Cell immortalization refers to cells that grow
in vitro induced by or under the influence of external factors and whose growth cycle is different from that of normal cells, and the trend of senescence is avoided in order to have high proliferation and long passage [
1]. Immortalization is a necessary stage in the process of malignant transformation from normal cells to cancer cells, so cell immortalization has become a research hotspot related to cancer. Meanwhile, the immortalization of B cells is an essential part of immunotherapy for cancer and one of the sources and bases for preparing specific monoclonal antibodies. They support the production of rare antibodies and can overcome the disadvantages of other production methods, such as the human naive library combined with a phage display platform [
2,
3,
4] or the single B cell platform [
5,
6], through screened monoclonal antibodies. Monoclonal antibodies of the same species do not cause immune antagonism and play an indispensable role in the treatment of diseases. Generally, the frequency of cell immortalization is very low, which is less than 1 × 10
−12 in human cells and 1 × 10
−6–1 × 10
−5 in rodent cells. Therefore, the spontaneous immortalization of animal cells is scarce [
7].
Currently, artificial methods are mainly used to induce cell immortalization. The immortalized mechanisms reported so far include radioactive mutations, telomere and telomerase activation, viral transformation, activation or inhibition of oncogenes and tumor suppressor genes, etc. [
8]. Although the mechanisms of cell immortalization are similar, the methods of immortalization are varied for different cells. The activation of human telomerase reverse transcriptase (hTERT) is an important step in cell immortalization [
9,
10,
11], as the stable expression of the hTERT gene in primary cultured cells can stabilize the length of telomeres and immortalize the cells of different species [
12,
13]. However, hTERT overexpression has never immortalized human B cells successfully. While cell lines transformed by Abelson murine leukemia virus (v-Abl cells) were first reported and used for studying the regulation of B cell development [
14], v-Abl blocks B cell development, resulting in B cells failing to mature and produce antibodies [
15]. Shortly afterward, Köler and Milstein discovered the immortalization of the murine B cells method—the hybridoma technology generating large amounts of monoclonal antibodies—which was a revolution in immunology [
16]. The immortalization of mouse B cells by fusing them with myeloma cells has since become widely practiced. Hybridomas based on B cells of other rodents (rat hybridomas are common) are very abundant and widely commercially available [
17]. In contrast, the immortalization of human B cells (and of non-rodent species) proved to be more challenging. There are no human myeloma cell lines that proved to be successful fusion partners. “Hetero-hybridomas” created by the fusion of human B cells with mouse myeloma cells were genetically unstable and could not be stably maintained in culture. So, it is necessary to look for efficient immortalization approaches for human B cells.
2. Immortalize Human Peripheral Blood B Lymphocytes by Epstein-Barr Virus
Epstein-Barr virus (EBV), a human herpesvirus causing infectious mononucleosis, is highly immunogenic having a more than 95% seropositive ratio in the world community and is associated with Burkitt lymphoma and nasopharyngeal carcinoma in etiology [
18,
19]. It contributes to 200,000 cancers per year around the world [
20], and after the establishment of latent infection in B lymphocytes, EBV can persist in the human host for life [
21]. EB virus transformation is a standard method to immortalize isolated human B cells [
22]. EBV can transform isolated mononuclear leukocytes, which contain human peripheral blood B lymphocytes, into immortal human lymphoblastoid cell lines (LCLs) in an appropriate
in vitro environment, and their biochemical and molecular biological characteristics remain unchanged [
23,
24]. The EBV genome is a linear double strand of about 172 kb containing more than 85 genes, but only a few are expressed in EBV-infected B lymphocytes, called latent genes [
24,
25]. Latent proteins can activate the interaction between cell growth factor and its receptor, and change the life cycle of B lymphocytes, thus immortalizing the cells.
Six latent virus genes, including Epstein-Barr nuclear antigen LP (EBNA-LP), nuclear antigen 3A (
EBNA3A), nuclear antigen 3C (
EBNA3C), nuclear antigen 1 (
EBNA1), nuclear antigen 2 (
EBNA2), and latent membrane protein 1 (
LMP1) [
26], are mainly expressed during the transformation of B cells into LCLs by EB virus [
27].
EBNA1 and
EBNA2 are the keys to inducing the immortalization of B cells, while
EBNA3A,
EBNA3C, and
LMP1 are also involved in the
in vitro transformation of B cells.
EBNA1 is a homologous dimer DNA-binding protein that can bind to multiple sites in the host genome [
28].
EBNA1′s expression in the latent and release periods of viruses mediates replication and segregation of the respective viral genomes [
29,
30], which is crucial for maintaining replication termination at OriP and viral episome maintenance [
31].
EBNA2 is a primary viral transcription activator and can up-regulate the expression of host genes and other EBNA genes [
32]. It activates the expression of viral oncoprotein
LMP1 during the initiation and maintenance of B cell immortalization [
33], which accelerates the immortalization of infected B cells into LCLs, enabling them to proliferate indefinitely during culture. Thus, the
LMP1 oncoprotein is essential for the continued growth and survival of LCLs [
34]. It simulates a pro-survival tumor necrosis factor receptor, which constitutively sends out signals through the NF-KB pathway to promote the proliferation of transformed cells and inhibit apoptosis [
35]. Inhibition of the activated NF-KB pathway downstream of
LMP1 resulted in the apoptosis of LCLs. However, studies have shown that B cells still proliferate rapidly with
LMP1 expression and low NF-KB activation levels in the early post-infection period [
35,
36]. In early infected B cells, the expression of
EBNA3A protein can induce the resistance to apoptosis of B lymphocytes and primary B cells by down-regulating the Bim gene [
37], thus reducing the “starting power” of infected cells to apoptosis. So, these early infected cells showed no apparent apoptosis, although the cell DNA damage response was strongly activated [
38]. In addition, the EBN3A family epigenetically down-regulates other human genes involved in cell cycle regulation [
39]. For example, the expression of two host proteins,
MCL-1 and
BFL-1, is controlled by
EBNA3A. Meanwhile,
EBNA3C and
EBNA3A inhibit the expression of tumor suppressor p16 in combination to maintain the continuous proliferation of B cells and transform B cells into immortalized LCLs [
40,
41]. In summary, the EBV latent gene mimics the critical factors of natural B cell growth, promotes its proliferation to form immortalized LCLs, and inhibits host innate tumor suppressor responses to uncontrollable proliferation (
Figure 1).
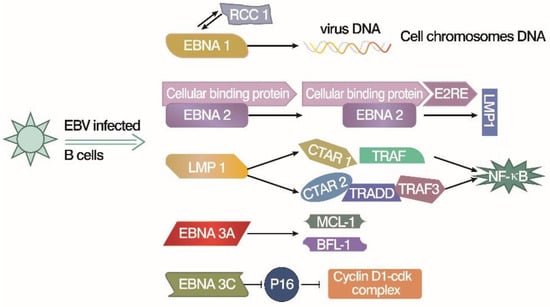
Figure 1. EBV latent gene products work on B cell immortalization [
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37].
EBNA1 may interact with RCC1, which leads the viral DNA onto the chromosomes and promotes virus replication.
EBNA2 upregulates the other viral EBNA genes, like
LMP1, by forming a complex with E2RE and cellular binding protein. LMP-1 activates NF-KB directly or indirectly through interaction with key intermediate proteins (TRAF and TRADD, etc.).
EBNA3A upregulates the
MCL-1 and
BFL-1 proteins, which are related to cell death, or apoptosis.
EBNA3C inhibits p16 to upregulate the Cyclins and B cell proliferation.
3. Immortalize Human Peripheral B Lymphocytes by Simian Virus 40
Simian virus 40 (SV40) was first identified in rhesus monkey kidney cell cultures used to produce the polio vaccine in 1960 [
55]. It is named for the effect on infected cells that produce a number of abnormal vacuoles, as SV40 may promote tumor growth in animal models and induce the transformation of primary cultured human cells [
56,
57]. SV40 infects rodent or mammalian cells to improve cell immortalization rate and is widely used in experimental models of mammalian cell replication and gene expression [
58,
59]. SV40 is a double-stranded DNA virus whose genome encodes seven proteins, three structural proteins, and four functional proteins in an overlapping reading frame [
58,
60]. Structural proteins are VP1, VP2, and VP3, and functional proteins include large T (LT) antigens and small T (ST) antigens essential to the virus life cycle and two small proteins whose functions are unknown [
59]. SV40 LT antigen, a multifunctional regulatory protein [
61], is classified as a member of the helicase superfamily and has the property of releasing double-stranded DNA and RNA, which play a decisive role in the initiation of virus-transformed cells [
62].
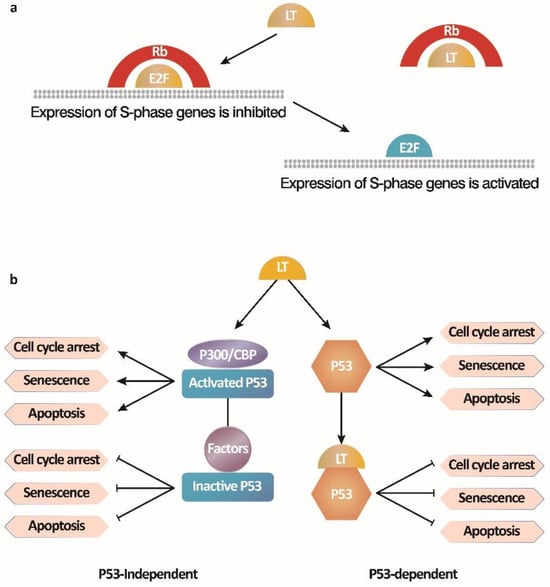
There are two main mechanisms by which the SV40 LT antigen immortalizes cells (
Figure 2) [
63]. One is the activation of E2F-mediated transcription by binding to the Rb-E2F complex. The other is to inhibit p53 activity by blocking p53-dependent transcriptional activation and p53-independent growth arrest. LT antigen binds to the Rb protein to release the inhibition of cell cycle regulation by the Rb-E2F complex, leading to the entry of the S phase with E2F-dependent gene transcription from the growth arrest pathway and resulting in continuous cell proliferation. During the cell transformation by SV40, the LT antigen interacts with the tumor suppressor p53, a transcriptional activator that mediates apoptosis under adverse conditions such as DNA damage, nucleotide deficiency, and abnormal inhibition of Rb protein, thereby transforming cells and extending their lifespan [
64,
65]. Moreover, the binding of LT to cellular factors, p300/CBP, also prevents apoptosis and leads to the survival of cells independent of direct interaction between LT and p53 [
66]. However, ST antigens can enhance cell transformation but are not necessary. In brief, LT antigens and ST antigens maintain cell transformation phenotypes together [
67].
Figure 2. Two mechanisms by which the SV40 LT antigen immortalizes B cells [
63]. (
a) SV40 LT antigen activates E2F-mediated transcription by binding to the Rb-E2F complex; (
b) SV40 LT antigen inhibits p53 activity by blocking p53-dependent transcriptional activation and p53-independent growth arrest, preventing B cell apoptosis.
4. Immortalize B Lymphocytes by In Vitro Gene Transduction
Lentiviral vectors have been designated as a tool for gene therapy because of their characteristic to induce all types of non-diving or slowly proliferating cells, which makes them very significant for clinical applications [
69]. Proto-oncogene Bcl-6 belongs to the B-cell lymphoma family of genes as a factor suppressing apoptosis, which is widely involved in the processes of cell differentiation, activation, cell cycle regulation, etc. Differentially expressed in cell types, Bcl-6 is expressed at high levels only in germinal center (GC) B cells and lymphomas with a germinal center B (GC-B) cell phenotype. Bcl-6 works by inhibiting transcriptional activity through the interaction with transcription factors; even the binding site of Bcl-6 is far from the initiation site [
70]. It inhibits apoptosis and participates in the cell cycle arrest response by directly inhibiting the transcription of the p53 tumor suppressor gene or by binding to the transcriptional activator protein inhibitor of activated STAT2 (PIAS2), thereby inhibiting the activation of the cell-cycle arrest gene p21. Therefore, Bcl-6 may enable GC-B cells to maintain physiological genotoxic stress associated with high proliferation but does not cause p53-dependent or p53-independent growth arrest and apoptotic responses [
71]. Studies on mice have shown that Bcl-6 is an essential factor for GC formation and is beneficial to the proliferation of isolated human B cells by inhibiting the differentiation of B cells into plasma cells [
72]. Plasma cells express the transcription factor B lymphocytes-induced mature protein-1 (
Blimp-1), which is necessary for plasma cell differentiation [
73], while Bcl-6 ectopic expression can inhibit the expression of
Blimp-1 by binding to Bcl-6 response elements in the
prdm1 gene, thus inhibiting plasma cell differentiation [
74]. Other studies have shown that the co-expression of Bcl-xl, Bcl-2, and Mcl1, a variety of Bcl-2 family genes, results in a strong inhibition of apoptosis in Bcl-6 transduced cells. Therein, Bcl-xl, an essential member of the Bcl-2 family that is mainly investigated in tumorigenesis and drug resistance, has the best effect on cell immortalization by inhibiting apoptosis through the interaction with various proteins [
75].
Co-cultured with CD40 ligand (CD40L) and interleukin-21 (IL-21), which are produced by follicular helper T cells, the isolated peripheral blood memory B cells transduced by Bcl-6 and Bcl-xl transgenes could be transformed into cell surface B cell receptor (BCR)-positive and Ig-secreting immortalized B cells. Kwakkenbos et al. set up the immortalized B cell culture system with mouse fibroblasts expressing cytokines and CD40L as the feeder layer and forced the expression of Bcl-6 by a retrovirus-mediated method that can prevent B cells from differentiating into plasma cells [
76]. When cell death is inhibited, Bcl-6/Bcl-xL-mediated B cells proliferate rapidly, stimulated by a variety of cytokines (including IL-4, IL-10, and IL-21) to immortalize B cells by
in vitro gene modification. The overexpression of Bcl-6 and Bcl-xl in combination with the CD40L/IL-21 culture system provided immortalized B cells with GC-B cell-like characteristics; thus, a variety of antiviral-specific antibodies were successfully screened, isolated, and generated [
77]. Studies have cloned respiratory syncytial virus-specific and influenza-specific B cell lines and used these cells as antibody sources to effectively neutralize the virus
in vivo. This method provides a new tool not only for studying B-cell biology and signal transduction by antigen-specific B-cell receptors, but also for the rapid preparation of high-affinity human monoclonal antibodies.
5. Immortalize B Lymphocytes by Activation of CD40 Signal
The starting point, species, approaches and final products of the human B cell immortalization methods are compiled in Table 1.
Table 1. Summary for human B cell immortalization methods.
|
Methods
|
Starting Point
|
Species
|
Approaches
|
Way to Maintain Cell Cultures
|
Final Products
|
References
|
|
EBV
|
Isolated mononuclear leukocytes
|
Human, some primates
|
Transformation
|
Latent genes, p53 tumor suppressor, telomerase, etc
|
LCLs
|
[23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]
|
|
SV40
|
Isolated human B lymphocytes
|
Mammalian
|
Infection
|
LT antigen regulated E2F and p53 down-stream gene, ST antigens
|
Permanently proliferative cell line
|
[65,68]
|
|
Gene transduction
|
Isolated human B cells
|
Multiple species
|
In vitro
|
Ectopic expression of Bcl-6 and Bcl-xl with proper feeder cells
|
Variable immortalized B-cell lineage
|
[72,75]
|
|
Activation of CD40 signal
|
PBMC
|
Human
|
Exogenous stimulation
|
Regular and repeated CD40 ligand/IL-4 stimulation plus cyclosporin A
|
Immortalized B cells with phenotypic characteristics of activated B cells
|
[82,83,84]
|