Alternative splicing changes are closely linked to aging, though it remains unclear if they are drivers or effects. As organisms age, splicing patterns change, varying gene isoform levels and functions. These changes may contribute to aging alterations rather than just reflect declining RNA quality control. Three main splicing types—intron retention, cassette exons, and cryptic exons—play key roles in age-related complexity. These events modify protein domains and increase nonsense-mediated decay, shifting protein isoform levels and functions. This may potentially drive aging or serve as a biomarker. Fluctuations in splicing factor expression also occur with aging. Somatic mutations in splicing genes can also promote aging and age-related disease.
1. Introduction
A wide range of changes in cellular mechanisms involving both transcriptional and post-transcriptional regulation have been linked to normal aging
[1]. While age-related variations in the cellular environment lead to eventual molecular changes, it is also possible that the molecular changes accelerate aging and age-related disorders (ranging from hypertension to cardiovascular disease, cancer, and neurodegeneration). Furthermore, different tissues and organs may experience different age-related alterations in transcriptional and post-transcriptional regulation.
In higher eukaryotic genomes, alternative splicing (AS) of both protein and non-coding genes not only profoundly contributes to increasing the functional diversity and complexity of the whole transcriptome
[2][3][4], but it also seems to be a master regulator of cellular and individual aging.
Although the majority of variations in alternative splicing events occur during development, it is estimated that approximately 30% of all alternative splicing alterations occur during aging
[5][6]. As rodents and humans consistently exhibit age- and tissue-related variations in the expression of genes involved in splicing
[6][7][8][9][10][11], age-related changes in splicing may be caused by the age-related decline in splicing factor expression. On the other hand, the main categories of genes with age-related altered splicing include those encoding genes with neuronal-specific activities such as synaptic transmission in the human brain
[11], as well as those implicated in collagen production and post-translational modification in the human Achilles tendon
[12][13]. These observations suggest that age-dependent splicing changes are more likely to occur in at least some of the same categories of tissue-specific genes that show transcriptional decline with aging.
Aging-dependent splicing alterations can explain why some genes show a tissue-specific decrease in expression. Splicing errors during pre-mRNA processing can result in the incorrect usage of alternative splice sites, leading to intron retention in the mature mRNA transcript rather than proper exon joining. Intron retention introduces premature termination codons that target the aberrant transcripts for degradation through nonsense-mediated decay (NMD). This differs from frameshift mutations caused by small insertions or deletions during splicing, which can also introduce premature stop codons but do not always trigger transcript degradation by NMD, and may allow some protein production from the altered transcripts
[14][15][16]. Considering that NMD might decline with aging
[17], this might affect the levels and impact of aging-related alternative splicing isoforms.
In addition, it has been observed that mutations within splice sites may activate cryptic splice sites, resulting in the erroneous processing of lamin A, a gene associated with laminopathies and implicated in premature aging and other aging-related disorders
[18].
2. Age-Related Alterations in Splicing
The multifaceted process of aging is linked to a progressive loss in physiological integrity, which causes functional decline and elevated morbidity. Aging can alter the balance of the proteins produced by a particular gene
[19][20].
This phenomenon may be considered as an evolved response to let cells change their transcriptome and proteome to adapt to the new condition. From this point of view, age-related changes in alternative splicing can contribute to and add a new degree of complexity to the control of gene expression (
Figure 1). On the other hand, the age-dependent variations in alternative splicing may be considered the negative side-effect of a progressive deterioration in quality control of the splicing machinery (
Figure 1), in a similar manner as has been observed at the proteomic level
[21][22].
Growing evidence suggests that aging is associated with changes in splicing fidelity, primarily intron retention
[23], a phenomenon conserved through evolution
[23][24][25]. However, it is unclear if this is a result of aging-related disruptions in cellular homeostasis or a cause.
Intriguingly, genes involved in metabolic processes exhibit intron inclusion with age, and dietary restriction induces intron retention in young organisms like Caenorhabditis elegans
[24] and Drosophila heads
[23], and in the hippocampus of mice
[16].
In addition, an increase in age-related intron retention in genes implicated in proteins and mRNA homeostasis has been correlated with aging-associated diseases, such as Alzheimer’s disease patients
[23].
Other studies have then showed that exon skipping is prevalent in aging skeletal muscle, particularly in genes tied to mitochondrial functions and inflammation
[26]. Additionally, a comparison of splicing types across tissues showed a bias towards intron retention upregulation and identified cassette exons as another frequent event
[27].
These observations emphasize that age-related changes in alternative splicing contribute to the complexity of aging.
Aging is a multifaceted process associated with the progressive loss of physiological integrity, leading to functional decline and increased morbidity. These observations highlight that age-related changes in alternative splicing contribute to the complexity of the aging process.
In conclusion, splicing changes are a dynamic and complex aspect of the aging process, involving various splicing events and tissue-specific effects, and underscore the importance of further research to elucidate the mechanisms and functional consequences of age-related splicing alterations that may provide insights into potential therapeutic strategies to mitigate age-related health issues.
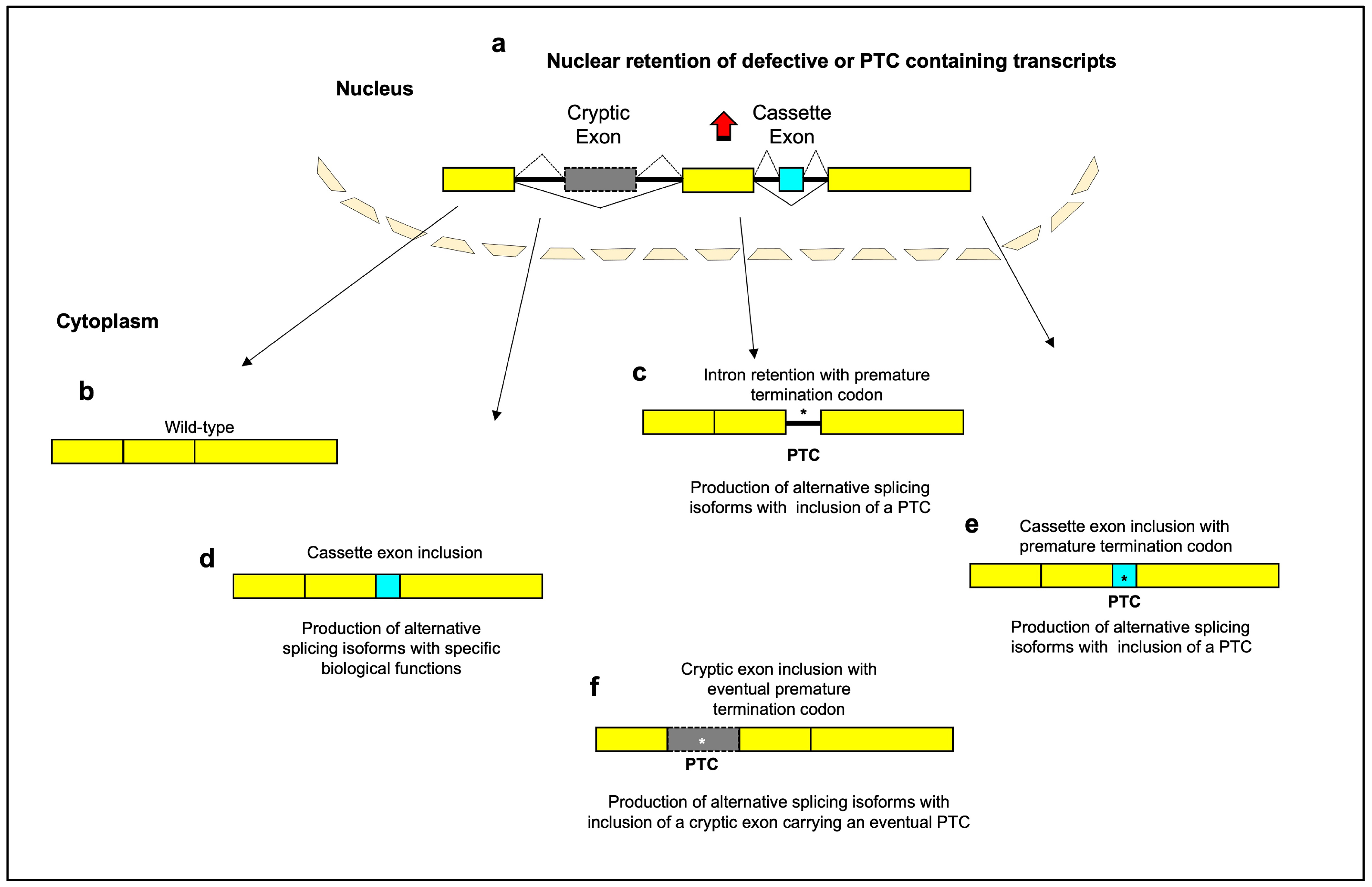
Figure 1. Schematic diagram of the main aging-related alternative splicing events. Most genes are split between exons and introns. Splicing-defective or premature termination codon (PTC)-containing transcripts are retained within nucleus, with inhibition of translation (
a). Normal patterns of splice site selection (indicated with solid black lines) join the constitutive exons together to create “wild type” mRNAs (
b). Intron-retaining transcripts can include a PTC and undergo degradation by activation of nonsense-mediated decay (
c). The inclusion of a cassette exon into mRNA (indicated with dashed black lines) can lead to a gain of functional domains (
d) or to the appearance of a PTC (
e). The inclusion of a cryptic exon into mRNA can lead to the introduction of frameshifts or PTC and subsequently to the loss of specific domains or to reduction in gene expression (
f). A portion of PTC-containing transcripts is recognized and retained in the nucleus before they have a chance to be exported to the cytoplasm for degradation by the NMD system. PTCs in different reading frames can lead to different levels of nuclear retention. This suggests that the mechanism of nuclear retention is not simply due to the presence of a stop codon, but is dependent on the specific sequence context of the PTC
[28][29]. The exact mechanisms by which PTC-containing transcripts are retained in the nucleus are still being investigated. One possible mechanism is alternative splicing—the presence of a PTC can affect the splicing of an mRNA, leading to the inclusion of exons that contain nuclear retention signals that prevent export to the cytoplasm. Another possibility is interaction with specific nuclear proteins or RNA binding proteins that bind to the PTC-containing transcripts and retain them in the nucleus, blocking their export. Changes to the mRNA structure induced by the PTC could also prevent transit to the cytoplasm if the folding prevents export. While further research is needed to elucidate the precise retention mechanisms, the nuclear retention itself may serve a protective purpose. By retaining PTC-containing transcripts in the nucleus, truncated proteins are prevented from being translated in the cytoplasm where they could have detrimental effects on the cell
[29][30]. Thus, nuclear retention may act more to protect the cell rather than facilitate mRNA repair pathways like splicing to excise the PTC or induce degradation of unrepairable mRNAs. Yellow box: constitutive exon; gray box: cryptic exon; aqua box: cassette exon; asterisk: premature termination codon.
3. Mining Databases for Splicing Factors Linked to Human Aging
Various available resources currently aim to support research on the genetics of human aging
[31][32][33][34][35]. The investigation revealed an overlap of 50 genes with the list of genes that either show increased or decreased expression during replicative senescence of human cells (
Table 1).
Table 1. Overlap between the genes categorized under Gene Ontology ID 0008380 and the list of genes that either show increased or decreased expression during replicative senescence of human cells available in the CellAge Database (
https://genomics.senescence.info/cells/, accessed on 11 December 2023). The table presents the results of matching the 1159 human orthologs of RNA binding proteins categorized under Gene Ontology ID 0008380 with the genes listed in the CellAge database. The trends of expression (over- or under-expression) associated with aging for these genes, as well as the effects (induction or inhibition) of these changes on the senescent phenotype, are shown.
| Gene Symbol |
Expression |
Senescence Effect |
| AHNAK2 |
Over-expressed |
|
| RBM24 |
Over-expressed |
|
| ALYREF |
Under-expressed |
|
| BUD13 |
Under-expressed |
|
| C1QBP |
Under-expressed |
|
| CWF19L1 |
Under-expressed |
|
| DAZAP1 |
Under-expressed |
|
| DCPS |
Under-expressed |
|
| DDX39A |
Under-expressed |
|
| DHX15 |
Under-expressed |
|
| EIF4A3 |
Under-expressed |
|
| FUS |
Under-expressed |
|
| GEMIN6 |
Under-expressed |
|
| HNRNPA1 |
Under-expressed |
Inhibits |
| HNRNPA2B1 |
Under-expressed |
|
| HNRNPA3 |
Under-expressed |
Inhibits |
| HNRNPC |
Under-expressed |
Induces |
| HNRNPF |
Under-expressed |
|
| HNRNPH1 |
Under-expressed |
|
| HNRNPH3 |
Under-expressed |
|
| HNRNPM |
Under-expressed |
|
| HNRNPU |
Under-expressed |
|
| KHDRBS1 |
Under-expressed |
|
| KHSRP |
Under-expressed |
|
| LSM2 |
Under-expressed |
|
| LSM3 |
Under-expressed |
|
| LSM5 |
Under-expressed |
|
| LSM6 |
Under-expressed |
|
| NPM1 |
Under-expressed |
Inhibits |
| PRPF38A |
Under-expressed |
|
| PRPF4 |
Under-expressed |
|
| PTBP1 |
Under-expressed |
|
| RBMX |
Under-expressed |
|
| RSRC1 |
Under-expressed |
|
| SF3B3 |
Under-expressed |
|
| SFPQ |
Under-expressed |
|
| SNRNP40 |
Under-expressed |
|
| SNRPA |
Under-expressed |
|
| SNRPB |
Under-expressed |
|
| SNRPB2 |
Under-expressed |
|
| SNRPC |
Under-expressed |
|
| SNRPD1 |
Under-expressed |
|
| SNRPE |
Under-expressed |
|
| SNRPF |
Under-expressed |
|
| SRSF3 |
Under-expressed |
|
| SRSF7 |
Under-expressed |
|
| SYNCRIP |
Under-expressed |
|
| THOC1 |
Under-expressed |
|
| TRA2B |
Under-expressed |
|
| TSEN15 |
Under-expresse |
However, it is crucial to recognize that the interplay between genes and biological processes is far from straightforward. The complexity of these relationships is exemplified in this analysis. Splicing-related genes are not uniform in their senescence-related effects; rather, they exhibit a range of roles. Some of these genes may act as activators of senescence, while others serve as inhibitors, indicating a nuanced interplay within the cellular context. The majority of these genes display under-expression during replicative senescence. This phenomenon suggests that these genes are subject to downregulation as cells progress towards replicative senescence, aligning with the established notion that changes in gene expression are a hallmark of cellular senescence.
Remarkably, the analysis also brought to light the presence of two genes, AHNAK2 and RBM24, which buck the trend by being over-expressed during replicative senescence. This discovery is particularly intriguing as it emphasizes that while downregulation of splicing-related genes is a common theme, there exist specific genes that undergo an increase in expression as senescence sets in. As such, further exploration of the roles played by AHNAK2 and RBM24 in senescence holds the promise of shedding light on the underlying molecular mechanisms.
The observation that splicing-related genes exhibit differential expression during senescence further substantiates the connection between splicing and the senescence process. This connection is reinforced by the understanding that alternative splicing is a pivotal step in the regulation of gene expression. Dysregulation of this process can have far-reaching effects, potentially impacting the transcriptome and proteome, and in turn, influencing pathways linked to senescence.
The intriguing aspect of a significant number of splicing-related genes experiencing under-expression during replicative senescence hints at the possibility of a broad-scale suppression of splicing machinery. This suppression could, in turn, impact the processing of pre-mRNA and the synthesis of functional proteins, ultimately leading to altered cellular functions and contributing to the senescence phenotype.
On the other hand, the analysis categorically outlines the associations of identified splicing-related genes with senescence induction or inhibition. Notably, HNRNPA1, HNRNPA3, and NPM1 are under-expressed and are associated with the inhibition of senescence. In contrast, hnRNPC stands out as the only splicing-related factor associated with senescence induction, despite being under-expressed. These observations underscore the intricacy of cellular senescence and emphasize the multifaceted roles of various genes in the regulation of this process. They underscore the notion that senescence is a delicately balanced phenomenon influenced by numerous genetic and molecular factors.
Understanding the roles of these splicing factors in senescence opens the door to potential clinical implications. Notably, if specific factors are linked to senescence induction and possess established roles in the process, they might be considered as viable targets for interventions aimed at modulating senescence. Such interventions hold promise in addressing aging-related conditions and age-related diseases, making this field of research highly relevant and potentially transformative.