
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sheryl A Zelenitsky | -- | 3638 | 2024-01-05 23:07:53 | | | |
| 2 | Lindsay Dong | -5 word(s) | 3633 | 2024-01-08 01:29:41 | | |
Video Upload Options
Appropriate surgical antimicrobial prophylaxis (SAP) is an important measure in preventing surgical site infections (SSIs). Although antimicrobial pharmacokinetics–pharmacodynamics (PKPD) is integral to optimizing antibiotic dosing for the treatment of infections, there is less research on preventing infections postsurgery. Whereas clinical studies of SAP dose, preincision timing, and redosing are informative, it is difficult to isolate their effect on SSI outcomes. Antimicrobial PKPD aims to explain the complex relationship between antibiotic exposure during surgery and the subsequent development of SSI. It accounts for the many factors that influence the PKs and antibiotic concentrations in patients and considers the susceptibilities of bacteria most likely to contaminate the surgical site.
1. Introduction
2. Dose–Response Relationship in Cefazolin Prophylaxis
Although clinical studies relating the SAP dose to SSI are informative, there are significant limitations that can impede the detection of a dose–SSI relationship. First, without the PKs, dose is a poor predictor of the antibiotic concentrations achieved in patients and antimicrobial activity in vivo. Furthermore, dose is only one component of the SAP regimen which also relies on appropriate preincision timing and redosing during surgery. Finally, SSI outcomes are affected by many other patient- and surgery-related factors such as obesity, diabetes, immune status, type of surgery, wound classification, and surgical duration. Heavier body weight (i.e., obesity) is a significant risk factor [23][24] which often complicates the study of cefazolin dosing for surgical prophylaxis. With the use of standard doses, there is a negative correlation between body weight and the mg/kg dose received by patients. For example, the mg/kg dose delivered by 2 g of cefazolin decreases as body weight increases to the cut-off of 120 kg, thereby confounding the effects of heavier body weight and lower dose as risk factors for SSI.
Abdel Jalil et al. conducted a prospective, observational study of the frequency and potential risk factors for SSI in caesarean surgery [25]. Compliance with a new institutional guideline to use 2 g instead of 1 g of cefazolin preoperatively for patients <120 kg was also evaluated. A dose–response relationship was observed where patients who developed SSI received a lower calculated mg/kg dose than those without infection (18.7 mg/kg versus 22.2 mg/kg, p = 0.037). Furthermore, a lower mg/kg dose of cefazolin independently associated with SSI (p = 0.035) in multivariable logistic regression analysis, as were longer hospital stay (p = 0.001), higher BMI (p = 0.003), and delivery beyond the 40th week (p = 0.005). As expected, the mg/kg dose was negatively correlated with BMI. The investigators concluded that using 2 g versus 1 g of cefazolin was beneficial, predicting that it would reduce the risk of infection by 36% in their typical patient undergoing caesarean surgery.
Morris et al. analyzed the relationship between cefazolin dose, body weight, and SSI in 38,288 cases of hip or knee arthroplasty, using data from a national (New Zealand) prospective, surveillance and quality improvement programme [26]. Underdosing was defined as 1 g for individuals ≥80 kg or <3 g for those ≥120 kg. The preoperative dose was administered within 1 h in 96% of cases, and surgery exceeded 4 h in only 1% of cases. The recommended cefazolin dose was given in 94.5% cases, noting that only 27% of patients ≥120 kg received 3 g. After multivariable analysis, underdosing (OR = 2.19, p < 0.0001), higher surgical risk score (p < 0.0001), revision (p < 0.0001) or hip surgery (p = 0.028), and male gender (p = 0.026) were independently associated with SSI. It was estimated that 83 heavier-weight individuals needed to be treated with the recommended dose to prevent one infection. The investigators suggested that 2 g and at least 3 g of cefazolin were appropriate for patients ≥80 kg and ≥120 kg, respectively, but were unable to address the potential need for higher doses in patients with morbid obesity undergoing arthroplasty.
In summary, available evidence supports an association between lower antibiotic concentrations in patients undergoing surgery, particularly at wound closure, and an increased risk of infection. However, the number of studies is relatively small. More research is needed to fully understand the principles of antimicrobial PKPD in surgical prophylaxis and realize its potential to improve SSI outcomes. Further study of antibiotic concentrations in serum/plasma and interstitial fluid during surgery can also help identify clinically meaningful targets for effective SAP.
3. Antibiotic Concentration–Response (SSI) Relationship in SAP
4. Relevance and Role of PKPD in SAP
4.1. Antimicrobial PKPD and Monte Carlo Simulations
4.2. PKPD Considerations for Improving Cefazolin Prophylaxis
4.2.1. Pharmacokinetics of Cefazolin Prophylaxis
4.2.2. Cefazolin Prophylaxis Dose and Obesity
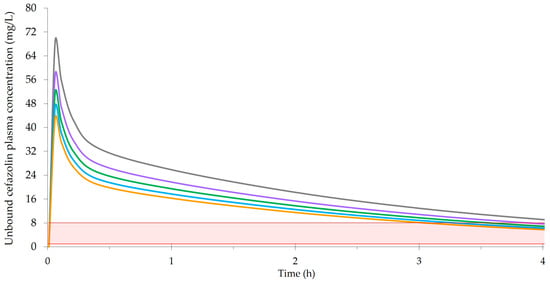
4.2.3. Preincision Timing of Cefazolin Prophylaxis
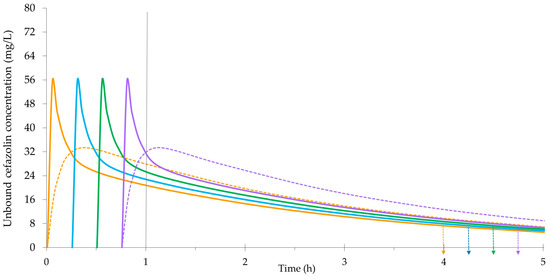
4.2.4. Redosing Cefazolin Prophylaxis during Surgery
4.2.5. Conclusions
References
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of Surgical Site Infection on Healthcare Costs and Patient Outcomes: A Systematic Review in Six European Countries. J. Hosp. Infect. 2017, 96, 1–15.
- Eckmann, C.; Kramer, A.; Assadian, O.; Flessa, S.; Huebner, C.; Michnacs, K.; Muehlendyck, C.; Podolski, K.M.; Wilke, M.; Heinlein, W.; et al. Clinical and Economic Burden of Surgical Site Infections in Inpatient Care in Germany: A Retrospective, Cross-Sectional Analysis from 79 Hospitals. PLoS ONE 2022, 17, e0275970.
- Gillespie, B.M.; Harbeck, E.; Rattray, M.; Liang, R.; Walker, R.; Latimer, S.; Thalib, L.; Andersson, A.E.; Griffin, B.; Ware, R.; et al. Worldwide Incidence of Surgical Site Infections in General Surgical Patients: A Systematic Review and Meta-Analysis of 488,594 Patients. Int. J. Surg. 2021, 95, 106136.
- Umscheid, C.A.; Mitchell, M.D.; Doshi, J.A.; Agarwal, R.; Williams, K.; Brennan, P.J. Estimating the Proportion of Healthcare-Associated Infections That Are Reasonably Preventable and the Related Mortality and Costs. Infect. Control Hosp. Epidemiol. 2011, 32, 101–114.
- Schreiber, P.W.; Sax, H.; Wolfensberger, A.; Clack, L.; Kuster, S.P. The Preventable Proportion of Healthcare-Associated Infections 2005–2016: Systematic Review and Meta-Analysis. Infect. Control Hosp. Epidemiol. 2018, 39, 1277–1295.
- Bowater, R.J.; Stirling, S.A.; Lilford, R.J. Is Antibiotic Prophylaxis in Surgery a Generally Effective Intervention?: Testing a Generic Hypothesis Over a Set of Meta-Analyses. Ann. Surg. 2009, 249, 551–556.
- Menz, B.D.; Charani, E.; Gordon, D.L.; Leather, A.J.; Moonesinghe, S.R.; Phillips, C.J. Surgical Antibiotic Prophylaxis in an Era of Antibiotic Resistance: Common Resistant Bacteria and Wider Considerations for Practice. IDR 2021, 14, 5235–5252.
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery. Am. J. Health-Syst. Pharm. 2013, 70, 195–283.
- Rodríguez-Gascón, A.; Solinís, M.Á.; Isla, A. The Role of PK/PD Analysis in the Development and Evaluation of Antimicrobials. Pharmaceutics 2021, 13, 833.
- Rao, G.G.; Landersdorfer, C.B. Antibiotic Pharmacokinetic/Pharmacodynamic Modelling: MIC, Pharmacodynamic Indices and Beyond. Int. J. Antimicrob. Agents 2021, 58, 106368.
- Velkov, T.; Bergen, P.J.; Lora-Tamayo, J.; Landersdorfer, C.B.; Li, J. PK/PD Models in Antibacterial Development. Curr. Opin. Microbiol. 2013, 16, 573–579.
- Rizk, M.L.; Bhavnani, S.M.; Drusano, G.; Dane, A.; Eakin, A.E.; Guina, T.; Jang, S.H.; Tomayko, J.F.; Wang, J.; Zhuang, L.; et al. Considerations for Dose Selection and Clinical Pharmacokinetics/Pharmacodynamics for the Development of Antibacterial Agents. Antimicrob. Agents Chemother. 2019, 63, e02309-18.
- Li, J.; Lovern, M.; Riccobene, T.; Carrothers, T.J.; Newell, P.; Das, S.; Talley, A.K.; Tawadrous, M. Considerations in the Selection of Renal Dosage Adjustments for Patients with Serious Infections and Lessons Learned from the Development of Ceftazidime-Avibactam. Antimicrob. Agents Chemother. 2020, 64, e02105-19.
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019, 58, 1407–1443.
- Crass, R.L.; Cojutti, P.G.; Pai, M.P.; Pea, F. Reappraisal of Linezolid Dosing in Renal Impairment To Improve Safety. Antimicrob. Agents Chemother. 2019, 63, e00605-19.
- Zelenitsky, S.A.; Ariano, R.E. An Updated Vancomycin Dosing Protocol for Initiating Therapy in Patients Undergoing Intermittent High-Flux Hemodialysis. Am. J. Health-Syst. Pharm. 2022, 79, 1006–1010.
- Parker, S.L.; Abdul-Aziz, M.H.; Roberts, J.A. The Role of Antibiotic Pharmacokinetic Studies Performed Post-Licensing. Int. J. Antimicrob. Agents 2020, 56, 106165.
- Mouton, J.W.; Brown, D.F.J.; Apfalter, P.; Cantón, R.; Giske, C.G.; Ivanova, M.; MacGowan, A.P.; Rodloff, A.; Soussy, C.-J.; Steinbakk, M.; et al. The Role of Pharmacokinetics/Pharmacodynamics in Setting Clinical MIC Breakpoints: The EUCAST Approach. Clin. Microbiol. Infect. 2012, 18, E37–E45.
- Ambrose, P.G.; Bhavnani, S.M.; Andes, D.R.; Bradley, J.S.; Flamm, R.K.; Pogue, J.M.; Jones, R.N. Old In Vitro Antimicrobial Breakpoints Are Misleading Stewardship Efforts, Delaying Adoption of Innovative Therapies, and Harming Patients. Open Forum Infect. Dis. 2020, 7, ofaa084.
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791.
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection, 2nd ed.; World Health Organization: Geneva, Switzerland, 2018; ISBN 978-92-4-155047-5.
- Calderwood, M.S.; Anderson, D.J.; Bratzler, D.W.; Dellinger, E.P.; Garcia-Houchins, S.; Maragakis, L.L.; Nyquist, A.-C.; Perkins, K.M.; Preas, M.A.; Saiman, L.; et al. Strategies to Prevent Surgical Site Infections in Acute-Care Hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2023, 44, 695–720.
- Winfield, R.D.; Reese, S.; Bochicchio, K.; Mazuski, J.E.; Bochicchio, G.V. Obesity and the Risk for Surgical Site Infection in Abdominal Surgery. Am. Surg. 2016, 82, 331–336.
- Thelwall, S.; Harrington, P.; Sheridan, E.; Lamagni, T. Impact of Obesity on the Risk of Wound Infection Following Surgery: Results from a Nationwide Prospective Multicentre Cohort Study in England. Clin. Microbiol. Infect. 2015, 21, 1008.e1–1008.e8.
- Abdel Jalil, M.H.; Abu Hammour, K.; Alsous, M.; Awad, W.; Hadadden, R.; Bakri, F.; Fram, K. Surgical Site Infections Following Caesarean Operations at a Jordanian Teaching Hospital: Frequency and Implicated Factors. Sci. Rep. 2017, 7, 12210.
- Morris, A.J.; Roberts, S.A.; Grae, N.; Frampton, C.M. Surgical Site Infection Rate Is Higher Following Hip and Knee Arthroplasty When Cefazolin Is Underdosed. Am. J. Health-Syst. Pharm. 2020, 77, 434–440.
- Goldmann, D.A.; Hopkins, C.C.; Karchmer, A.W.; Abel, R.M.; McEnany, M.T.; Akins, C.; Buckley, M.J.; Moellering, R.C. Cephalothin Prophylaxis in Cardiac Valve Surgery. A Prospective, Double-Blind Comparison of Two-Day and Six-Day Regimens. J. Thorac. Cardiovasc. Surg. 1977, 73, 470–479.
- Zelenitsky, S.A.; Ariano, R.E.; Harding, G.K.M.; Silverman, R.E. Antibiotic Pharmacodynamics in Surgical Prophylaxis: An Association between Intraoperative Antibiotic Concentrations and Efficacy. Antimicrob. Agents Chemother. 2002, 46, 3026–3030.
- Zelenitsky, S.A.; Silverman, R.E.; Duckworth, H.; Harding, G.K.M. A Prospective, Randomized, Double-Blind Studyof Single High Dose versus Multiple Standard Dose Gentamicin Both in Combination Withmetronidazole for Colorectal Surgicalprophylaxis. J. Hosp. Infect. 2000, 46, 135–140.
- Zelenitsky, S.A.; Calic, D.; Arora, R.C.; Grocott, H.P.; Lakowski, T.M.; Lillico, R.; Ariano, R.E. Antimicrobial Prophylaxis for Patients Undergoing Cardiac Surgery: Intraoperative Cefazolin Concentrations and Sternal Wound Infections. Antimicrob. Agents Chemother. 2018, 62, e01360-18.
- Calic, D.; Ariano, R.E.; Arora, R.C.; Grocott, H.P.; Lakowski, T.M.; Lillico, R.; Zelenitsky, S.A. Evaluation of Cefazolin Antimicrobial Prophylaxis during Cardiac Surgery with Cardiopulmonary Bypass. J. Antimicrob. Chemother. 2018, 73, 768–771.
- Takayama, Y.; Komatsu, T.; Nakamura, T.; Tomoda, Y.; Toda, M.; Miura, H.; Sato, T.; Atsuda, K.; Okamoto, H.; Hanaki, H. Association of Serum and Fat Tissue Antibiotic Concentrations with Surgical Site Infections in Lower Gastrointestinal Surgery. Surgery 2022, 171, 1000–1005.
- Mouton, J.W.; Theuretzbacher, U.; Craig, W.A.; Tulkens, P.M.; Derendorf, H.; Cars, O. Tissue Concentrations: Do We Ever Learn? J. Antimicrob. Chemother. 2007, 61, 235–237.
- Jager, N.G.L.; Van Hest, R.M.; Lipman, J.; Roberts, J.A.; Cotta, M.O. Antibiotic Exposure at the Site of Infection: Principles and Assessment of Tissue Penetration. Expert Rev. Clin. Pharmacol. 2019, 12, 623–634.
- Dadhwal, U.S. A Few Comments on “Association of Serum and Fat Tissue Antibiotic Concentrations with Surgical Site Infections in Lower Gastrointestinal Surgery” by Yoko Takayama et al. Surgery 2023, 173, 558–559.
- Chua, H.C.; Tam, V.H. Optimizing Clinical Outcomes Through Rational Dosing Strategies: Roles of Pharmacokinetic/Pharmacodynamic Modeling Tools. Open Forum Infect. Dis. 2022, 9, ofac626.
- De Velde, F.; Mouton, J.W.; De Winter, B.C.M.; Van Gelder, T.; Koch, B.C.P. Clinical Applications of Population Pharmacokinetic Models of Antibiotics: Challenges and Perspectives. Pharmacol. Res. 2018, 134, 280–288.
- Grayson, M.L.; Cosgrove, S.; Crowe, S.; Hope, W.; McCarthy, J.; Mills, J.; Mouton, J.W.; Paterson, D. (Eds.) Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs, 7th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-4796-7.
- Rondon, A.J.; Kheir, M.M.; Tan, T.L.; Shohat, N.; Greenky, M.R.; Parvizi, J. Cefazolin Prophylaxis for Total Joint Arthroplasty: Obese Patients Are Frequently Underdosed and at Increased Risk of Periprosthetic Joint Infection. J. Arthroplast. 2018, 33, 3551–3554.
- Karamian, B.A.; Toci, G.R.; Lambrechts, M.J.; Siegel, N.; Sherman, M.; Canseco, J.A.; Hilibrand, A.S.; Kepler, C.K.; Vaccaro, A.R.; Schroeder, G.D. Cefazolin Prophylaxis in Spine Surgery: Patients Are Frequently Underdosed and at Increased Risk for Infection. Spine J. 2022, 22, 1442–1450.
- Coates, M.; Shield, A.; Peterson, G.M.; Hussain, Z. Prophylactic Cefazolin Dosing in Obesity—A Systematic Review. Obes. Surg. 2022, 32, 3138–3149.
- Ahmadzia, H.K.; Patel, E.M.; Joshi, D.; Liao, C.; Witter, F.; Heine, R.P.; Coleman, J.S. Obstetric Surgical Site Infections: 2 Grams Compared With 3 Grams of Cefazolin in Morbidly Obese Women. Obstet. Gynecol. 2015, 126, 708–715.
- Peppard, W.J.; Eberle, D.G.; Kugler, N.W.; Mabrey, D.M.; Weigelt, J.A. Association between Pre-Operative Cefazolin Dose and Surgical Site Infection in Obese Patients. Surg. Infect. 2017, 18, 485–490.
- Hussain, Z.; Curtain, C.; Mirkazemi, C.; Gadd, K.; Peterson, G.M.; Zaidi, S.T.R. Prophylactic Cefazolin Dosing and Surgical Site Infections: Does the Dose Matter in Obese Patients? Obes. Surg. 2019, 29, 159–165.
- Hites, M.; Deprez, G.; Wolff, F.; Ickx, B.; Verleije, A.; Closset, J.; Loi, P.; Prévost, J.; Taccone, F.S.; Racapé, J.; et al. Evaluation of Total Body Weight and Body Mass Index Cut-Offs for Increased Cefazolin Dose for Surgical Prophylaxis. Int. J. Antimicrob. Agents 2016, 48, 633–640.
- Classen, D.C.; Evans, R.S.; Pestotnik, S.L.; Horn, S.D.; Menlove, R.L.; Burke, J.P. The Timing of Prophylactic Administration of Antibiotics and the Risk of Surgical-Wound Infection. N. Engl. J. Med. 1992, 326, 281–286.
- Steinberg, J.P.; Braun, B.I.; Hellinger, W.C.; Kusek, L.; Bozikis, M.R.; Bush, A.J.; Dellinger, E.P.; Burke, J.P.; Simmons, B.; Kritchevsky, S.B. Timing of Antimicrobial Prophylaxis and the Risk of Surgical Site Infections: Results From the Trial to Reduce Antimicrobial Prophylaxis Errors. Ann. Surg. 2009, 250, 10–16.
- De Jonge, S.W.; Gans, S.L.; Atema, J.J.; Solomkin, J.S.; Dellinger, P.E.; Boermeester, M.A. Timing of Preoperative Antibiotic Prophylaxis in 54,552 Patients and the Risk of Surgical Site Infection: A Systematic Review and Meta-Analysis. Medicine 2017, 96, e6903.
- Koch, C.G.; Nowicki, E.R.; Rajeswaran, J.; Gordon, S.M.; Sabik, J.F.; Blackstone, E.H. When the Timing Is Right: Antibiotic Timing and Infection after Cardiac Surgery. J. Thorac. Cardiovasc. Surg. 2012, 4, 931–937.e4.
- Koch, C.G.; Li, L.; Hixson, E.; Tang, A.; Gordon, S.; Longworth, D.; Phillips, S.; Blackstone, E.; Henderson, M.J. Is It Time to Refine? An Exploration and Simulation of Optimal Antibiotic Timing in General Surgery. J. Am. Coll. Surg. 2013, 217, 628.
- Sommerstein, R.; Atkinson, A.; Kuster, S.P.; Thurneysen, M.; Genoni, M.; Troillet, N.; Marschall, J.; Widmer, A.F.; Swissnoso; Balmelli, C.; et al. Antimicrobial Prophylaxis and the Prevention of Surgical Site Infection in Cardiac Surgery: An Analysis of 21,007 Patients in Switzerland†. Eur. J. Cardio-Thorac. Surg. 2019, 56, 800–806.
- Weber, W.P.; Mujagic, E.; Zwahlen, M.; Bundi, M.; Hoffmann, H.; Soysal, S.D.; Kraljević, M.; Delko, T.; Von Strauss, M.; Iselin, L.; et al. Timing of Surgical Antimicrobial Prophylaxis: A Phase 3 Randomised Controlled Trial. Lancet Infect. Dis. 2017, 17, 605–614.
- De Jonge, S.W.; Boldingh, Q.J.J.; Koch, A.H.; Daniels, L.; De Vries, E.N.; Spijkerman, I.J.B.; Ankum, W.M.; Kerkhoffs, G.M.M.J.; Dijkgraaf, M.G.; Hollmann, M.W.; et al. Timing of Preoperative Antibiotic Prophylaxis and Surgical Site Infection: TAPAS, An Observational Cohort Study. Ann. Surg. 2021, 274, e308–e314.
- Paasch, C.; Schildberg, C.; Lünse, S.; Heisler, S.; Meyer, J.; Kirbach, J.; Kobelt, E.; Hunger, R.; Haller, I.-E.; Helmke, C.; et al. Optimal Timing for Antimicrobial Prophylaxis to Reduce Surgical Site Infections: A Retrospective Analysis of 531 Patients. Sci. Rep. 2023, 13, 9405.
- Weber, W.P.; Marti, W.R.; Zwahlen, M.; Misteli, H.; Rosenthal, R.; Reck, S.; Fueglistaler, P.; Bolli, M.; Trampuz, A.; Oertli, D.; et al. The Timing of Surgical Antimicrobial Prophylaxis. Ann. Surg. 2008, 247, 918.
- Naik, B.I.; Roger, C.; Ikeda, K.; Todorovic, M.S.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Comparative Total and Unbound Pharmacokinetics of Cefazolin Administered by Bolus versus Continuous Infusion in Patients Undergoing Major Surgery: A Randomized Controlled Trial. Br. J. Anaesth. 2017, 118, 876–882.
- Douglas, A.; Udy, A.A.; Wallis, S.C.; Jarrett, P.; Stuart, J.; Lassig-Smith, M.; Deans, R.; Roberts, M.S.; Taraporewalla, K.; Jenkins, J.; et al. Plasma and Tissue Pharmacokinetics of Cefazolin in Patients Undergoing Elective and Semielective Abdominal Aortic Aneurysm Open Repair Surgery. Antimicrob. Agents Chemother. 2011, 55, 5238–5242.
- Himebauch, A.S.; Nicolson, S.C.; Sisko, M.; Moorthy, G.; Fuller, S.; Gaynor, J.W.; Zuppa, A.F.; Fox, E.; Kilbaugh, T.J. Skeletal Muscle and Plasma Concentrations of Cefazolin during Cardiac Surgery in Infants. J. Thorac. Cardiovasc. Surg. 2014, 148, 2634–2641.
- Himebauch, A.S.; Sankar, W.N.; Flynn, J.M.; Sisko, M.T.; Moorthy, G.S.; Gerber, J.S.; Zuppa, A.F.; Fox, E.; Dormans, J.P.; Kilbaugh, T.J. Skeletal Muscle and Plasma Concentrations of Cefazolin during Complex Paediatric Spinal Surgery. Br. J. Anaesth. 2016, 117, 87–94.
- Caruso, T.J.; Wang, E.Y.; Colletti, A.A.; Sharek, P.J. Intraoperative Antibiotic Redosing Compliance and the Extended Postoperative Recovery Period: Often Overlooked Areas That May Reduce Surgical Site Infections. Pediatr. Anesth. 2019, 29, 290–291.
- Wolfhagen, N.; Boldingh, Q.J.J.; De Lange, M.; Boermeester, M.A.; De Jonge, S.W. Intraoperative Redosing of Surgical Antibiotic Prophylaxis in Addition to Preoperative Prophylaxis Versus Single-Dose Prophylaxis for the Prevention of Surgical Site Infection: A Meta-Analysis and GRADE Recommendation. Ann. Surg. 2022, 275, 1050–1057.
- Lanoiselée, J.; Chaux, R.; Hodin, S.; Bourayou, S.; Gibert, A.; Philippot, R.; Molliex, S.; Zufferey, P.J.; Delavenne, X.; Ollier, E. Population Pharmacokinetic Model of Cefazolin in Total Hip Arthroplasty. Sci. Rep. 2021, 11, 19763.





