
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Viseslav Popadic | -- | 2786 | 2023-12-29 08:19:34 | | | |
| 2 | Fanny Huang | Meta information modification | 2786 | 2023-12-29 10:19:25 | | |
Video Upload Options
Coronary microvascular dysfunction (CMD) is a clinical entity linked with various risk factors that significantly affect cardiac morbidity and mortality. Hypertension, one of the most important, causes both functional and structural alterations in the microvasculature, promoting the occurrence and progression of microvascular angina. Endothelial dysfunction and capillary rarefaction play the most significant role in the development of CMD among patients with hypertension. CMD is also related to several hypertension-induced morphological and functional changes in the myocardium in the subclinical and early clinical stages, including left ventricular hypertrophy, interstitial myocardial fibrosis, and diastolic dysfunction. This indicates the fact that CMD, especially if associated with hypertension, is a subclinical marker of end-organ damage and heart failure, particularly that with preserved ejection fraction.
1. Introduction
2. Diagnostics for Coronary Microvascular Dysfunction in Patients with Hypertension
2.1. Non-Invasive Diagnostics
2.1.1. Echocardiography
2.1.2. Computerized Tomography (CT)
2.1.3. Single-Photon Emission Computed Tomography (SPECT)
2.1.4. Positron Emission Tomography (PET)
2.1.5. Cardiac Magnetic Resonance (CMR)
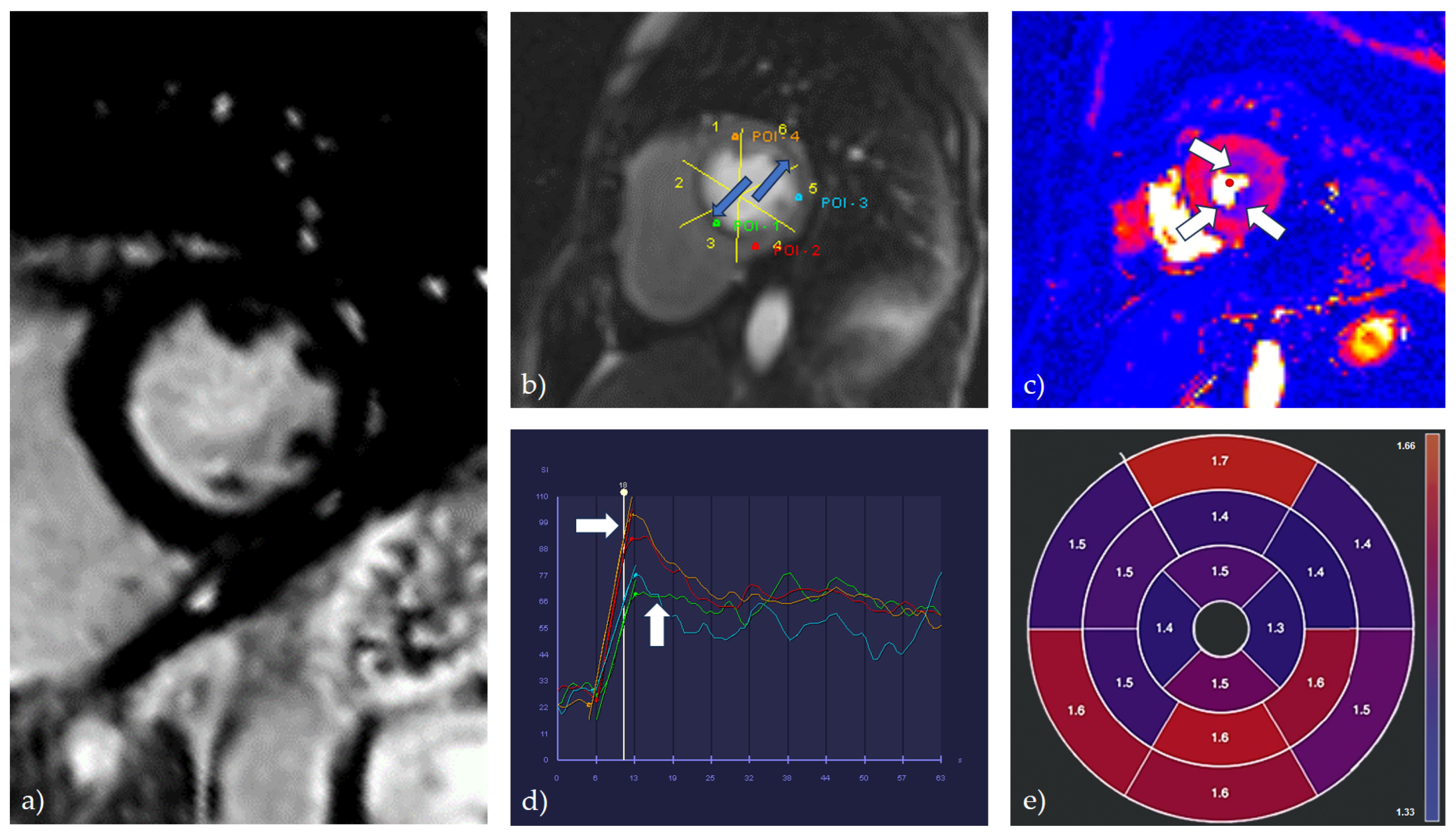
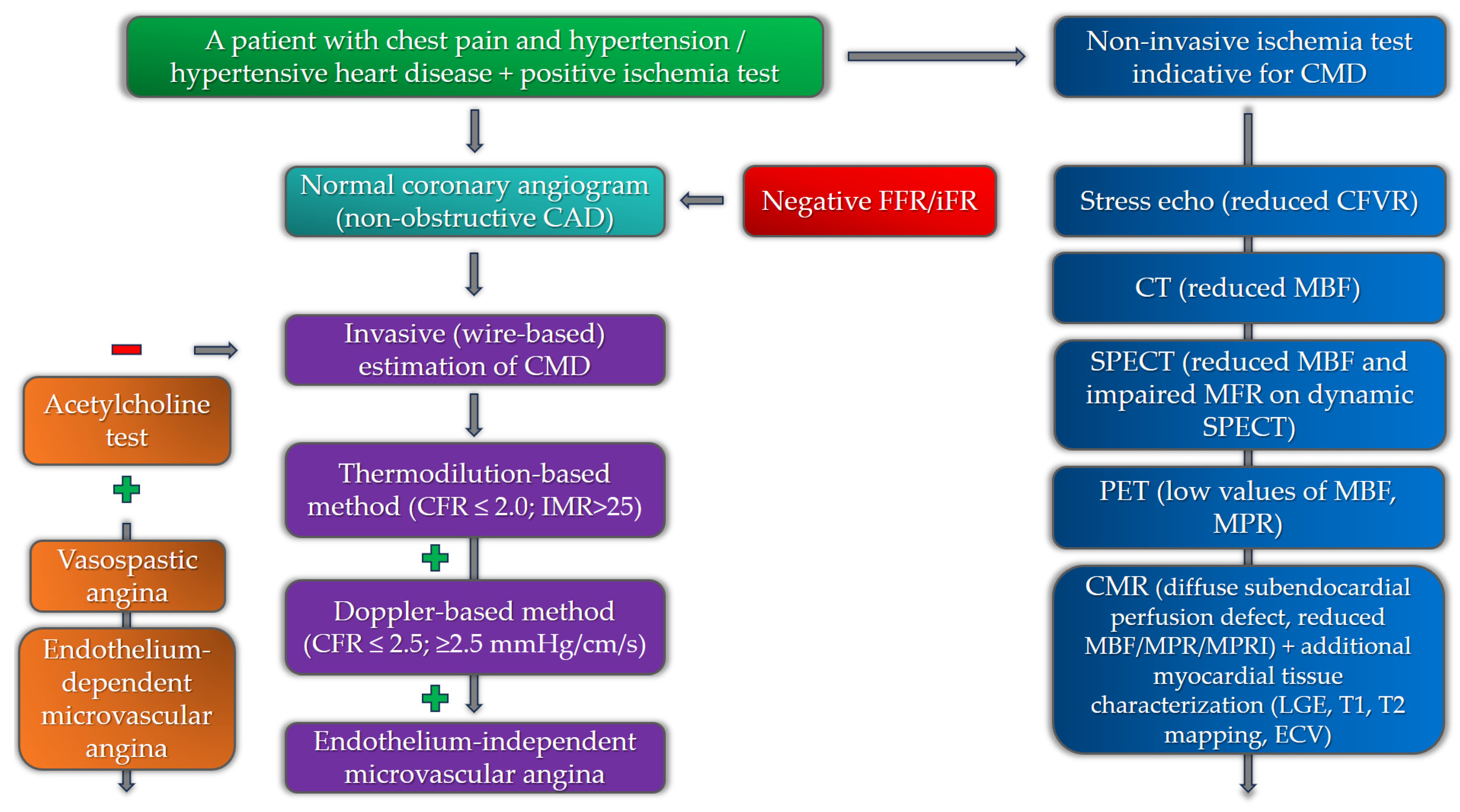
2.2. Invasive Diagnostics
References
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802.
- Vasan, R.S.; Song, R.J.; Xanthakis, V.; Beiser, A.; DeCarli, C.; Mitchell, G.F.; Seshadri, S. Hypertension-Mediated Organ Damage: Prevalence, Correlates, and Prognosis in the Community. Hypertension 2022, 79, 505–515.
- Benas, D.; Triantafyllidi, H.; Birmpa, D.; Fambri, A.; Schoinas, A.; Thymis, I.; Kostelli, G.; Ikonomidis, I. Hypertension-Mediated Organ Damage in Young Patients with First-Diagnosed And Never Treated Systolic Hypertension. Curr. Vasc. Pharmacol. 2023, 21, 197–204.
- Chen, C.; Wei, J.; AlBadri, A.; Zarrini, P.; Merz, C.N.B. Coronary Microvascular Dysfunction—Epidemiology, Pathogenesis, Prognosis, Diagnosis, Risk Factors and Therapy. Circ. J. 2016, 81, 3–11.
- Bradley, C.; Berry, C. Definition and epidemiology of coronary microvascular disease. J. Nucl. Cardiol. 2022, 29, 1763–1775.
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880.
- Godo, S.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Endothelium in Coronary Macrovascular and Microvascular Diseases. J. Cardiovasc. Pharmacol. 2021, 78 (Suppl. S6), S19–S29.
- Labazi, H.; Trask, A.J. Coronary microvascular disease as an early culprit in the pathophysiology of diabetes and metabolic syndrome. Pharmacol. Res. 2017, 123, 114–121.
- Horton, W.B.; Barrett, E.J. Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr. Rev. 2021, 42, 29–55.
- Kibel, A.; Selthofer-Relatic, K.; Drenjancevic, I.; Bacun, T.; Bosnjak, I.; Kibel, D.; Gros, M. Coronary microvascular dysfunction in diabetes mellitus. J. Int. Med. Res. 2017, 45, 1901–1929.
- Lee, M.P.; Glynn, R.J.; Schneeweiss, S.; Lin, K.J.; Patorno, E.; Barberio, J.; Levin, R.; Evers, T.; Wang, S.V.; Desai, R.J. Risk Factors for Heart Failure with Preserved or Reduced Ejection Fraction Among Medicare Beneficiaries: Application of Competing Risks Analysis and Gradient Boosted Model. Clin. Epidemiol. 2020, 12, 607–616.
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271.
- Lanza, G.A.; Morrone, D.; Pizzi, C.; Tritto, I.; Bergamaschi, L.; De Vita, A.; Villano, A.; Crea, F. Diagnostic approach for coronary microvascular dysfunction in patients with chest pain and no obstructive coronary artery disease. Trends Cardiovasc. Med. 2022, 32, 448–453.
- Fordyce, C.B.; Newby, D.E.; Douglas, P.S. Diagnostic Strategies for the Evaluation of Chest Pain: Clinical Implications from SCOT-HEART and PROMISE. J. Am. Coll. Cardiol. 2016, 67, 843–852.
- Carbone, A.; D’andrea, A.; Sperlongano, S.; Tagliamonte, E.; Mandoli, G.E.; Santoro, C.; Evola, V.; Bandera, F.; Morrone, D.; Malagoli, A.; et al. Echocardiographic assessment of coronary microvascular dysfunction: Basic concepts, technical aspects, and clinical settings. Echocardiography 2021, 38, 993–1001.
- Schroder, J.; Prescott, E. Doppler Echocardiography Assessment of Coronary Microvascular Function in Patients with Angina and No Obstructive Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 723542.
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.-S.; Beussink-Nelson, L.; Faxén, U.L.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450.
- Völz, S.; Svedlund, S.; Andersson, B.; Li-Ming, G.; Rundqvist, B. Coronary flow reserve in patients with resistant hypertension. Clin. Res. Cardiol. 2016, 106, 151–157.
- Clemmensen, T.S.; Christensen, M.; Løgstrup, B.B.; Kronborg, C.J.S.; Knudsen, U.B. Reduced coronary flow velocity reserve in women with previous pre-eclampsia: Link to increased cardiovascular disease risk. Ultrasound Obstet. Gynecol. 2020, 55, 786–792.
- Gaibazzi, N.; Bergamaschi, L.; Pizzi, C.; Tuttolomondo, D. Resting global longitudinal strain and stress echocardiography to detect coronary artery disease burden. Eur. Heart J. Cardiovasc. Imaging 2023, 24, e86–e88.
- Tagliamonte, E.; Sperlongano, S.; Montuori, C.; Riegler, L.; Scarafile, R.; Carbone, A.; Forni, A.; Radmilovic, J.; Di Vilio, A.; Astarita, R.; et al. Coronary microvascular dysfunction affects left ventricular global longitudinal strain response to dipyridamole stress echocardiography: A pilot study. Heart Vessel. 2023, 38, 470–477.
- Jovanovic, I.; Tesic, M.; Giga, V.; Dobric, M.; Boskovic, N.; Vratonjic, J.; Orlic, D.; Gudelj, O.; Tomasevic, M.; Dikic, M.; et al. Impairment of coronary flow velocity reserve and global longitudinal strain in women with cardiac syndrome X and slow coronary flow. J. Cardiol. 2020, 76, 1–8.
- Nieman, K.; Balla, S. Dynamic CT myocardial perfusion imaging. J. Cardiovasc. Comput. Tomogr. 2020, 14, 303–306.
- Seitun, S.; Clemente, A.; De Lorenzi, C.; Benenati, S.; Chiappino, D.; Mantini, C.; Sakellarios, A.I.; Cademartiri, F.; Bezante, G.P.; Porto, I. Cardiac CT perfusion and FFRCTA: Pathophysiological features in ischemic heart disease. Cardiovasc. Diagn. Ther. 2020, 10, 1954–1978.
- Ihdayhid, A.R.; Fairbairn, T.A.; Gulsin, G.S.; Tzimas, G.; Danehy, E.; Updegrove, A.; Jensen, J.M.; Taylor, C.A.; Bax, J.J.; Sellers, S.L.; et al. Cardiac computed tomography-derived coronary artery volume to myocardial mass. J. Cardiovasc. Comput. Tomogr. 2022, 16, 198–206.
- Van Rosendael, S.E.; van Rosendael, A.R.; Kuneman, J.H.; Patel, M.R.; Nørgaard, B.L.; Fairbairn, T.A.; Nieman, K.; Akasaka, T.; Berman, D.S.; Koweek, L.M.H.; et al. Coronary Volume to Left Ventricular Mass Ratio in Patients with Hypertension. Am. J. Cardiol. 2023, 199, 100–109.
- Djaïleb, L.; Riou, L.; Piliero, N.; Carabelli, A.; Vautrin, E.; Broisat, A.; Leenhardt, J.; Machecourt, J.; Fagret, D.; Vanzetto, G.; et al. SPECT myocardial ischemia in the absence of obstructive CAD: Contribution of the invasive assessment of microvascular dysfunction. J. Nucl. Cardiol. 2018, 25, 1017–1022.
- Zhang, H.; Caobelli, F.; Che, W.; Huang, Y.; Zhang, Y.; Fan, X.; Hu, X.; Xu, C.; Fei, M.; Zhang, J.; et al. The prognostic value of CZT SPECT myocardial blood flow (MBF) quantification in patients with ischemia and no obstructive coronary artery disease (INOCA): A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1940–1953.
- Mathew, R.C.; Bourque, J.M.; Salerno, M.; Kramer, C.M. Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC Cardiovasc. Imaging 2020, 13, 1577–1590.
- Zhou, W.; Brown, J.M.; Bajaj, N.S.; Chandra, A.; Divakaran, S.; Weber, B.; Bibbo, C.F.; Hainer, J.; Taqueti, V.R.; Dorbala, S.; et al. Hypertensive coronary microvascular dysfunction: A subclinical marker of end organ damage and heart failure. Eur. Heart J. 2020, 41, 2366–2375.
- Patel, A.R.; Salerno, M.; Kwong, R.Y.; Singh, A.; Heydari, B.; Kramer, C.M. Stress Cardiac Magnetic Resonance Myocardial Perfusion Imaging: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 1655–1668.
- Zdravkovic, M.; Klasnja, S.; Popovic, M.; Djuran, P.; Mrda, D.; Ivankovic, T.; Manojlovic, A.; Koracevic, G.; Lovic, D.; Popadic, V. Cardiac Magnetic Resonance in Hypertensive Heart Disease: Time for a New Chapter. Diagnostics 2022, 13, 137.
- Liang, L.; Wang, X.; Yu, Y.; Zhang, Y.; Liu, J.; Chen, M.; Zhang, L.; Jiang, T. T1 Mapping and Extracellular Volume in Cardiomyopathy Showing Left Ventricular Hypertrophy: Differentiation between Hypertrophic Cardiomyopathy and Hypertensive Heart Disease. Int. J. Gen. Med. 2022, 15, 4163–4173.
- Engblom, H.; Xue, H.; Akil, S.; Carlsson, M.; Hindorf, C.; Oddstig, J.; Hedeer, F.; Hansen, M.S.; Aletras, A.H.; Kellman, P.; et al. Fully quantitative cardiovascular magnetic resonance myocardial perfusion ready for clinical use: A comparison between cardiovascular magnetic resonance imaging and positron emission tomography. J. Cardiovasc. Magn. Reson. 2017, 19, 78.
- Zorach, B.; Shaw, P.W.; Bourque, J.; Kuruvilla, S.; Balfour, P.C.; Yang, Y.; Mathew, R.; Pan, J.; Gonzalez, J.A.; Taylor, A.M.; et al. Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. J. Cardiovasc. Magn. Reson. 2018, 20, 14.
- Thomson, L.E.; Wei, J.; Agarwal, M.; Haft-Baradaran, A.; Shufelt, C.L.; Mehta, P.K.; Gill, E.B.; Johnson, B.D.; Kenkre, T.; Handberg, E.M.; et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ. Cardiovasc. Imaging 2015, 8, e002481.
- Rahman, H.; Scannell, C.M.; Demir, O.M.; Ryan, M.; McConkey, H.; Ellis, H.; Masci, P.G.; Perera, D.; Chiribiri, A. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2021, 14, 978–986.
- Chang, A.; Kang, N.; Chung, J.; Gupta, A.R.; Parwani, P. Evaluation of Ischemia with No Obstructive Coronary Arteries (INOCA) and Contemporary Applications of Cardiac Magnetic Resonance (CMR). Medicina 2023, 59, 1570.
- Scarsini, R.; Shanmuganathan, M.; De Maria, G.L.; Borlotti, A.; Kotronias, R.A.; Burrage, M.K.; Terentes-Printzios, D.; Langrish, J.; Lucking, A.; Fahrni, G.; et al. Coronary Microvascular Dysfunction Assessed by Pressure Wire and CMR After STEMI Predicts Long-Term Outcomes. JACC Cardiovasc. Imaging 2021, 14, 1948–1959.
- Knott, K.D.; Seraphim, A.; Augusto, J.B.; Xue, H.; Chacko, L.; Aung, N.; Petersen, S.E.; Cooper, J.A.; Manisty, C.; Bhuva, A.N.; et al. The Prognostic Significance of Quantitative Myocardial Perfusion: An Artificial Intelligence Based Approach Using Perfusion Mapping. Circulation 2020, 141, 1282–1291.
- Levelt, E.; Piechnik, S.K.; Liu, A.; Wijesurendra, R.S.; Mahmod, M.; Ariga, R.; Francis, J.M.; Greiser, A.; Clarke, K.; Neubauer, S.; et al. Adenosine stress CMR T1-mapping detects early microvascular dysfunction in patients with type 2 diabetes mellitus without obstructive coronary artery disease. J. Cardiovasc. Magn. Reson. 2017, 19, 81.
- Travieso, A.; Jeronimo-Baza, A.; Faria, D.; Shabbir, A.; Mejia-Rentería, H.; Escaned, J. Invasive evaluation of coronary microvascular dysfunction. J. Nucl. Cardiol. 2022, 29, 2474–2486.
- Mangiacapra, F.; Viscusi, M.M.; Verolino, G.; Paolucci, L.; Nusca, A.; Melfi, R.; Ussia, G.P.; Grigioni, F. Invasive Assessment of Coronary Microvascular Function. J. Clin. Med. 2021, 11, 228.
- Geng, Y.; Wu, X.; Liu, H.; Zheng, D.; Xia, L. Index of microcirculatory resistance: State-of-the-art and potential applications in computational simulation of coronary artery disease. J. Zhejiang Univ. Sci. B 2022, 23, 123–140.
- Fearon, W.F.; Kobayashi, Y. Invasive Assessment of the Coronary Microvasculature: The Index of Microcirculatory Resistance. Circ. Cardiovasc. Interv. 2017, 10, e005361.
- Toya, T.; Nagatomo, Y.; Ikegami, Y.; Masaki, N.; Adachi, T. Coronary microvascular dysfunction in heart failure patients. Front. Cardiovasc. Med. 2023, 10, 1153994.
- Dryer, K.; Gajjar, M.; Narang, N.; Lee, M.; Paul, J.; Shah, A.P.; Nathan, S.; Butler, J.; Davidson, C.J.; Fearon, W.F.; et al. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H1033–H1042.
- Chirinos, J.A.; Mitchell, G.F.; Parise, H.; Benjamin, E.J.; Larson, M.G.; Keyes, M.J.; Vita, J.A.; Vasan, R.S.; Levy, D.; Hashimoto, J.; et al. Large Artery Stiffness, Microvascular Function, and Cardiovascular Risk. Circ. Cardiovasc. Imaging 2016, 9, e005903.
- Ikonomidis, I.; Lekakis, J.; Papadopoulos, C.; Triantafyllidi, H.; Paraskevaidis, I.; Georgoula, G.; Tzortzis, S.; Revela, I.; Kremastinos, D.T. Incremental value of pulse wave velocity in the determination of coronary microcirculatory dysfunction in never-treated patients with essential hypertension. Am. J. Hypertens. 2008, 21, 806–813.
- Sakalidis, A.; Dimitriadis, K.; Leontsinis, I.; Dri, E.; Mantzouranis, E.; Bora, M.; E Karanikola, A.; Iliakis, P.; Vlachakis, P.; Siafi, E.; et al. Increased arterial stiffness in patients with ischemia and no obstructive coronary artery disease. Eur. Heart J. 2023, 44, ehad655.2139.
- Aursulesei Onofrei, V.; Ceasovschih, A.; Anghel, R.C.; Roca, M.; Marcu, D.T.M.; Adam, C.A.; Mitu, O.; Cumpat, C.; Mitu, F.; Crisan, A.; et al. Subendocardial Viability Ratio Predictive Value for Cardiovascular Risk in Hypertensive Patients. Medicina 2022, 59, 24.




