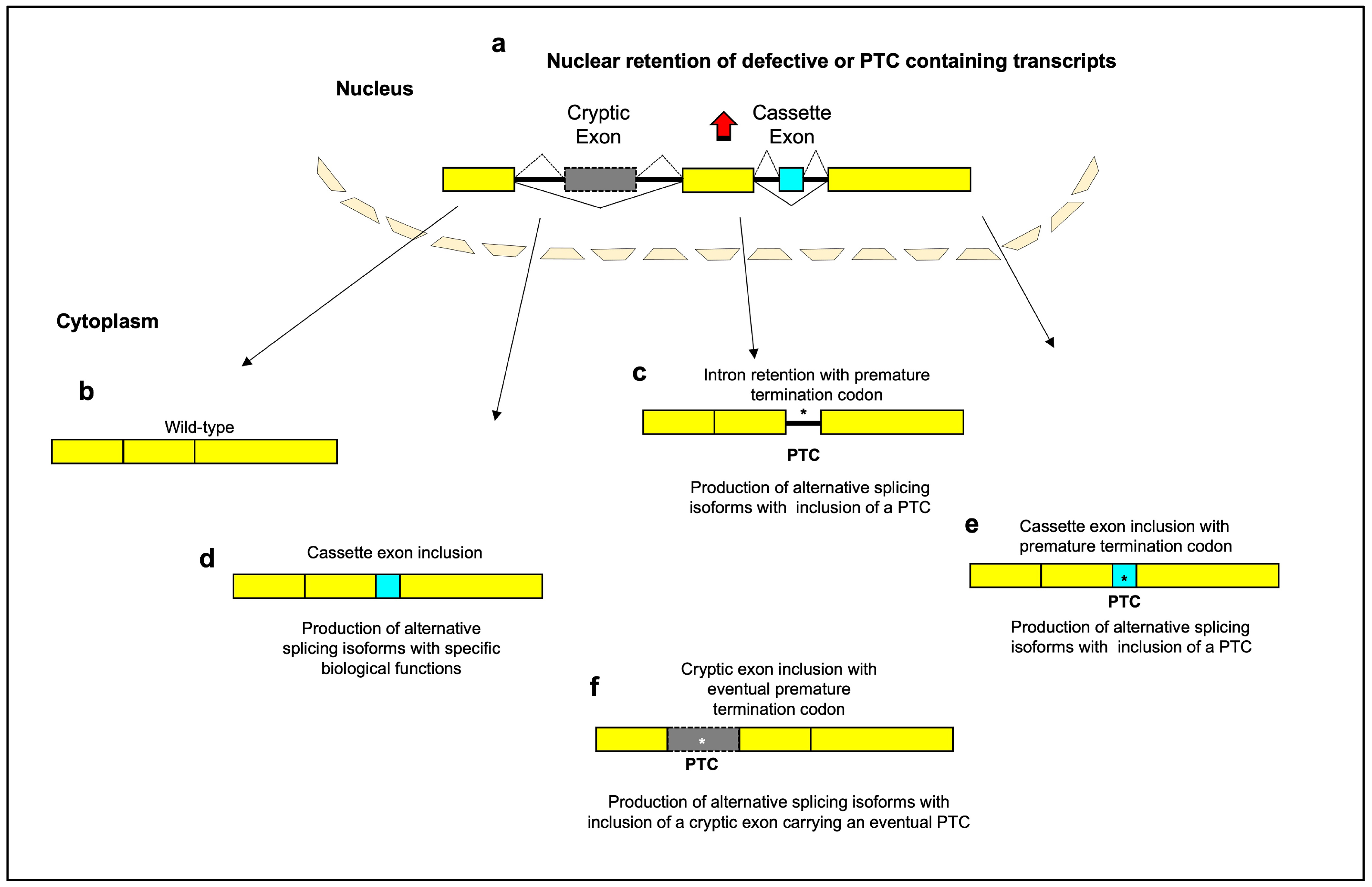
Alternative splicing changes are closely linked to aging, though it remains unclear if they are drivers or effects. As organisms age, splicing patterns change, varying gene isoform levels and functions. These changes may contribute to aging alterations rather than just reflect declining RNA quality control. Three main splicing types—intron retention, cassette exons, and cryptic exons—play key roles in age-related complexity. These events modify protein domains and increase nonsense-mediated decay, shifting protein isoform levels and functions. This may potentially drive aging or serve as a biomarker. Fluctuations in splicing factor expression also occur with aging. Somatic mutations in splicing genes can also promote aging and age-related disease.
- aging
- alternative splicing
- senescence
- age-related diseases
- splicing factors
1. Introduction
2. Age-Related Alterations in Splicing
3. Mining Databases for Splicing Factors Linked to Human Aging
| Gene Symbol | Expression | Senescence Effect |
|---|---|---|
| AHNAK2 | Over-expressed | |
| RBM24 | Over-expressed | |
| ALYREF | Under-expressed | |
| BUD13 | Under-expressed | |
| C1QBP | Under-expressed | |
| CWF19L1 | Under-expressed | |
| DAZAP1 | Under-expressed | |
| DCPS | Under-expressed | |
| DDX39A | Under-expressed | |
| DHX15 | Under-expressed | |
| EIF4A3 | Under-expressed | |
| FUS | Under-expressed | |
| GEMIN6 | Under-expressed | |
| HNRNPA1 | Under-expressed | Inhibits |
| HNRNPA2B1 | Under-expressed | |
| HNRNPA3 | Under-expressed | Inhibits |
| HNRNPC | Under-expressed | Induces |
| HNRNPF | Under-expressed | |
| HNRNPH1 | Under-expressed | |
| HNRNPH3 | Under-expressed | |
| HNRNPM | Under-expressed | |
| HNRNPU | Under-expressed | |
| KHDRBS1 | Under-expressed | |
| KHSRP | Under-expressed | |
| LSM2 | Under-expressed | |
| LSM3 | Under-expressed | |
| LSM5 | Under-expressed | |
| LSM6 | Under-expressed | |
| NPM1 | Under-expressed | Inhibits |
| PRPF38A | Under-expressed | |
| PRPF4 | Under-expressed | |
| PTBP1 | Under-expressed | |
| RBMX | Under-expressed | |
| RSRC1 | Under-expressed | |
| SF3B3 | Under-expressed | |
| SFPQ | Under-expressed | |
| SNRNP40 | Under-expressed | |
| SNRPA | Under-expressed | |
| SNRPB | Under-expressed | |
| SNRPB2 | Under-expressed | |
| SNRPC | Under-expressed | |
| SNRPD1 | Under-expressed | |
| SNRPE | Under-expressed | |
| SNRPF | Under-expressed | |
| SRSF3 | Under-expressed | |
| SRSF7 | Under-expressed | |
| SYNCRIP | Under-expressed | |
| THOC1 | Under-expressed | |
| TRA2B | Under-expressed | |
| TSEN15 | Under-expresse |
This entry is adapted from the peer-reviewed paper 10.3390/cells12242819
References
- Johnson, F.B.; Sinclair, D.A.; Guarente, L. Molecular Biology of Aging. Cell 1999, 96, 291–302.
- Liu, Y.; Gonzàlez-Porta, M.; Santos, S.; Brazma, A.; Marioni, J.C.; Aebersold, R.; Venkitaraman, A.R.; Wickramasinghe, V.O. Impact of Alternative Splicing on the Human Proteome. Cell Rep. 2017, 20, 1229–1241.
- Kim, E.; Magen, A.; Ast, G. Different Levels of Alternative Splicing among Eukaryotes. Nucleic Acids Res. 2007, 35, 125–131.
- Lee, Y.; Rio, D.C. Mechanisms and Regulation of Alternative Pre-mRNA Splicing. Annu. Rev. Biochem. 2015, 84, 291–323.
- Somel, M.; Guo, S.; Fu, N.; Yan, Z.; Hu, H.Y.; Xu, Y.; Yuan, Y.; Ning, Z.; Hu, Y.; Menzel, C.; et al. MicroRNA, mRNA, and Protein Expression Link Development and Aging in Human and Macaque Brain. Genome Res. 2010, 20, 1207–1218.
- Holly, A.C.; Melzer, D.; Pilling, L.C.; Fellows, A.C.; Tanaka, T.; Ferrucci, L.; Harries, L.W. Changes in Splicing Factor Expression Are Associated with Advancing Age in Man. Mech. Ageing Dev. 2013, 134, 356–366.
- Lee, B.P.; Pilling, L.C.; Emond, F.; Flurkey, K.; Harrison, D.E.; Yuan, R.; Peters, L.L.; Kuchel, G.A.; Ferrucci, L.; Melzer, D.; et al. Changes in the Expression of Splicing Factor Transcripts and Variations in Alternative Splicing Are Associated with Lifespan in Mice and Humans. Aging Cell 2016, 15, 903–913.
- Wood, S.H.; Craig, T.; Li, Y.; Merry, B.; de Magalhães, J.P. Whole Transcriptome Sequencing of the Aging Rat Brain Reveals Dynamic RNA Changes in the Dark Matter of the Genome. Age 2013, 35, 763–776.
- Harries, L.W.; Hernandez, D.; Henley, W.; Wood, A.; Holly, A.C.; Bradley-Smith, R.M.; Yaghootkar, H.; Dutta, A.; Murray, A.; Frayling, T.M.; et al. Human Aging Is Characterized by Focused Changes in Gene Expression and Deregulation of Alternative Splicing. Aging Cell 2011, 10, 868–878.
- Mazin, P.; Xiong, J.; Liu, X.; Yan, Z.; Zhang, X.; Li, M.; He, L.; Somel, M.; Yuan, Y.; Phoebe Chen, Y.-P.; et al. Widespread Splicing Changes in Human Brain Development and Aging. Mol. Syst. Biol. 2013, 9, 633.
- Tollervey, J.R.; Wang, Z.; Hortobágyi, T.; Witten, J.T.; Zarnack, K.; Kayikci, M.; Clark, T.A.; Schweitzer, A.C.; Rot, G.; Curk, T.; et al. Analysis of Alternative Splicing Associated with Aging and Neurodegeneration in the Human Brain. Genome Res. 2011, 21, 1572–1582.
- Peffers, M.J.; Fang, Y.; Cheung, K.; Wei, T.K.J.; Clegg, P.D.; Birch, H.L. Transcriptome Analysis of Ageing in Uninjured Human Achilles Tendon. Arthritis Res. Ther. 2015, 17, 33.
- Swindell, W.R.; Johnston, A.; Sun, L.; Xing, X.; Fisher, G.J.; Bulyk, M.L.; Elder, J.T.; Gudjonsson, J.E. Meta-Profiles of Gene Expression during Aging: Limited Similarities between Mouse and Human and an Unexpectedly Decreased Inflammatory Signature. PLoS ONE 2012, 7, e33204.
- Maquat, L.E.; Kinniburgh, A.J.; Rachmilewitz, E.A.; Ross, J. Unstable Beta-Globin mRNA in mRNA-Deficient Beta o Thalassemia. Cell 1981, 27, 543–553.
- Losson, R.; Lacroute, F. Interference of Nonsense Mutations with Eukaryotic Messenger RNA Stability. Proc. Natl. Acad. Sci. USA 1979, 76, 5134–5137.
- Tabrez, S.S.; Sharma, R.D.; Jain, V.; Siddiqui, A.A.; Mukhopadhyay, A. Differential Alternative Splicing Coupled to Nonsense-Mediated Decay of mRNA Ensures Dietary Restriction-Induced Longevity. Nat. Commun. 2017, 8, 306.
- Son, H.G.; Seo, M.; Ham, S.; Hwang, W.; Lee, D.; An, S.W.A.; Artan, M.; Seo, K.; Kaletsky, R.; Arey, R.N.; et al. RNA Surveillance via Nonsense-Mediated mRNA Decay Is Crucial for Longevity in Daf-2/Insulin/IGF-1 Mutant C. elegans. Nat. Commun. 2017, 8, 14749.
- Coppedè, F. Mutations Involved in Premature-Ageing Syndromes. Appl. Clin. Genet. 2021, 14, 279–295.
- Anisimova, A.S.; Meerson, M.B.; Gerashchenko, M.V.; Kulakovskiy, I.V.; Dmitriev, S.E.; Gladyshev, V.N. Multifaceted Deregulation of Gene Expression and Protein Synthesis with Age. Proc. Natl. Acad. Sci. USA 2020, 117, 15581–15590.
- Stegeman, R.; Weake, V.M. Transcriptional Signatures of Aging. J. Mol. Biol. 2017, 429, 2427–2437.
- Anisimova, A.S.; Alexandrov, A.I.; Makarova, N.E.; Gladyshev, V.N.; Dmitriev, S.E. Protein Synthesis and Quality Control in Aging. Aging 2018, 10, 4269–4288.
- Stoeger, T.; Grant, R.A.; McQuattie-Pimentel, A.C.; Anekalla, K.R.; Liu, S.S.; Tejedor-Navarro, H.; Singer, B.D.; Abdala-Valencia, H.; Schwake, M.; Tetreault, M.-P.; et al. Aging Is Associated with a Systemic Length-Associated Transcriptome Imbalance. Nat. Aging 2022, 2, 1191–1206.
- Adusumalli, S.; Ngian, Z.-K.; Lin, W.-Q.; Benoukraf, T.; Ong, C.-T. Increased Intron Retention Is a Post-Transcriptional Signature Associated with Progressive Aging and Alzheimer’s Disease. Aging Cell 2019, 18, e12928.
- Heintz, C.; Doktor, T.K.; Lanjuin, A.; Escoubas, C.; Zhang, Y.; Weir, H.J.; Dutta, S.; Silva-García, C.G.; Bruun, G.H.; Morantte, I.; et al. Splicing Factor 1 Modulates Dietary Restriction and TORC1 Pathway Longevity in C. elegans. Nature 2017, 541, 102–106.
- Rollins, J.A.; Shaffer, D.; Snow, S.S.; Kapahi, P.; Rogers, A.N. Dietary Restriction Induces Posttranscriptional Regulation of Longevity Genes. Life Sci. Alliance 2019, 2, e201800281.
- Ubaida-Mohien, C.; Lyashkov, A.; Gonzalez-Freire, M.; Tharakan, R.; Shardell, M.; Moaddel, R.; Semba, R.D.; Chia, C.W.; Gorospe, M.; Sen, R.; et al. Discovery Proteomics in Aging Human Skeletal Muscle Finds Change in Spliceosome, Immunity, Proteostasis and Mitochondria. eLife 2019, 8, e49874.
- Wang, K.; Wu, D.; Zhang, H.; Das, A.; Basu, M.; Malin, J.; Cao, K.; Hannenhalli, S. Comprehensive Map of Age-Associated Splicing Changes across Human Tissues and Their Contributions to Age-Associated Diseases. Sci. Rep. 2018, 8, 10929.
- Shi, M.; Zhang, H.; Wang, L.; Zhu, C.; Sheng, K.; Du, Y.; Wang, K.; Dias, A.; Chen, S.; Whitman, M.; et al. Premature Termination Codons Are Recognized in the Nucleus in A Reading-Frame Dependent Manner. Cell Discov. 2015, 1, 15001.
- Jacob, A.G.; Smith, C.W.J. Intron Retention as a Component of Regulated Gene Expression Programs. Hum. Genet. 2017, 136, 1043–1057.
- Monteuuis, G.; Wong, J.J.L.; Bailey, C.G.; Schmitz, U.; Rasko, J.E.J. The Changing Paradigm of Intron Retention: Regulation, Ramifications and Recipes. Nucleic Acids Res. 2019, 47, 11497–11513.
- Tacutu, R.; Thornton, D.; Johnson, E.; Budovsky, A.; Barardo, D.; Craig, T.; Diana, E.; Lehmann, G.; Toren, D.; Wang, J.; et al. Human Ageing Genomic Resources: New and Updated Databases. Nucleic Acids Res. 2018, 46, D1083–D1090.
- de Magalhães, J.P.; Budovsky, A.; Lehmann, G.; Costa, J.; Li, Y.; Fraifeld, V.; Church, G.M. The Human Ageing Genomic Resources: Online Databases and Tools for Biogerontologists. Aging Cell 2009, 8, 65–72.
- Aging Atlas Consortium. Aging Atlas: A Multi-Omics Database for Aging Biology. Nucleic Acids Res. 2021, 49, D825–D830.
- Chatsirisupachai, K.; Palmer, D.; Ferreira, S.; de Magalhães, J.P. A Human Tissue-Specific Transcriptomic Analysis Reveals a Complex Relationship between Aging, Cancer, and Cellular Senescence. Aging Cell 2019, 18, e13041.
- Avelar, R.A.; Ortega, J.G.; Tacutu, R.; Tyler, E.J.; Bennett, D.; Binetti, P.; Budovsky, A.; Chatsirisupachai, K.; Johnson, E.; Murray, A.; et al. A Multidimensional Systems Biology Analysis of Cellular Senescence in Aging and Disease. Genome Biol. 2020, 21, 91.
