在叶片中具有较高的底物特异性和催化效率,表明单一酶Gj-CCD4a, could drive the synthesis of crocin [9]. 可以驱动藏红花素的合成[98]。Xie et al. [6] combined the strategy of fusion with the 等[102]将融合策略与2A polypeptide connection and successfully constructed a multi-gene vector containing four genes to N. benthamiana, which transformed 多肽连接相结合,成功构建了包含4个基因的多基因载体,将GjCCD4a and two downstream glycosyltransferase genes 和2个下游糖基转移酶基因Gj-UGT74F8 and 和Gj-UGT94E13, to achieve higher substrate conversion efficiency that solved the problem of the low proportion of the main active components crocin I and crocin II, especially crocin I, as evidenced in previous research for synthesizing crocin in transgenic tobacco, and transformed ALDH introduced into tobacco for the first time.转化,实现了更高的底物转化效率,解决了主要活性成分藏红花素I和藏红花素II比例低的问题, 特别是藏红花素I,如先前在转基因烟草中合成藏红花素的研究所证明的那样,并首次将ALDH转化为烟草。
A related species, 一个相关的物种,N. glauca, contains carotenoid pigments in its petals. ,在其花瓣中含有类胡萝卜素色素。Huang et al. [3] expressed the 等[108]在藏红花的花瓣和叶片中组成型表达Bd-CCD4.1 enzyme from B. davidii constitutively in its petals and leaves and obtained 酶,分别获得321.6 ± 21.3 µμg/g and 和302.7 ± 25.6 µμg/g DCW crocin, respectively. Interestingly, in their transgenic lines expressing 藏红花素。有趣的是,在表达CsCCD2L, the difference in the accumulation of crocin between leaves and petals may have been due to the relatively higher accumulation of zeaxanthin or the tissue specificity of CCD in leaves [3]. 的转基因株系中,叶子和花瓣之间藏红花素积累的差异可能是由于玉米黄质的积累相对较高或CCD在叶片中的组织特异性[108]。Martí et al. used tobacco etch virus to drive the expression of 等人利用烟草蚀刻病毒驱动本氏猪笼草中Cs-CCD2L and 和Bd-CCD4.1 in N. benthamiana and found that only 的表达,发现在13天内,只有Cs-CCD2L could produce 可以产生2.18 ± 0.23 mg of crocins and 8.24 ± 2.93 mg of picrocrocin per gram (DCW) over 13 days. The study also found that CCD can intercept the metabolic flux in leaves and reduce the synthesis of lutein, which sharply increases the expression levels of phytoene and drives the carotenoid metabolic pathway in the direction of crocin synthesis [21].±0.23mg藏红花素和8.24±2.93mg苦霉素/克(DCW)。该研究还发现,CCD可以拦截叶片中的代谢通量,减少叶黄素的合成,从而急剧增加八氢番茄红素的表达水平,并带动类胡萝卜素代谢途径向藏红花素合成方向发展[52]。
Frusciante et al. introduced 等人通过农杆菌介导的瞬时表达将CCD2 into zeaxanthin-rich maize endosperm by Agrobacterium-mediated transient expression and found that, unlike in E. coli, where only crocetin dialdehyde could be detected, zeaxanthin was not only converted to crocin dialdehyde but also further oxidized to crocetin. This is likely because maize endosperm possesses an endogenous aldehyde dehydrogenase to facilitate the oxidation step.引入富含玉米黄质的玉米胚乳中,发现与大肠杆菌只能检测到藏红花素二醛不同,玉米黄质不仅转化为藏红花素二醛,而且进一步氧化为藏红花素。这可能是因为玉米胚乳具有内源性醛脱氢酶,以促进氧化步骤。
Due to the presence of vacuoles, plant cells are large compared with those of E. coli and S. cerevisiae. Accordingly, when using comparable culture volumes, it is difficult to improve production with plant cell cultures by increasing the number of cells. As a result, relatively low yields of recombinant protein product (由于液泡的存在,与大肠杆菌和酿酒酵母相比,植物细胞很大。因此,当使用可比的培养体积时,很难通过增加细胞数量来提高植物细胞培养物的产量。因此,重组蛋白产物在植物细胞系统中的产量相对较低(0.01 to -10 mg/L) are achieved in plant cell systems [14][15]. In addition, not all plant species can be adapted to suspension cell culture in a fermenter due to the presence of exogenous plant enzymes [22].mg/L)[110,111]。此外,由于存在外源植物酶,并非所有植物物种都能适应发酵罐中的悬浮细胞培养[117]。
3. Microbial Biosynthesis of Crocins藏红花素的微生物生物合成
Another approach to the production of crocins is combinatorial biosynthesis, which consists of combining enzyme-encoding genes from different species and designing a new set of gene clusters to produce bioactive compounds in a heterologous host. The commonly used microbial hosts for crocin production are E. coli among the prokaryotes and the yeast S. cerevisiae among the eukaryotes [1][2]. It is very important to select a suitable host organism for the optimization of product yield and quality, and there are pros and cons for both bacteria and yeast in this context. Bacterial hosts have a short life cycle, offer easy genetic manipulation and handling, and have a higher growth rate and excellent potential for protein and enzyme overexpression生产藏红花素的另一种方法是组合生物合成,它包括结合来自不同物种的酶编码基因,并设计一组新的基因簇以在异源宿主中产生生物活性化合物。生产藏红花素的常用微生物宿主是原核生物中的大肠杆菌和真核生物中的酿酒酵母[7,62]。选择合适的宿主生物对于优化产品产量和质量非常重要,在这种情况下,细菌和酵母各有利弊。细菌宿主生命周期短,易于基因操作和处理,具有较高的生长速率和极好的蛋白质和酶过表达潜力; however, they are not as beneficial for large proteins and proteins requiring post-translational modifications, which may be essential for correct folding and functional activity [19]. The S. cerevisiae is also well characterized and easy to grow and manipulate like E. coli but in addition can express proteins with appropriate post-translational modifications and offers better expression of membrane proteins然而,它们对大蛋白和需要翻译后修饰的蛋白质没有那么有益,而翻译后修饰对于正确的折叠和功能活性可能是必不可少的[115]。酿酒酵母也具有很好的特征,像大肠杆菌一样易于生长和操作,但除此之外,还可以通过适当的翻译后修饰表达蛋白质,并提供更好的膜蛋白表达; moreover, it has food-grade status (generally recognized as safe此外,它具有食品级状态(通常被认为是安全的; GRAS) [23]. However, it results in lower yields than bacteria and can add a large number of mannose residues to recombinant proteins, resulting in protein misfolding and problems with activity [22].GRAS)[118]。然而,它导致的产量低于细菌,并且会在重组蛋白中添加大量甘露糖残基,导致蛋白质错误折叠和活性问题[117]。
All in all, heterologous production of crocins in microorganisms is highly advantageous since they can grow on inexpensive substrates and, compared to plants, are easier to manipulate and have very rapid production cycles, allowing crocins to be produced faster and possibly in larger amounts [24]. Thus, taking into consideration all the pros and cons, the commercial application of heterologous production of crocins by microorganisms is the more attractive route.总而言之,藏红花素在微生物中的异源生产是非常有利的,因为它们可以在廉价的基质上生长,并且与植物相比,它们更容易操作并且具有非常快的生产周期,使藏红花素的生产速度更快,可能生产量更大[119]。因此,考虑到所有的利弊,微生物异源生产藏红花素的商业应用是更具吸引力的途径。
3.1. Biosynthesis of Crocins in E. coli藏红花素在大肠杆菌中的生物合成
Wild野生型大肠杆菌本身不具备合成类胡萝卜素的能力,但经过代谢工程后,可以成功合成β-type E. coli does not have the ability to synthesize carotenoids itself胡萝卜素和其他各种类胡萝卜素[120, but after metabolic engineering121, it can successfully synthesize β-carotene and various other carotenoids [25][26][27]. Therefore, E. coli has the potential to synthesize crocin after appropriate pathway modification.122]。因此,大肠杆菌在经过适当的途径修饰后具有合成藏红花素的潜力。
These were the first reports to demonstrate functional expression of a carotene gene cluster in E. coli: 这是第一篇证明胡萝卜素基因簇在大肠杆菌中功能表达的报道:Perry et al. [28] and 等[123]和Tuveson et al. [29] introduced a 12.4 kb carotene gene cluster from Erwinia herbicola (reclassified as 等[124]将欧文氏菌(重新分类为Pantoea agglomerans))的12.4 into E. coli and successfully produced yellow pigmentation. kb胡萝卜素基因簇引入大肠杆菌中,并成功产生黄色色素沉着。Misawa et al. [30] isolated a 等[125]从上述基因组片段中分离出一个6.9 kb yellow-pigment-producing gene cluster fragment from the above genome segment and found six open reading frames: 的产生黄色素的基因簇片段,发现了6个开放阅读框:CrtE, 、CrtI, CrtB, CrtX, 、CrtB、CrtX、CrtY, and 和CrtZ. It was confirmed that the yellow substance produced using this gene cluster was zeaxanthin and that the recombinant E. coli could also synthesize phytoene, lycopene, β-carotene, zeaxanthin, and basic carotenoids with 。证实利用该基因簇产生的黄色物质为玉米黄质,重组大肠杆菌还能以GGPP as a substrate. In recent years, E. coli has often been used as a host strain for the production of various carotenoids, which thus provides a theoretical basis for the heterologous synthesis of crocin in prokaryotes.为底物合成八氢番茄红素、番茄红素、β-胡萝卜素、玉米黄质和碱性类胡萝卜素。近年来,大肠杆菌常被用作生产各种类胡萝卜素的宿主菌株,从而为原核生物中藏红花素的异源合成提供了理论依据。
In terms of crocins synthesis, 在藏红花素合成方面,Wang et al. [1] introduced the 等[7]将苜蓿的Cs-CCD2L gene of C. sativus and the glycosyltransferases 基因和茉莉花的糖基转移酶Gj-UGT94E5 and 和Gj-UGT75L6 of G. jasminoides into E. coli, which was then capable of producing zeaxanthin and crocetin dialdehyde. This strain was able to produce crocetin after further engineering with the pTrc-ALD8 gene from 引入大肠杆菌中,从而产生玉米黄质和藏红花素二醛。该菌株在使用来自Neurospora crassa. Finally, the glycosyltransferases of 的pTrc-ALD8基因进行进一步工程后能够产生藏红花素。最后,将Bs-YjiC, 、Bs-YdhE, and 和Bs-YojK were introduced into the expression system to obtain crocin V with a yield of 的糖基转移酶引入表达体系,得到藏红花素V,收率为4.42 mg/L. This was the first time that a heterologous crocetin and crocin synthesis pathway was successfully constructed in E. coli [1]. 。这是首次在大肠杆菌中成功构建异源藏红花素和藏红花素合成途径[7]。Ding et al. [31] successfully mined two microbially derived glycosyltransferases with higher heterologous production and catalytic efficiency to improve crocin production. It was found that 等[101]成功提取了两种微生物衍生的糖基转移酶,具有更高的异源产生和催化效率,提高了藏红花素的产生。结果表明,枯草芽孢杆菌168的Bs-GT glycosyltransferase from Bacillus subtilis 16糖基转移酶最高可达到8 could achieve a maximum crocetin glycosylation conversion efficiency of 81.9% and a yield of 的藏红花素糖基化转化效率,藏红花素V和藏红花素III的得率为476.8 mg/L crocin V and crocin III. 。Bc-GTA showed a much lower conversion efficiency and specificity than Bs-GT [31]. 的转换效率和特异性远低于Bs-GT[101]。Pu et al. [32] found that 等[61]发现,G.-jasminoides-derived 衍生的Gj-UGT74F8和Gj-UGT74F8 and Gj-UGT94E13 gave whole-cell biotransformation rates as high as 66.1% and 59.6% for 50 mg/L crocin, respectively, which was higher than was achieved using UGTs from microorganisms. By precisely controlling the glycosylation time course, a high concentration of crocin with a specific degree of glycosylation can be obtained. Further optimization of gardenia UGTs may provide a valuable tool for the industrial production of crocin [33]. At the same time, 对50 mg/L藏红花素的全细胞生物转化率分别高达66.1%和59.6%,高于使用微生物UGTs实现的全细胞生物转化率。通过精确控制糖基化时间过程,可以获得具有特定糖基化程度的高浓度藏红花素。栀子花UGTs的进一步优化可能为藏红花素的工业化生产提供有价值的工具[126]。同时,Pu et al. also found that the glucose content in the culture environment is one of the key factors for obtaining crocin. When the endogenous 等人还发现,培养环境中的葡萄糖含量是获得藏红花素的关键因素之一。当工程菌株的内源性UDPG supply in engineered strains is insufficient for the efficient production of crocin, appropriate supplementation with a certain concentration of glucose can improve the catalytic activity of heterologously expressed UGTs to maintain efficient and sustainable production [32].供应不足以高效生产藏红花素时,适当补充一定浓度的葡萄糖可以提高异源表达UGTs的催化活性,以维持高效和可持续的生产[61]。
The synthesis of crocin in E. coli has been well studied. However, like other prokaryotes, the E. coli does not have a complex internal membrane system, as do eukaryotes. Thus, heterologous production of various eukaryotic enzymes in E. coli may result in differences in folding and functional group modification, which may in turn lead to reduced catalytic efficiency or no enzyme activity. Since C. sativus is a eukaryote, the synthesis of crocin involves the transfer of metabolites between multiple subcellular compartments, e.g., from plastids to vacuoles, and the cooperation of various related enzymes [34]. Lack of these enzymes or use of structurally defective enzymes may affect crocin production or produce toxic byproducts. 大肠杆菌中藏红花素的合成已得到充分研究。然而,与其他原核生物一样,大肠杆菌没有像真核生物那样复杂的内部膜系统。因此,大肠杆菌中各种真核酶的异源产生可能导致折叠和官能团修饰的差异,进而可能导致催化效率降低或酶活性降低。由于苜蓿是一种真核生物,藏红花素的合成涉及代谢物在多个亚细胞区室之间的转移,例如从质体到液泡,以及各种相关酶的合作[8]。缺乏这些酶或使用结构有缺陷的酶可能会影响藏红花素的产生或产生有毒的副产物。Zheng et al. [9] obtained crocetin dialdehyde in vitro by incubating 等[98]将β-apo载脂蛋白-8′-carotene as a substrate with crude lysates of E. coli cells that expressed 胡萝卜素作为底物,与表达Gj-CCD4a, showing that 的大肠杆菌细胞粗裂解物一起孵育,在体外获得藏红花素二醛,表明大肠杆菌中表达的Gj-CCD4a expressed in E. coli has enzyme activity. However, although E. coli itself has no endogenous ALDs, it has been reported that the properties of ALDs from microbial sources expressed in E. coli are better than those of endogenous C. sativus ALDs, while other plant-derived ALDs are expressed at very low levels in E. coli [1]. Further investigation and optimization of candidate ALDs is required.具有酶活性。然而,虽然大肠杆菌本身没有内源性原子酒精性沉积,但据报道,在大肠杆菌中表达的微生物来源的原子层沉积的性质优于内源性苜蓿酒精性脂肪酸,而其他植物来源的原子酒精性沉积在大肠杆菌中的表达水平非常低[7]。需要对候选原子层沉积进行进一步研究和优化。
3.2. Biosynthesis of Crocins in S. cerevisiae酿酒酵母藏红花素的生物合成
On account of its 由于其GRAS status, S. cerevisiae is often used in the field of food processing地位,酿酒酵母经常用于食品加工领域。与大肠杆菌的细菌模型不同,酿酒酵母是一种真核微生物,因此具有一套完整的细胞内膜,包括核膜和各种细胞器膜,与植物和哺乳动物细胞中的细胞膜相似,为外源基因提供了完整的转录、翻译和修饰环境[127]. Unlike the bacterial model of E. coli, S. cerevisiae is a eukaryotic microorganism and thus has a complete set of intracellular membranes, including nuclear membranes and various organelle membranes, which are similar to those in plant and mammalian cells and provide a complete transcription, translation, and modification environment for foreign genes [35]. The various compartments in the cell interior can also provide transport and storage space for gene expression products and metabolites. Since S. cerevisiae does not have an endogenous biochemical pathway for the synthesis of carotenoids, it is necessary to redesign the enzymes of crocin synthesis that initiate the 细胞内部的各个隔室还可以为基因表达产物和代谢物提供运输和储存空间。由于酿酒酵母没有合成类胡萝卜素的内源性生化途径,因此有必要重新设计启动MVA pathway to increase the levels of substrates to those required by the downstream pathway [36][37]. 途径的藏红花素合成酶,以将底物水平提高到下游途径所需的水平[105,128]。Shimada et al. redirected the ergosterol biosynthetic pathway in S. cerevisiae by introducing three genes required for lycopene synthesis, namely 等人通过引入番茄红素合成所需的三个基因,即CrtE, 、CrtB, and 和CrtI, and they were thus able to synthesize lycopene with a yield of ,重新定向了酿酒酵母的麦角甾醇生物合成途径,从而能够以1.1 mg/g dry cell weight (的干细胞重量(DCW). Ergosterol is a type of isoprene that shares a precursor with )的产率合成番茄红素。麦角甾醇是一种异戊二烯,与β-carotene and can provide abundant substrate for the production of crocin [38]. 胡萝卜素共享前体,可为藏红花素的生产提供丰富的底物[129]。Lv et al. [39] designed a dual-metabolic pathway in S. cerevisiae that simultaneously uses acetyl-Co等[130]在酿酒酵母中设计了一种双重代谢途径,同时在细胞质和线粒体中使用乙酰辅酶A in th。在提高前体利用率和扩大异戊二烯生产方面,表明这种双代谢途径比重组菌株中仅使用线粒体途径或细胞质途径具有优势。
Me cytoplasm and mitochondria. In terms of improving the utilization rate of precursors and expanding the production of isoprene, it was shown that this dual-metabolic pathway has advantages over those that only use the mitochondrial pathway or the cytoplasmic pathway in recombinant strains.
When 等[131]最初发现,当将Eu-CrtZ was introduced into S. cerevisiae, along with knock-out of the genes 引入酿酒酵母中,同时敲除负责将法呢基焦磷酸盐引向麦角甾醇合成的基因Lpp1 and 和Dpp1, which are responsible for directing farnesyl pyrophosphate towards ergosterol synthesis, Mei et al. [40] initially found that zeaxanthin production was only increased by a small amount, but a high yield of 时,最初发现玉米黄质的产量仅少量增加,但当使用三个拷贝的GAL1高强度启动子时,玉米黄质的产量高达96.2 mg/L of zeaxanthin was achieved when three copies of the GAL1 high-strength promoter were used. Improvement of Zeaxanthin Production by Multiple-Copy Integration of 。通过Eu-crtZ [41]. 的多拷贝整合提高玉米黄质的产生[89]。通过将Enhancing zeaxanthin production in Y. lipolytica was achieved by integrating the Eu-crtZ gene, in which the gene led to the highest titer and content for producing the target molecule, the expression cassette, into the 基因整合到26S rDNA region. 区域,提高了溶脂耶氏菌玉米黄质的产生,其中该基因导致产生靶分子(表达盒)的最高滴度和含量。Xie et al. [41] achieved a 等[89]玉米黄质滴度比单拷贝增加4.02-fold increase in the titer of zeaxanthin and a 倍,玉米黄质含量增加721% increase in the content of zeaxanthin than the single copy and achieved a 21.98 ± ,玉米黄质滴度为21.98±1.80 mg/L zeaxanthin titer. This high-yield engineered strain was named SyBE。这种高产工程菌株被命名为SyBE-Sc0123Z020.。 Chai et al. [2] selected three key enzymes, namely 等[62]从不同物种中选取了3种关键酶,即CrtZ, 、CCD, and ALD, from different species for expression in the 和ALD,用于酿酒酵母菌株S. cerevisiae strain SyBE-SC0014CY06, which was capable of producing β-carotene. The best combination of the three genes was Ps-SCrtZ from 0014CY06中表达,该菌株能够产生β-胡萝卜素。3个基因的最佳组合分别是来自Pantoea stewartii, C的Ps-CrtZ、来自CD2L from C. sativus的Cs, and -CCD2L和来自集胞藻属的Syn-ALD from Synechocystis sp. 。PCC6803, which together produced ,两者合计产生0.633 mg/L crocin. Tan et al. [42] designed, optimized, and synthesized a new 藏红花素。Tan等[66]设计、优化合成了一种新的Cs-ALD enz酶,并将其引入酿酒酵母Syme and introduced it into S. cerevisiae SyBEBE-Sc02070187-189,中,该酶能够产生玉米黄质,得到62.79 which was then capable of producinμg zeaxanthin, obtaining a yield of 62.79 µg/g DCW crocetin dialdehyde. Song et al. [36] knocked out 藏红花素二醛的收率。Song等[105]敲除了CIT2 and 和MLS1, two genes that consume acetyl-Co这两个在细胞质中消耗乙酰辅酶A in the cytoplasm, and increased the production of lycopene by 50%. They then constructed a fusion enzyme composed of 的基因,使番茄红素的产生增加了50%。然后,他们构建了一种由Ps-CrtZ and 和CsCCD2, which increased the concentration of crocin by 44%, yielding 12.43 ± 组成的融合酶,使藏红花素的浓度提高了44%,产生了12.43±0.62 mg/L crocin, which was twice as high as that produced by the initial strain SyBE藏红花素,是初始菌株SyBE-Sc0123C050 [2]. The above examples suggest that crocin production in S. cerevisiae is feasible, and this could provide a safe and efficient route of crocin production in eukaryotes.产生的浓度的两倍[62]。上述例子表明,酿酒酵母藏红红花素的产生是可行的,这可以为真核生物藏红花素的生产提供一条安全有效的途径。
However, S. cerevisiae contains five characterized endogenous 然而,酿酒酵母含有五个表征的内源性ALDH genes and a large number of other endogenous 基因和大量其他内源性ALDH genes that have not been fully characterized and are difficult to remove. These endogenous ALDH genes will seriously interfere with the expression and function of exogenous ALDH genes, significantly reducing crocin productivity [43]. 基因,这些基因尚未完全表征且难以去除。这些内源性ALDH基因会严重干扰外源性ALDH基因的表达和功能,显著降低藏红花素的生产力[63]。扩增酿酒酵母外源Amplifying the copy number of exogenous ALDH genes in S. cerevisiae can competitively inhibit the expression and function of the endogenous 基因的拷贝数可以竞争性抑制内源ALDH genes, improving the expression and specificity of the exogenous 基因的表达和功能,提高外源ALDH genes, thereby increasing the production of crocetin [43]. When 基因的表达和特异性,从而增加藏红花素的产生[63]。Chai et al. [43] used the multicopy 等[63]使用多拷贝质粒plasmid pRS426 to increase the copy number of 增加Cs-CCD2L and 和Syn-ALD, the production of crocin was further increased to 的拷贝数后,藏红花素的产量进一步提高到1.219 mg/L, which was twice the yield obtained with a single-copy plasmid.,是单拷贝质粒产量的2倍。
3.3. Biosynthesis of Crocins in Microalgal Hosts微藻宿主中藏红花素的生物合成
Microalgae are microscopic photosynthetic eukaryotes that live in aquatic environments [44]. As single-celled organisms and the ancestors of land plants originating about 微藻是生活在水生环境中的微观光合真核生物[132]。微藻作为起源于约100 million years ago, microalgae nevertheless have a carotenoid synthesis pathway similar to that of higher plants [45][46]. Thus, homologs of 亿年前的单细胞生物和陆地植物的祖先,其类胡萝卜素合成途径与高等植物相似[85,133]。因此,微藻中存在CCD1, 、CCD7, CCD8, and NCED are present in microalgae such that heterologous synthesis of crocin from 、CCD8和NCED的同源物,因此可以从β-carotene is possible [45][47]. Indeed, the complex carotenoid metabolism system in microalgae can synthesize a variety of carotenoids that are found in land plants, such as lutein, astaxanthin, fucoxanthin, and 胡萝卜素异源合成藏红花素[85,134]。事实上,微藻中复杂的类胡萝卜素代谢系统可以合成多种存在于陆地植物中的类胡萝卜素,如叶黄素、虾青素、岩藻黄质和β-carotene [48]. Based on the background of Chlamydomonas 胡萝卜素[135]。基于衣藻β-carotene synthesis pathway, it can greatly reduce the building line of the crocin synthesis pathway module and workload.胡萝卜素合成途径的背景,可以大大减少藏红花素合成途径模块的构建线和工作量。
Microalgae are characterized by a fast growth rate, relatively easy modification of endogenous metabolic pathways, and a complement of silent genes or genes expressed at low levels微藻的特点是生长速度快,内源性代谢途径相对容易修饰,无声基因或低水平表达基因的补体; this simplifies their metabolic engineering for use as a crocin bioreactor [49]. Carotenoids from microalgae have already been used for commercial purposes. For example, C. vulgaris can use lycopene as a precursor for the synthesis of 这简化了它们用作藏红花素生物反应器的代谢工程[136]。微藻中的类胡萝卜素已用于商业目的。例如,寻常梭菌可利用番茄红素作为前体,在不同培养条件下合成β-carotene, zeaxanthin, astaxanthin, and other substances under different culture conditions [49]. D. salina, which can survive in extremely high-salt environments, produces 胡萝卜素、玉米黄质、虾青素等物质[136]。盐碱可以在极高盐的环境中生存,自然产生β-carotene naturally. One benefit of the high-salinity culture environment is that it can effectively inhibit contamination by other microorganisms, thereby reducing culture costs [50].胡萝卜素。高盐度培养环境的一个好处是可以有效抑制其他微生物的污染,从而降低培养成本[137]。
In the 在1960s, C. vulgaris became the first single-celled green alga to be exploited on a large scale because of its simple structure, fast growth, and low maintenance costs [51]. C. vulgaris has been used as a cell factory and can synthesize various nutrients through photosynthesis年代,寻常梭菌因其结构简单、生长迅速、维护成本低而成为第一个被大规模开发的单细胞绿藻[138]。寻常梭菌已被用作细胞工厂,可以通过光合作用合成各种营养物质; it is also capable of synthesizing proteins, carbohydrates, carotenoids, and lipids. Its protein content can be as high as 它还能够合成蛋白质、碳水化合物、类胡萝卜素和脂质。其蛋白质含量可高达68%, and it is widely used in human health foods and additives as well as for animal feed in aquaculture [52][53][54][55]. However,广泛用于人类保健食品和添加剂以及水产养殖中的动物饲料[139,140,141, unbalanced cellular metabolic fluxes and competition between intermediate and precursor metabolites are challenges for the heterologous expression of crocin in microalgae. Lycopene 142]。然而,不平衡的细胞代谢通量以及中间体和前体代谢物之间的竞争是微藻中藏红花素异源表达的挑战。番茄红素ε-cyclase (LCYE) is a crucial enzyme that cyclizes lycopene to 环化酶(LCYE)是一种将番茄红素环化为α-carotene and provides a large pool of substrate for the synthesis of lutein [56]. The enzyme of 胡萝卜素的关键酶,为叶黄素的合成提供了大量的底物[143]。LCYE is encoded by the 的酶由CvLCYE gene, whose nucleotide sequence is highly conserved in a variety of green algae [4]. Overexpression of the 基因编码,其核苷酸序列在多种绿藻中高度保守[109]。CvLCYE gene can greatly improve lutein production in 基因的过表达可以大大改善寻常梭菌叶黄素的产生[109]。通过阻断或沉默C. vulgaris [4]. By blocking or silencing the expression of CvLCYE gene, more lycopene can flow to 基因的表达,更多的番茄红素可以流向β-carotene synthesis, thereby providing more substrate for the synthesis of crocin.胡萝卜素合成,从而为藏红花素的合成提供更多的底物。
Based on this characteristic of C. vulgaris, 基于寻常梭菌的这一特性,Lou et al. [57] used Agrobacterium-mediated transient expression of the 等[103]利用农杆菌介导的雨生红球藻CrtRB gene from Haematococcus pluvialis and the 基因和寻常梭菌柱头的ZCD1 gene from the stigma of C. sativus in C. vulgaris and successfully detected the accumulation of crocin. This was the first report to demonstrate crocin production in microalgae. 基因瞬时表达,成功检测了藏红花素的积累。这是第一份证明微藻中藏红花素产生的报告。ZCD1 is a 13-amino-acid mutant of 是Cs-ZCD, which originally lacked the residues and domains necessary for zeaxanthin dioxygenase activity; this modification restores the activity [57][58], which is important for modifying the weak activity of 的13个氨基酸突变体,最初缺乏玉米黄质双加氧酶活性所需的残基和结构域;这种修饰恢复了活性[96,103],这对于修饰CCD and increasing the production of crocin.的弱活性和增加藏红花素的产生具有重要意义。
D. salina is a free-moving, single-celled green microalga with flagella but without a rigid cell wall [59]. The intracellular glycerol content of D. salina is more than 盐藻是一种自由移动的单细胞绿色微藻,有鞭毛,但没有刚性细胞壁[144]。盐水楠的细胞内甘油含量超过其重量的50% its weight, which allows it to regulate the osmotic pressure by changing the intracellular glycerol concentration [60]. Therefore, it can survive in salt solutions of ,这使得它可以通过改变细胞内甘油浓度来调节渗透压[145]。因此,它可以在0.5% to -35%, i.e., up to nearly saturated solutions [60]. It is one of the most salt-tolerant eukaryotes known [61]. The optimal-growth salt concentration range for D. salina is 的盐溶液中存活,即在接近饱和的溶液中存活[145]。它是已知最耐盐的真核生物之一[146]。盐碱的最佳生长盐浓度范围为1.0–2.0 M NaCl [62]. Under high salt-stress conditions, i.e., [147]。在高盐胁迫条件下,即3.0–-4.0 M NaCl, the synthesis of chlorophyll and cell growth are inhibited [63]. However, when operating at optimal salt concentrations, contamination by most non-halotolerant bacteria or protists is minimal, thus reducing production costs and helping to maintain an axenic environment [64]. Compared with higher plants, microalgae grow fast. Most higher plants depend on photosynthesis for their growth and reproduction [60][65]. On the other hand, D. salina has the highest known content of ,叶绿素的合成和细胞生长受到抑制[148]。然而,当在最佳盐浓度下操作时,大多数非盐素耐受细菌或原生生物的污染最小,从而降低了生产成本并有助于维持无菌环境[149]。与高等植物相比,微藻生长迅速。大多数高等植物的生长和繁殖都依赖于光合作用[145,150]。另一方面,盐碱菜的β-carotene in the plant kingdom [66][67]. It is rich in lutein, zeaxanthin, and 胡萝卜素含量在植物界中含量最高[151,152]。它富含叶黄素、玉米黄质和β-carotene, the latter of which accounts for 胡萝卜素,后者占DCW的14% of DCW [68]. D. salina is one of the most widely used algal species for the commercial production of [153]。盐碱是商业化生产β-carotene胡萝卜素应用最广泛的藻类之一,在藏红花素合成方面也具有很强的潜力[144,154, and it also has strong potential for crocin synthesis [59][69][70][71]. Due to their versatility in adapting to a variety of growing conditions and climates (e.g.155, glacial to tro156]。由于微藻在适应各种生长条件和气候(例如,冰川到热带,淡水到高盐)和不同的pical and freshwater to highly saline) and different pH values, microalgae show distinct advantages over higher plants, reducing the need for sophisticated culture equipment and thereby reducing costs. Microalgae generally have higher carotenoid contents than higher plants. The major carotenoids in D. salina include H值方面具有多功能性,因此与高等植物相比,微藻显示出明显的优势,减少了对复杂培养设备的需求,从而降低了成本。微藻通常比高级植物具有更高的类胡萝卜素含量。盐藻中的主要类胡萝卜素包括9- or 或9′'-cis-顺式β-carotene and all-trans-胡萝卜素和全反式-β-carotene, which is preferentially absorbed compared to the 胡萝卜素,与9-cis-顺式-β-isomer [66]. Nevertheless, the 异构体相比,胡萝卜素优先被吸收[151]。然而,与反式键相比,由于顺式键的反应性更高,因此9-cis-顺式β-isomer has a higher antioxidant activity due to the higher reactivity of the cis bond compared to the trans bond [66]. Among all natural sources studied to date, D. salina possesses the highest content of 异构体具有更高的抗氧化活性[151]。在迄今为止研究的所有天然来源中,盐水草的9-cis-顺式β-carotene, reaching levels of up to 胡萝卜素含量最高,DCW含量高达100 g/kgg/kg[151,152]。这将为盐水苜蓿生产藏红花素提供一个大型底物池[157]。用促有丝分裂抑制剂(丙噼胺和氯丙虫胺)处理10 of DCW [66][67]. This would provide a large substrate pool for the production of crocin by D. salina [72]. The relative carotenoid content (% of total carotenoids) of octahydro-lycopene increased more than 后,盐碱中八氢番茄红素的相对类胡萝卜素含量(占总类胡萝卜素的百分比)增加了48-fold in D. salina after treatment with mitogenic inhibitors (propyzamide and chlorpropham) for 10 h [72]. The production of lycopene and 倍以上[157]。番茄红素和β-carotene was also significantly increased after exposure to red light. This is due to the accumulation of the more readily degraded 胡萝卜素的产生在红光照射后也显著增加。这是由于在高强度红光条件下更容易降解的9-cis 顺式β-carotene under high-intensity red-light conditions胡萝卜素的积累; such conditions are associated with high rates of photooxidation, which in turn increases the activity of 这种情况与高光氧化速率有关,这反过来又增加了β-carotene isomerases, the gene transcripts of which are induced by light stress [73]. These characteristics of D. salina provide some conditions for the synthesis of crocin by transgenic technology.胡萝卜素异构酶的活性,其基因转录本是由光胁迫诱导的[158]。盐水斩斩的这些特性为转基因技术合成藏红花素提供了一定的条件。
By mining the transcriptome and genome of D. salina using deep sequencing, Lou et al. [74][159] found that, under high-light and high-salinity stress, D. salina activates an endogenous miRNA, m0533-3p, which in response to the stress signals inhibits malate dehydrogenase. This is likely to lead to a reduced flow of acetyl-CoA into the tricarboxylic acid cycle and instead greater participation of acetyl-CoA in the synthesis of GGPP, with a concomitant increase in β-carotene levels. However, as salt concentration increases, D. salina is more inclined to divert β-carotene to α-ionone and β-ionone synthesis to improve stress resistance, resulting in a decrease in β-carotene reserves, thus affecting the conversion efficiency of crocin [75][76][77][160,161,162]. Therefore, to balance these two opposing fluxes, the optimal salt concentration for D. salina should be 1.5 M NaCl [75][76][160,161]. Hou [5][104] introduced CrtRB, Cs-ZCD, and CCD2 as target genes into D. salina by the glass-bead method and successfully detected trace amounts of crocetin dialdehyde.
D. salina has many applications in the pharmaceutical, nutraceutical, and cosmeceutical industries. However, although there are thorough and comprehensive research methods for using microalgae to produce other carotenoid products, they are still in the initial stages as hosts for the production of crocin; still, they have great potential for this application [78][79][42,163]. Nevertheless, it will not be enough to identify and modify the key enzymes in engineered pathways; there will also be a requirement for increased investment in the optimization of algal strains and for further investigation and optimization of culture conditions, methods of exogenous gene transformation, and the selection of transcription and translation-related factors [80][164].
