
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yihong Hu | -- | 2348 | 2024-01-04 08:04:02 | | | |
| 2 | Peter Tang | Meta information modification | 2348 | 2024-01-04 08:41:31 | | |
Video Upload Options
Human B cell immortalization that maintains the constant growth characteristics and antibody expression of B cells in vitro is very critical for the development of antibody drugs and products for the diagnosis and bio-therapeutics of human diseases. Human B cell immortalization methods include Epstein-Barr virus (EBV) transformation, Simian virus 40 (SV40) virus infection, in vitro genetic modification, and activating CD40, etc. Immortalized human B cells produce monoclonal antibodies (mAbs) very efficiently, and the antibodies produced in this way can overcome the immune rejection caused by heterologous antibodies. It is an effective way to prepare mAbs and an important method for developing therapeutic monoclonal antibodies.
1. Introduction
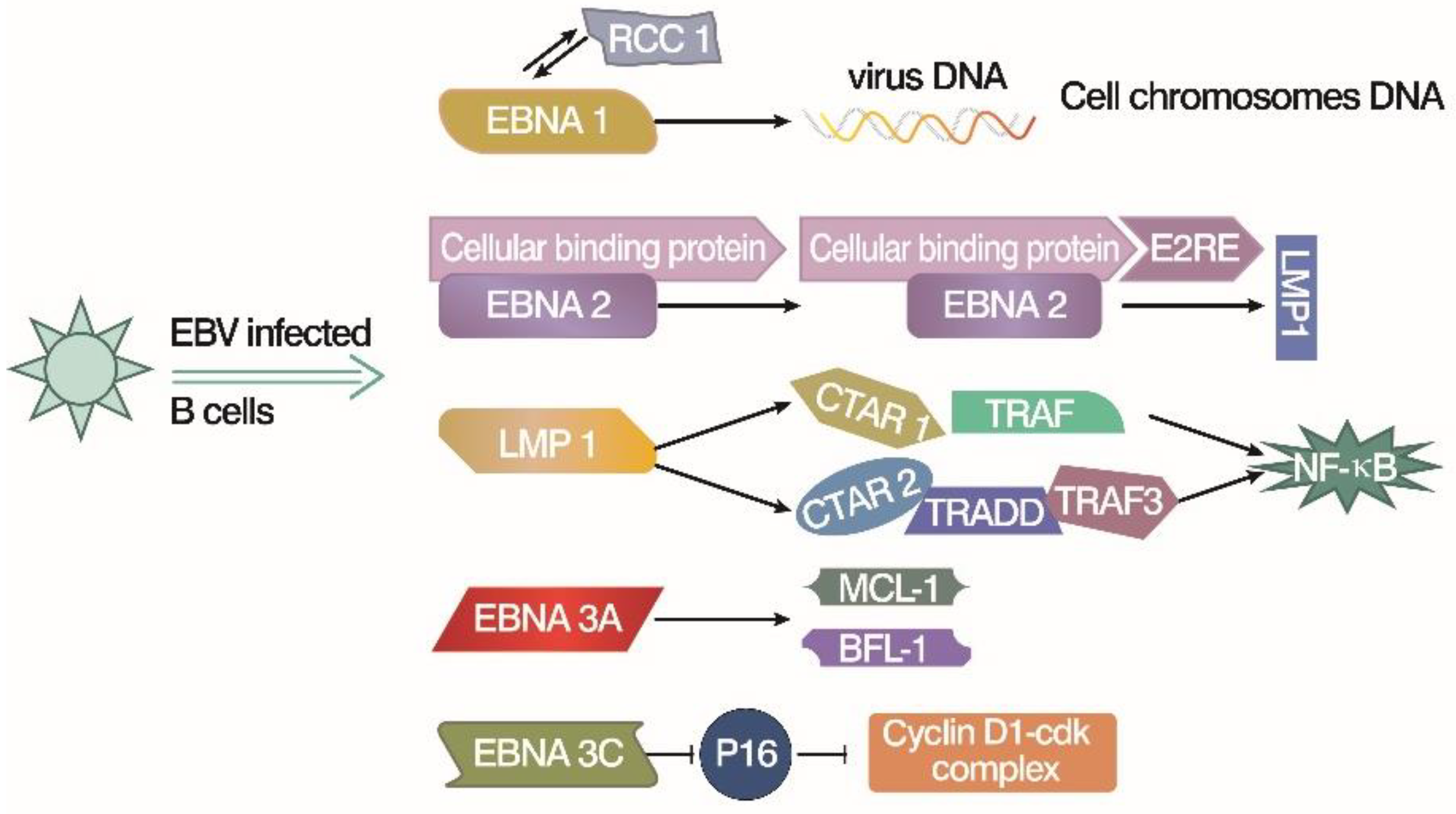
2. Immortalize Human Peripheral Blood B Lymphocytes by Epstein-Barr Virus
3. Immortalize Human Peripheral B Lymphocytes by Simian Virus 40

4. Immortalize B Lymphocytes by In Vitro Gene Transduction
5. Immortalize B Lymphocytes by Activation of CD40 Signal
|
Methods |
Starting Point |
Species |
Approaches |
Way to Maintain Cell Cultures |
Final Products |
References |
|---|---|---|---|---|---|---|
|
EBV |
Isolated mononuclear leukocytes |
Human, some primates |
Transformation |
Latent genes, p53 tumor suppressor, telomerase, etc |
LCLs |
[23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][64][65][66][67][68][69][70][71][72][73][74][75] |
|
SV40 |
Isolated human B lymphocytes |
Mammalian |
Infection |
LT antigen regulated E2F and p53 down-stream gene, ST antigens |
Permanently proliferative cell line |
|
|
Gene transduction |
Isolated human B cells |
Multiple species |
In vitro |
Ectopic expression of Bcl-6 and Bcl-xl with proper feeder cells |
Variable immortalized B-cell lineage |
|
|
Activation of CD40 signal |
PBMC |
Human |
Exogenous stimulation |
Regular and repeated CD40 ligand/IL-4 stimulation plus cyclosporin A |
Immortalized B cells with phenotypic characteristics of activated B cells |
References
- Obinata, M. The immortalized cell lines with differentiation potentials: Their establishment and possible application. Cancer Sci. 2007, 98, 275–283.
- Chan, S.K.; Rahumatullah, A.; Lai, J.Y.; Lim, T.S. Naive Human Antibody Libraries for Infectious Diseases. Adv. Exp. Med. Biol. 2017, 1053, 35–59.
- Lai, J.Y.; Lim, T.S. Construction of Naive and Immune Human Fab Phage Display Library. Methods Mol. Biol. 2023, 2702, 39–58.
- Omar, N.; Lim, T.S. Construction of Naive and Immune Human Fab Phage-Display Library. Methods Mol. Biol. 2018, 1701, 25–44.
- Weitkamp, J.H.; Kallewaard, N.; Kusuhara, K.; Feigelstock, D.; Feng, N.; Greenberg, H.B.; Crowe, J.E., Jr. Generation of recombinant human monoclonal antibodies to rotavirus from single antigen-specific B cells selected with fluorescent virus-like particles. J. Immunol. Methods 2003, 275, 223–237.
- von Boehmer, L.; Liu, C.; Ackerman, S.; Gitlin, A.D.; Wang, Q.; Gazumyan, A.; Nussenzweig, M.C. Sequencing and cloning of antigen-specific antibodies from mouse memory B cells. Nat. Protoc. 2016, 11, 1908–1923.
- Katakura, Y.; Alam, S.; Shirahata, S. Immortalization by gene transfection. Methods Cell Biol. 1998, 57, 69–91.
- Zhong, Z.; Yu, S.; Ge, S. Advances in immortalization of human B cells. Sheng Wu Gong Cheng Xue Bao 2021, 37, 30–39.
- Colgin, L.M.; Reddel, R.R. Telomere maintenance mechanisms and cellular immortalization. Curr. Opin. Genet. Dev. 1999, 9, 97–103.
- Lundberg, A.S.; Hahn, W.C.; Gupta, P.; Weinberg, R.A. Genes involved in senescence and immortalization. Curr. Opin. Cell Biol. 2000, 12, 705–709.
- Fridman, A.L.; Tainsky, M.A. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene 2008, 27, 5975–5987.
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352.
- Ma, C.; Li, X.; Ding, W.; Zhang, X.; Chen, H.; Feng, Y. Effects of hTERT transfection on the telomere and telomerase of Periplaneta americana cells in vitro. AMB Express 2023, 13, 118.
- Sklar, M.D.; White, B.J.; Rowe, W.P. Initiation of oncogenic transformation of mouse lymphocytes in vitro by Abelson leukemia virus. Proc. Natl. Acad. Sci. USA 1974, 71, 4077–4081.
- Muljo, S.A.; Schlissel, M.S. A small molecule Abl kinase inhibitor induces differentiation of Abelson virus-transformed pre-B cell lines. Nat. Immunol. 2003, 4, 31–37.
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497.
- Parray, H.A.; Shukla, S.; Samal, S.; Shrivastava, T.; Ahmed, S.; Sharma, C.; Kumar, R. Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. Int. Immunopharmacol. 2020, 85, 106639.
- Ko, Y.H. EBV and human cancer. Exp. Mol. Med. 2015, 47, e130.
- Saha, A.; Robertson, E.S. Mechanisms of B-Cell Oncogenesis Induced by Epstein-Barr Virus. J. Virol. 2019, 93, e00238-19.
- Burton, E.M.; Gewurz, B.E. Epstein-Barr virus oncoprotein-driven B cell metabolism remodeling. PLoS Pathog. 2022, 18, e1010254.
- Yetming, K.D.; Lupey-Green, L.N.; Biryukov, S.; Hughes, D.J.; Marendy, E.M.; Miranda, J.L.; Sample, J.T. The BHLF1 Locus of Epstein-Barr Virus Contributes to Viral Latency and B-Cell Immortalization. J. Virol. 2020, 94, e01215-20.
- Steinitz, M.; Klein, G.; Koskimies, S.; Makel, O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature 1977, 269, 420–422.
- Neitzel, H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum. Genet. 1986, 73, 320–326.
- Hatton, O.L.; Harris-Arnold, A.; Schaffert, S.; Krams, S.M.; Martinez, O.M. The interplay between Epstein-Barr virus and B lymphocytes: Implications for infection, immunity, and disease. Immunol. Res. 2014, 58, 268–276.
- Manivannan, A.C.; Devaraju, V.; Velmurugan, P.; Sathiamoorthi, T.; Sivakumar, S.; Subbiah, S.K.; Ravi, A.V. Tumorigenesis and diagnostic practice applied in two oncogenic viruses: Epstein Barr virus and T-cell lymphotropic virus-1-Mini review. Biomed. Pharmacother. 2021, 142, 111974.
- Price, A.M.; Dai, J.; Bazot, Q.; Patel, L.; Nikitin, P.A.; Djavadian, R.; Winter, P.S.; Salinas, C.A.; Barry, A.P.; Wood, K.C.; et al. Epstein-Barr virus ensures B cell survival by uniquely modulating apoptosis at early and late times after infection. Elife 2017, 6, e22509.
- Dolcetti, R.; Dal Col, J.; Martorelli, D.; Carbone, A.; Klein, E. Interplay among viral antigens, cellular pathways and tumor microenvironment in the pathogenesis of EBV-driven lymphomas. Semin. Cancer Biol. 2013, 23, 441–456.
- Ai, P.; Li, Z.; Jiang, Y.; Song, C.; Zhang, L.; Hu, H.; Wang, T. Tumor microenvironment contributes to Epstein-Barr virus anti-nuclear antigen-1 antibody production in nasopharyngeal carcinoma. Oncol. Lett. 2017, 14, 2458–2462.
- Deschamps, T.; Bazot, Q.; Leske, D.M.; MacLeod, R.; Mompelat, D.; Tafforeau, L.; Lotteau, V.; Marechal, V.; Baillie, G.S.; Gruffat, H.; et al. Epstein-Barr virus nuclear antigen 1 interacts with regulator of chromosome condensation 1 dynamically throughout the cell cycle. J. Gen. Virol. 2017, 98, 251–265.
- Zimber-Strobl, U.; Strobl, L.J. EBNA2 and Notch signalling in Epstein-Barr virus mediated immortalization of B lymphocytes. Semin. Cancer Biol. 2001, 11, 423–434.
- Dheekollu, J.; Wiedmer, A.; Ayyanathan, K.; Deakyne, J.S.; Messick, T.E.; Lieberman, P.M. Cell-cycle-dependent EBNA1-DNA crosslinking promotes replication termination at oriP and viral episome maintenance. Cell 2021, 184, 643–654.e13.
- Kashuba, E.; Pokrovskaja, K.; Klein, G.; Szekely, L. Epstein-Barr virus-encoded nuclear protein EBNA-3 interacts with the epsilon-subunit of the T-complex protein 1 chaperonin complex. J. Hum. Virol. 1999, 2, 33–37.
- Kaye, K.M.; Izumi, K.M.; Kieff, E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 1993, 90, 9150–9154.
- Cahir-McFarland, E.D.; Davidson, D.M.; Schauer, S.L.; Duong, J.; Kieff, E. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 2000, 97, 6055–6060.
- Price, A.M.; Tourigny, J.P.; Forte, E.; Salinas, R.E.; Dave, S.S.; Luftig, M.A. Analysis of Epstein-Barr virus-regulated host gene expression changes through primary B-cell outgrowth reveals delayed kinetics of latent membrane protein 1-mediated NF-kappaB activation. J. Virol. 2012, 86, 11096–11106.
- Nikitin, P.A.; Price, A.M.; McFadden, K.; Yan, C.M.; Luftig, M.A. Mitogen-induced B-cell proliferation activates Chk2-dependent G1/S cell cycle arrest. PLoS ONE 2014, 9, e87299.
- Paschos, K.; Smith, P.; Anderton, E.; Middeldorp, J.M.; White, R.E.; Allday, M.J. Epstein-barr virus latency in B cells leads to epigenetic repression and CpG methylation of the tumour suppressor gene Bim. PLoS Pathog. 2009, 5, e1000492.
- Allday, M.J. EBV finds a polycomb-mediated, epigenetic solution to the problem of oncogenic stress responses triggered by infection. Front. Genet. 2013, 4, 212.
- Styles, C.T.; Bazot, Q.; Parker, G.A.; White, R.E.; Paschos, K.; Allday, M.J. EBV epigenetically suppresses the B cell-to-plasma cell differentiation pathway while establishing long-term latency. PLoS Biol. 2017, 15, e2001992.
- Bazot, Q.; Paschos, K.; Skalska, L.; Kalchschmidt, J.S.; Parker, G.A.; Allday, M.J. Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57KIP2. PLoS Pathog. 2015, 11, e1005031.
- Kamranvar, S.A.; Masucci, M.G. Regulation of Telomere Homeostasis during Epstein-Barr virus Infection and Immortalization. Viruses 2017, 9, 217.
- Li, D.; Zhao, R.; Lilyestrom, W.; Gai, D.; Zhang, R.; DeCaprio, J.A.; Fanning, E.; Jochimiak, A.; Szakonyi, G.; Chen, X.S. Structure of the replicative helicase of the oncoprotein SV40 large tumour antigen. Nature 2003, 423, 512–518.
- Poulin, D.L.; DeCaprio, J.A. Is there a role for SV40 in human cancer? J. Clin. Oncol. 2006, 24, 4356–4365.
- Pipas, J.M. SV40: Cell transformation and tumorigenesis. Virology 2009, 384, 294–303.
- Fanning, E.; Knippers, R. Structure and function of simian virus 40 large tumor antigen. Annu. Rev. Biochem. 1992, 61, 55–85.
- Sullivan, C.S.; Pipas, J.M. T antigens of simian virus 40: Molecular chaperones for viral replication and tumorigenesis. Microbiol. Mol. Biol. Rev. 2002, 66, 179–202.
- Imperiale, M.J. The human polyomaviruses, BKV and JCV: Molecular pathogenesis of acute disease and potential role in cancer. Virology 2000, 267, 1–7.
- Ahuja, D.; Saenz-Robles, M.T.; Pipas, J.M. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 2005, 24, 7729–7745.
- Simmons, D.T. SV40 large T antigen functions in DNA replication and transformation. Adv. Virus Res. 2000, 55, 75–134.
- Alwin Prem Anand, A.; Gowri Sankar, S.; Kokila Vani, V. Immortalization of neuronal progenitors using SV40 large T antigen and differentiation towards dopaminergic neurons. J. Cell Mol. Med. 2012, 16, 2592–2610.
- DeCaprio, J.A. How the Rb tumor suppressor structure and function was revealed by the study of Adenovirus and SV40. Virology 2009, 384, 274–284.
- Alaribe, F.N.; Mazzoni, E.; Rigolin, G.M.; Rizzotto, L.; Maniero, S.; Pancaldi, C.; Manfrini, M.; Martini, F.; Tognon, M.G. Extended lifespan of normal human B lymphocytes experimentally infected by SV40 or transfected by SV40 large T antigen expression vector. Leuk. Res. 2013, 37, 681–689.
- Ali, S.H.; DeCaprio, J.A. Cellular transformation by SV40 large T antigen: Interaction with host proteins. Semin. Cancer Biol. 2001, 11, 15–23.
- Levine, A.J. The common mechanisms of transformation by the small DNA tumor viruses: The inactivation of tumor suppressor gene products: p53. Virology 2009, 384, 285–293.
- Gutierrez-Guerrero, A.; Cosset, F.L.; Verhoeyen, E. Lentiviral Vector Pseudotypes: Precious Tools to Improve Gene Modification of Hematopoietic Cells for Research and Gene Therapy. Viruses 2020, 12, 1016.
- Chang, C.C.; Ye, B.H.; Chaganti, R.S.; Dalla-Favera, R. BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc. Natl. Acad. Sci. USA 1996, 93, 6947–6952.
- Klein, U.; Dalla-Favera, R. Germinal centres: Role in B-cell physiology and malignancy. Nat. Rev. Immunol. 2008, 8, 22–33.
- Diehl, S.A.; Schmidlin, H.; Nagasawa, M.; van Haren, S.D.; Kwakkenbos, M.J.; Yasuda, E.; Beaumont, T.; Scheeren, F.A.; Spits, H. STAT3-mediated up-regulation of BLIMP1 Is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J. Immunol. 2008, 180, 4805–4815.
- Shapiro-Shelef, M.; Lin, K.I.; McHeyzer-Williams, L.J.; Liao, J.; McHeyzer-Williams, M.G.; Calame, K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 2003, 19, 607–620.
- Tunyaplin, C.; Shaffer, A.L.; Angelin-Duclos, C.D.; Yu, X.; Staudt, L.M.; Calame, K.L. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J. Immunol. 2004, 173, 1158–1165.
- Li, M.; Wang, D.; He, J.; Chen, L.; Li, H. Bcl-XL: A multifunctional anti-apoptotic protein. Pharmacol. Res. 2020, 151, 104547.
- Kwakkenbos, M.J.; van Helden, P.M.; Beaumont, T.; Spits, H. Stable long-term cultures of self-renewing B cells and their applications. Immunol. Rev. 2016, 270, 65–77.
- Kwakkenbos, M.J.; Diehl, S.A.; Yasuda, E.; Bakker, A.Q.; van Geelen, C.M.; Lukens, M.V.; van Bleek, G.M.; Widjojoatmodjo, M.N.; Bogers, W.M.; Mei, H.; et al. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat. Med. 2010, 16, 123–128.
- Stepanenko, A.A.; Kavsan, V.M. Immortalization and malignant transformation of eukaryotic cells. Tsitol. Genet. 2012, 46, 36–75.
- Egbuniwe, O.; Idowu, B.D.; Funes, J.M.; Grant, A.D.; Renton, T.; Di Silvio, L. P16/p53 expression and telomerase activity in immortalized human dental pulp cells. Cell Cycle 2011, 10, 3912–3919.
- Kamranvar, S.A.; Chen, X.; Masucci, M.G. Telomere dysfunction and activation of alternative lengthening of telomeres in B-lymphocytes infected by Epstein-Barr virus. Oncogene 2013, 32, 5522–5530.
- Pratt, Z.L.; Zhang, J.; Sugden, B. The latent membrane protein 1 (LMP1) oncogene of Epstein-Barr virus can simultaneously induce and inhibit apoptosis in B cells. J. Virol. 2012, 86, 4380–4393.
- Traggiai, E.; Becker, S.; Subbarao, K.; Kolesnikova, L.; Uematsu, Y.; Gismondo, M.R.; Murphy, B.R.; Rappuoli, R.; Lanzavecchia, A. An efficient method to make human monoclonal antibodies from memory B cells: Potent neutralization of SARS coronavirus. Nat. Med. 2004, 10, 871–875.
- Nogales-Gadea, G.; Saxena, A.; Hoffmann, C.; Hounjet, J.; Coenen, D.; Molenaar, P.; Losen, M.; Martinez-Martinez, P. Generation of Recombinant Human IgG Monoclonal Antibodies from Immortalized Sorted B Cells. J. Vis. Exp. 2015, e52830.
- Tousizadeh, B.; Moghim, S.; Chaleshtori, A.R.S.; Ghanbarian, M.; Mirian, M.; Salehi, M.; Tousizadeh, S.; Zaboli, F. Application of Epstein-Barr Virus for Optimization of Immortalized B-lymphocyte Production as a Positive Control in Genetic Studies. Adv. Biomed. Res. 2017, 6, 80.
- McFadden, K.; Hafez, A.Y.; Kishton, R.; Messinger, J.E.; Nikitin, P.A.; Rathmell, J.C.; Luftig, M.A. Metabolic stress is a barrier to Epstein-Barr virus-mediated B-cell immortalization. Proc. Natl. Acad. Sci. USA 2016, 113, E782–E790.
- Chen, X.; Kamranvar, S.A.; Masucci, M.G. Oxidative stress enables Epstein-Barr virus-induced B-cell transformation by posttranscriptional regulation of viral and cellular growth-promoting factors. Oncogene 2016, 35, 3807–3816.
- Hafez, A.Y.; Messinger, J.E.; McFadden, K.; Fenyofalvi, G.; Shepard, C.N.; Lenzi, G.M.; Kim, B.; Luftig, M.A. Limited nucleotide pools restrict Epstein-Barr virus-mediated B-cell immortalization. Oncogenesis 2017, 6, e349.
- Wang, C.; Liu, X.; Liang, J.; Narita, Y.; Ding, W.; Li, D.; Zhang, L.; Wang, H.; Leong, M.M.L.; Hou, I.; et al. A DNA tumor virus globally reprograms host 3D genome architecture to achieve immortal growth. Nat. Commun. 2023, 14, 1598.
- Zhao, B. Epstein-Barr Virus B Cell Growth Transformation: The Nuclear Events. Viruses 2023, 15, 832.
- Bright, R.K.; Kimchi, E.T.; Shearer, M.H.; Kennedy, R.C.; Pass, H.I. SV40 Tag-specific cytotoxic T lymphocytes generated from the peripheral blood of malignant pleural mesothelioma patients. Cancer Immunol. Immunother. 2002, 50, 682–690.
- Hu, B.T.; Lee, S.C.; Marin, E.; Ryan, D.H.; Insel, R.A. Telomerase is up-regulated in human germinal center B cells in vivo and can be re-expressed in memory B cells activated in vitro. J. Immunol. 1997, 159, 1068–1071.
- Wiesner, M.; Zentz, C.; Mayr, C.; Wimmer, R.; Hammerschmidt, W.; Zeidler, R.; Moosmann, A. Conditional immortalization of human B cells by CD40 ligation. PLoS ONE 2008, 3, e1464.
- Banchereau, J.; de Paoli, P.; Valle, A.; Garcia, E.; Rousset, F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science 1991, 251, 70–72.





