Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mona Saleh | -- | 5662 | 2023-12-28 13:32:37 | | | |
| 2 | Jason Zhu | Meta information modification | 5662 | 2024-01-02 06:16:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Akram, N.; El-Matbouli, M.; Saleh, M. Salmonids' Immune Response to Myxozoan Parasite Myxobolus cerebralis. Encyclopedia. Available online: https://encyclopedia.pub/entry/53223 (accessed on 08 February 2026).
Akram N, El-Matbouli M, Saleh M. Salmonids' Immune Response to Myxozoan Parasite Myxobolus cerebralis. Encyclopedia. Available at: https://encyclopedia.pub/entry/53223. Accessed February 08, 2026.
Akram, Naveed, Mansour El-Matbouli, Mona Saleh. "Salmonids' Immune Response to Myxozoan Parasite Myxobolus cerebralis" Encyclopedia, https://encyclopedia.pub/entry/53223 (accessed February 08, 2026).
Akram, N., El-Matbouli, M., & Saleh, M. (2023, December 28). Salmonids' Immune Response to Myxozoan Parasite Myxobolus cerebralis. In Encyclopedia. https://encyclopedia.pub/entry/53223
Akram, Naveed, et al. "Salmonids' Immune Response to Myxozoan Parasite Myxobolus cerebralis." Encyclopedia. Web. 28 December, 2023.
Copy Citation
Salmonids are affected by the economically significant whirling disease (WD) caused by the myxozoan parasite Myxobolus cerebralis. In the past, it was endemic to Eurasia, but it has now spread to different regions of North America, Europe, New Zealand, and South Africa. Among salmonids, rainbow trout is considered the most highly susceptible host. Upon entering to the host’s body, the parasite invades the spine and cranium, resulting in whirling behaviour, a blackened tail, and destruction of cartilage. The disease is characterized by the infiltration of numerous inflammatory cells, primarily lymphocytes and macrophages, with the onset of fibrous tissue infiltration.
myxozoa
M. cerebralis
immune modulation
rt-PCR
flow cytometry
innate immunity
1. Introduction
Fish, being a potential source of nutrition, play an important part in meeting global food demands [1]. Climate change has created favourable conditions for the parasitic lifecycle, posing a serious threat to aquaculture, including salmonid fishes [2]. Among these threats, myxozoan parasites, particularly Myxobolus cerebralis, is a major challenge for salmonids. It is the causative agent of the serious disease named as whirling disease (WD) [3][4][5]. Studies have suggested that M. cerebralis stands out as the most notable and economically impactful parasite, leading to an estimated loss of $35–60 million in the US alone [6][7]. While previously believed to be enzootic only in Eurasia, WD has now been detected in various salmonid-rearing regions across Europe, the USA, Canada, South Africa, and New Zealand [8][9].
Whirling disease affects multiple salmonid fishes with varying severity and disease onset [10][11]. Clinical signs can be observed in Chinook salmon (Oncorhynchus tshawytscha), brook trout (Salvelinus fontinalis), sock eye salmon (Oncorhynchus nerka), rainbow trout (Oncorhynchus mykiss), and brown trout (Salmo trutta) [10][12][13]. Although different studies have revealed clinical progression in brown trout, rainbow trout is considered the most highly susceptible host [14][15][16]. The clinical signs caused by WD include a blackened tail, whirling behaviour, and death in infected fish [17][18][19]. Tubifex tubifex, as an obligate invertebrate host, releases triactinomyxon spores (TAMs) [20], which invade fish through mucous cell openings in skin, gill epithelium, and the oral route [21]. Following penetration, the sporoplasm travels within the skin and gill epithelium, subsequently invading epithelial cells [22]. As a result of internal budding within a cytoplasmic vacuole, the sporoplasm produces primary and secondary cells [21]. These primary cells, containing vegetative nuclei and generative cells, are named as plasmodia. They migrate through the peripheral nerves to spinal cord and brain and reside in the cartilage, leading to destruction of the ossification processes in the entire skeleton and necrosis due to granulomatous inflammation [14][22]. This cartilage damage activates the fish host’s immune system [16].
The fish immune system comprises two main components: the innate and adaptive immune systems [23]. Innate immunity is activated by pathogens and serves as the host’s first line of defence against infections [24]. Myxosporean spp. often provoke minimal or no host responses [21][25]. In fact, it is believed that they employ such a manipulative strategy to evade the fish immune system, avoiding local immune responses and potential inflammatory reactions at the point of entry [26][27]. Specifically, M. cerebralis uses an immune-privileged pathway to invade the head cartilage via the central nervous system and peripheral nerves, where there is a minimum of immune response [21]. TAMs exhibit a higher affinity for rainbow trout compared to brown trout. On the other hand, brown trout triggers more protective immune mechanisms that contribute to disease resistance [14]. The attachment of TAMs is influenced by mucosal factors, and a cellular protective response can be observed through the presence of eosinophils in the root ganglia of infected fish [15].
Limited information is available regarding resistance and susceptibility mechanisms among M. cerebralis and fish host species [28]. However, recent investigations have used fluorescence-activated cell sorting (FACS) [29] to reveal certain aspects of cellular and cytokine responses against M. cerebralis, thereby enhancing understanding of the factors underlying susceptibility and resistance to WD [29][30][31][32]. Previously, both innate and acquired immune mechanisms have been reviewed in myxozoans [33][34]. Various efforts have also been made to investigate the role of STAT3 in Th 17 cell differentiation [35] and to study the potential inhibitory actions of suppressors of cytokine signalling (SOCS1 and SOCS3) on IFNγ and IL-1β [30] during WD. Moreover, the expression levels of natural resistance-associated macrophage proteins (Nramp α and β) [28] have been studied to stimulate the immune systems. An effort has been made to silence M. cerebralis serine protease (MyxSP-1) within the T. tubifex host using short-interfering RNA techniques to disrupt the life cycle of M. cerebralis [36]. Nevertheless, a complete understanding of fish immune mechanisms against M. cerebralis remains to be uncovered [4][37]. Although, the aforementioned research studies have investigated the expressions of different immune related genes, offering insights into the role of these genes in immunity against WD, further exploration is still required to fully comprehend the whole function of the immune system of host in defence mechanism against WD.
2. Clinical and Histopathological Changes in Diseased Fish
The parasite migrates to the spine and cranium, causing inflammation and lesions resulting in cranium destruction, which leads to damage and deformation of the skeleton in fish [38]. Moreover, it causes spinal cord constriction and brain stem compression, resulting in whirling behaviour and pressure on nerves, which control the pigment cells causing a black tail [8][39]. Numerous intra- and intercellular sporoplasm cells are found in the epithelia of the buccal cavity and epidermis (Figure 1a). Myxobolus cerebralis spores can also be found in gill arches [14]. Parasitic stages can be observed between the nerve fibres of the spinal cord (Figure 1b) [16], as well as in the head cartilage, vertebrae and gills [40]. The presence of cartilaginous necrotic foci rich in parasitic stages and vegetative cells characterizes the cranial lesions (Figure 2). The disease is characterized by the infiltration of numerous inflammatory cells, primarily lymphocytes and macrophages, with onset of fibrous tissue infiltration [41]. Reduced ability to maintain an upright orientation of the body was suggested to be due to the damage of the vestibular-auditory apparatus [42]. Following penetration through the integumentary system, the parasite attacks the nervous system directly, so scientists have focused more on immune studies in the cartilage and nervous system of salmonids.
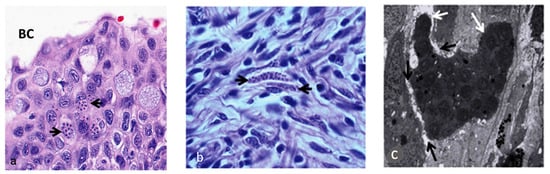
Figure 1. Presence of parasitic stages of M. cerebralis (black arrows) in buccal cavity mucosa, ×700 (a), and in nerve fibres and spinal cord, ×1050 (b) lesions of the epidermis, ×2800 (c) (Adapted from Sarker et al. 2015) [16].
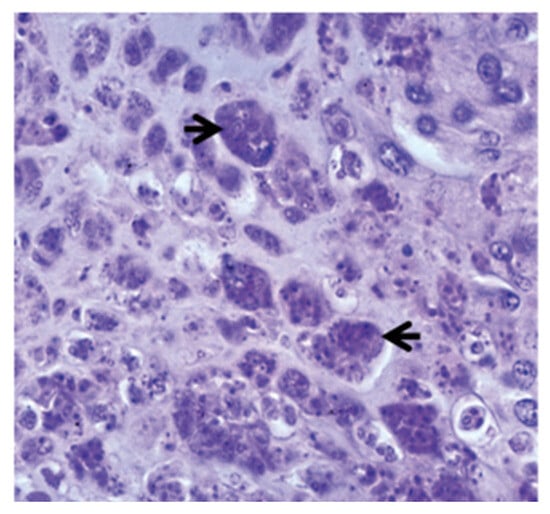
Figure 2. Aggregates of parasitic stages (black arrows) in the cartilage, 12 weeks post exposure, ×700 [16].
3. Innate Immune Response
Two immune mechanisms are involved in fish immunity: innate immunity and adaptive immunity, also named the non-specific and specific immune systems, respectively [43]. Being efficient, innate immunity is considered the primary component in combating disease-causing pathogens in comparison with the acquired immune system [44]. Innate immunity is a rapidly responding strategy but does not deliver protection for long period of time [45]. The categorization of the innate immune mechanism is based on three compartments: the physical barrier and cellular and humoral factors [26][46].
3.1. Role of Physical Barrier in Immunity against M. cerebralis
The physical component of the immune system is a significant part of the innate immune system in fish. It includes scales, the mucous layer and epithelium present in the skin, gills and gastrointestinal tract, providing resistance to various infections [47][48][49]. Further, immune cells such as macrophages, lymphocytes, and eosinophilic granular cells are also found in the epidermis [47][50][51]. The triactinomyxon spores of M. cerebralis enter the fish body through these physical barriers [22][52] and encounter mucous barriers that consist of a complex of mucins containing lectins, lysozymes, C-reactive proteins, complements, haemolysins commensal microbiota, pentraxins and immunoglobulins (IgM) [53][54][55][56][57]. These biochemicals substances have biocidal or biostatic functions [58]. A study revealed the destruction of M. cerebralis within the cytoplasm of fish epithelial cells [21]. There is another report about presence of parasitic developmental stages in cytoplasm of phagocytes in the epidermis of the rainbow trout [59]. During the first few hours of intrapiscine development, M. cerebralis proteases were upregulated in the fins and gills after invasion. Genes encoding the serine protease (MyxSP-1) and cysteine protease (MyxCP-1) were assessed post infection. Upregulation in the expression of these genes was reported in the gills and dorsal fin tissues [60][61]. There was additional identification of the serine protease gene (MyxSubtSP) from M. cerebralis [62]. Sarker et al. [36] used short-interfering RNA (siRNA) to induce RNA interference (RNAi), targeting the MyxSP-1 for development of intervention strategy. The study silenced M. cerebralis MyxSP-1 in its annelid host T. tubifex via siRNA-induced RNAi. T. tubifex were infected with M. cerebralis myxospores and were subjected to treatment with MyxSP-1 siRNA or negative control siRNA, both at 2 μM concentration for 24 h at 15 °C. This treatment occurred at 24 h post infection (hpi), 48 hpi, 72 hpi, 96 hpi, 1 month post infection (mpi), 2 mpi and 3 mpi, respectively. After the final siRNA treatment at 3 mpi, the siRNA-treated T. tubifex were collected, and the expression of the MyxSP-1 gene was assessed using qPCR (Figure 3). When it was applied to rainbow trout fry, it prevented whirling disease and induced sustained RNAi in T. tubifex, representing a promising RNAi-based therapy for whirling disease in salmonids [36]. It would be beneficial to explore the optimization of siRNA methods and dosages to increase the efficiency of MyxSP-1 gene silencing in T. tubifex. Moreover, adapting RNAi therapy for diverse salmonid species and environments is essential for a comprehensive whirling disease strategy.
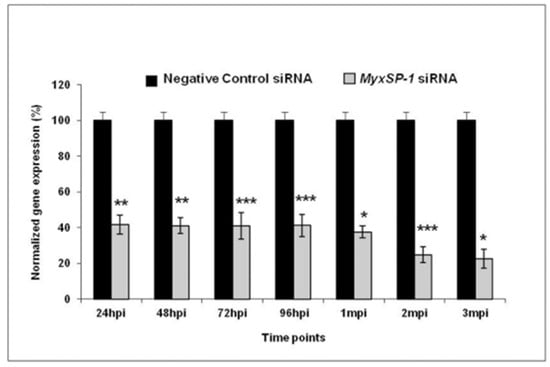
Figure 3. Normalized gene expression of MyxSP-1 gene after siRNA treatment of T. tubifex at different time points post infection (* p < 0.0001; ** p < 0.001; *** p < 0.005) [36].
3.2. Cellular Immunity
Like mammals, the immune cell populations in fish include macrophages, lymphocytes, neutrophils, eosinophilic granular cells, basophils, and dendritic cells. Additionally, fish possess melanomacrophage centres and rodlet cells (RC) [63].
3.2.1. Macrophages
Macrophages have a central role in immunity owing to their function in phagocytosis and lymphocyte activation. They have distinct receptors with the ability to recognize β-glucan, which allows immunostimulants to intensify leukocytes’ respiratory burst by producing reactive oxygen species possessing bactericidal properties [64]. In macrophages, nitric oxide is produced in ample amounts by inducible nitric oxide synthase iNOS, and assumes a pivotal role in the process of inflammation [65]. Previous studies have reported increased iNOS expression after M. cerebralis infection within progressive time periods of the disease. These observations were in the infected susceptible Trout lodge (TL) strain, while in the case of the resistant Hofer strain (HO), greater expression was evident only at 8 days post exposure (PE) [66]. Arginase is the distinctive enzyme involved in promoting the macrophage’s proinflammatory response [67]. There are two different isoforms of arginase, arginase-1 and arginase-2 [68]. In fish, arginase-2 is subjected to distinct regulatory mechanisms and is implicated in inducing an alternative activation state in fish macrophages [67]. A qRT-PCR-based study measured the expression level of arginase-2 and iNOS after exposure to M. cerebralis infection in rainbow trout. The susceptible fish strain TL exhibited increased levels of arginase-2 at 2 h, while in the case of resistant strain HO, the increase was observed 8 days after exposure [66]. The expression level of iNOS was upregulated in the susceptible strain from 24 h to 8 days post exposure, whereas in the resistant strain, this upregulation was observed only at 8 days post exposure. As a result of increased iNOS expression, it is expected that the inflammatory response and tissue damage will be more significant than strain H and strain T being unable to mount an effective immune response compared to resistant strains [69]. A research study compared the post-exposure expression level of Nramp α and β to M. cerebralis. Natural resistance-associated macrophage proteins (Nramp) have been considered a central figure in the innate immune response that arouses macrophage activity and boosts the macrophage’s capability to kill phagocytized pathogens. Downregulation of both genes Nramp α and Nramp β was noted in the vulnerable American TL strain at day 14 and day 40 following exposure, respectively [28]. This discovery indicated a possible Nramp involvement in the negative feedback mechanism [70]. Other studies have also reported similar gene expression in response to pathogens, but the cause of this regulation still needs to be disclosed [71][72][73]. Exploring the immune functional role of Nramp in the host through the regulation of the negative feedback mechanism would be a captivating endeavour.
3.2.2. Lymphocytes
Lymphocytes are defensive cells analogous to B cells, T cells, macrophages, cytotoxic cells, and leukocytes [47][74][75]. Haematological responses against triactinomyxon spores of M. cerebralis have been investigated [76]. Lower numbers of lymphocytes were reported in infected fish. In salmonid fish, the presence of lower number of lymphocytes has been reported in Saprolegina-infected brown trout [77], in rainbow trout infected with Vibrio anguillarum [78], and post exposure to copper [79]. Physiological processes leading to stress [80][81], alterations in dynamics of lymphocytes due to the direct interaction of the fish immune system, lymphocyte destruction by pathogenic agents, and migration of lymphocytes from peripheral blood to invaded tissue are multiple factors involved in the cause of lymphopenia [78][82].
3.2.3. Granulocytes
Fish granulocytes are traditionally referred to with the terms neutrophil, basophil, and eosinophil. However, other terms such as eosinophilic granulocytes or fine granulocytes have also been used [83]. In mucosal infections (skin, gills, and intestines), granulocytes and phagocytes are typically the most prevalent immune system cells [63].
A wide range of hosts and variability in host susceptibility to M. cerebralis are evident [15][22]. It is thought that brown trout’s resilience compared to other salmonid hosts [84][85] is a result of this host’s evolutionary relationship with M. cerebralis in its native European habitat [86]. However, assuming that co-adaptation is the only cause of resistance based solely on geographic proximity is difficult to reconcile with the robust resilience of indigenous Trout lodge species like coho salmon [15]. Despite the fact that precise defence mechanisms remain incompletely elucidated, the existence of eosinophilic granular leucocytes in the root ganglia of afflicted brown trout, as opposed to rainbow trout, suggests the potential for a cellular protective response against the parasite [58].
3.2.4. Mast Cells
Being a part of natural immunity, mast cells are found close to the skin’s blood vessels, gills, gastrointestinal tract, and ovaries [87]. These cells are identified using electron microscopy as exhibiting numerous cytoplasmic electron-dense granules. Various proteins like desmin, CD117, and S100 proteins are expressed by mast cell granules [88]. A number of factors, notably chronic inflammation and infection by parasites, lead to the induction of mast cells in fish tissues and organs [89].
A study concluded that coho salmon are immune to infection and the emergence of symptoms associated with WD [10]. Coho salmon, unlike rainbow trout or chinook salmon, which are susceptible to M. cerebralis infection, exhibited an abundance of eosinophilic granule cells (EGC) or mast cells in parasite-induced lesions or ganglia containing parasitic stages [90]. Brown trout species that are mildly resistant to M. cerebralis have shown similar mast cell responses [83][84]. For this reason, EGCs were suggested to have a role in the immunity of salmonids to whirling disease, but in general, their role is still unresolved [91], and detailed investigation could be helpful for future endeavours.
3.2.5. Rodlet Cells
Rodlet cells are recognized by their distinctive thicker capsule-like cell borders and the presence of rodlet cytoplasmic inclusions resembling rods, and are closely related to inflammatory cells (eosinophile granule cells, mesothelial cells, epithelioid cells) [92] involved in the response against myxosporea [93][94]. Most often, these cells are found within the cardiac region, kidney, spleen, thymus, skin, gills, pancreas, gall bladder, and blood vessel endothelium [87]. They are responsible for various functions, having a secretory defensive role [95] and carrying out pH control, osmoregulation, electrolyte, and ion transport. They are mostly observed during parasitic infections, and can be triggered by any form of tissue damage, which ultimately stimulates leukocyte reaction due to chemotactic stimuli [94][96]. Disparity in the distribution of rodlet cells can be seen among various fish families. In salmonids, helminth infections are thought to be the reason for the induction of local recruitment of rodlet cells in affected epithelial cells [95]. Although some reports on rodlet cells and their defensive role in salmonids and other fish families against various parasites are available [97], the role of these cells during whirling disease still needs to be explored, and this would be fascinating development.
3.3. Humoral Immunity
Humoral immune parameters are a variety of soluble compounds that act as preventive agents by limiting the growth of microbes and neutralizing the enzymes on which the pathogen depends for pathogenesis [63]. Multiple nonspecific protective substances, including lectins, transferrin, antimicrobial peptides, and lysozymes, inhibit or suppress microbial growth [46]. In the case of myxosporean infections, multiple humoral innate factors such as lysozymes, peroxidases, and compliments are engaged in the eradication of pathogens [98]. During the development of whirling disease, parasites degenerate after entry into the host’s skin, and do not reach the peripheral nervous system [21]. Humoral immunity is thought to be involved in eliminating the M. cerebralis parasite from the fish’s skin, but the actual mechanisms involved should be interrogated [59].
3.4. Cytokine Response
Cytokines, being signalling low-molecular-weight secretory proteins, are considered regulators of the immune mechanism [31][99]. They are produced at the entry sites of pathogens to control phagocytes and neutralize entering microorganisms [100]. In general, fish have been found to possess various cytokines, including interleukin-1β (IL-1β), transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), chemokines, and interferon (IFN) [101][102][103][104]. The IFN-γ related inflammatory response is modulated by SOCS proteins, and is involved in host’s response to M. cerebralis infection. Differential modulation of several interleukins (IL-17A, IL17-C, IL-21) and RORγ after M. cerebralis infection indicates the function of these molecules in rainbow trout immunity against the parasite [30]. Other studies have reported increased post-exposure expression of pro-inflammatory cytokines like IFNγ and IL-1β in brown trout and rainbow trout against M. cerebralis [35][105]. The increase in gene expression indicates their role in host protection against the infection [30]. The expression induction of TGF-β1b, SOCS1, and SOCS3 in brown trout following M. cerebralis exposure signifies their importance in mediating proper immune protection and restraining excessive inflammatory responses during the course of the infection [30].
The gene expression levels of SOCS1 and SOCS3 genes have been investigated following exposure to M. cerebralis. The parasite triggered the expression of SOCS1, IL-6-dependent SOCS3, IL-10, and Treg-associated transcription factor FOXP3 in the TL susceptible strain, which caused limited STAT1 and STAT3 stimulation, thereby impacting the Th17-mediated immune response [32]. The expression of SOCS1 and SOCS3 was instigated, which inhibits the stimulation of STAT1 and STAT3 in American TL strain, thus resulting in an imbalance of Th17/Treg17 and rendering the host incapable of launching a defensive reaction or regulating inflammatory responses, increasing vulnerability to WD. Conversely, within the resistant HO rainbow trout strain, the expression of SOCS1 and SOCS3 was controlled, while STAT1 and IL-23-mediated STAT3 expression enabled a more protective immune reaction. Fish immunity was promoted by the successful balancing of Th17/Treg17 responses, which was maintained by increased expression of STAT1 and IL-23-mediated STAT3. Therefore, the study demonstrated the key role of SOCS1 and SOCS3 in modulating the activation and significance of host immunity in rainbow trout [32]. Future investigations into the mechanisms of WD’s resistance to disease should focus on STAT3 and other factors influencing Th17/Treg cell distribution and balance [32][35].
4. Adaptive Immune Responses to M. cerebralis
The adaptive immune response in fish depends on T and B cells, as well as the diversity and specificity of their antigen receptors, which are called antibodies and T cell receptors, respectively [106][107]. For many years, it was believed that fish could not mount an adaptive immune response to myxosporea [108][109][110]. However, it has now been clearly confirmed that fish with various myxosporean infections, including whirling disease, have expressed particular antibodies [59][111][112].
4.1. T Cells
T cells are a type of lymphocytes that bear a surface T cell receptor, which identifies antigens in conjunction with MHC molecules [37]. Fish T cells include CD8+ cytotoxic T lymphocytes (CTL) and CD4+ T helper (Th) cells [37][113]. CTLs express the membrane bound glycoprotein CD8 and are capable of killing cells of the host that are infected [114]. Th cells express CD4+ molecules and release cytokines that control the activity of other cells of immune system. Fish CD4+ cells functionally differentiate into the effector subtypes Th1, Th2, Th17 and Treg [114][115][116]. By promoting CTL proliferation and macrophage activation, Th1 cells in fish facilitate the coordination of the immune response to ensure protection against intracellular infections [117]. Th2 cells aid in the promotion of B cell proliferation and antibody-mediated production, and are linked to immunity to external parasites [118][119]. Just like in mammals, in fish, Th1 and Th2 responses interact with IL4, lowering Th1 proliferation, and with IFNγ, hampering Th2 proliferation [120][121][122]. Th 17 cells are involved in mucosal immunity against extracellular pathogens including fungi and bacteria; they secrete IL17, IL21, and IL22 [115]. Regulatory T cells produce the anti-inflammatory cytokines IL10 and TGF, which help regulate the immune response [123].
Interferon-related genes (IFNγ and IRF1) were upregulated in rainbow trout infected with M. cerebralis, indicating an activation of the innate immune system in both strains. At 24 h and later time points, TL strain showed greater up-regulation of these two genes than the resistant HO strain [35]. This trend of increased transcription may be harmful to the susceptible TL strain, as it is necessary for IFNγ to keep a balance between anti-pathogenic effect and host inflammatory tissue damage [124]. In brown trout, the expression of IFNγ was greater in HK, SP, and CF. STAT3 expression was comparatively higher in the caudal fin of resistant HO fish, indicating it may induce resistance in the HO strain through activation of Th17 cells [35]. Th17 cells produce IL-17 and are thought to be the key player in resisting M. cerebralis at the epithelia [30]. Using flow cytometry, increased CD8+ and CD8− (presumably CD4+) T cells were observed in the CF, spleen, and HK in the resistant HO fish strain. The resistant strain exhibits a significantly more robust T cell response than the susceptible TL strain [29].
4.2. B Cells
Antibodies or immunoglobulins (IGs) are the primary elements of the immune response to infections [26]. Pathogen clearance through phagocytosis, virus and toxin neutralization, and complement cascade activation are a few of the immunological mechanisms mediated by IGs [125][126]. In teleost, the main three B cell lineages have been identified, resulting in the production of three different isotypes of immunoglobulins: IgM, IgD, and IgT/Z [127]. IgT was detected in rainbow trout and identified as IgZ in zebra fish [63]. Rainbow trout have expressed three subcategories of IgT. The IgT1 subclass has been reported in gut [128] and mucosal lymphoid tissues (gills) [129]. Similarly, IgT2 and IgT3 have been observed to be present in lymphoid organs and serum, respectively [128]. IgM has a role in systemic immunity, with IgM+ B cells predominating within both the blood and various systemic lymphoid organs, and during infection, their proliferation increases in the mucosal surface of skin and intestine [130][131].
Using flow cytometry, an increased cell count of IgM+ B was identified within the spleen, head kidney, and caudal fin in the M. cerebralis-resistant HO rainbow trout strain [29]. An experiment conducted by Ryce et al. (2003) investigated the acquired immune response of rainbow trout to M. cerebralis. It was determined that initial immunization exposure provides th fish with immunity to subsequent exposures [132][133]. Previously, a study detected antibodies against triactinomyxon spores’ antigen via Western blot and ELISA [133]. Samples from infected rainbow trout in the wild or in controlled laboratory experiments showed positive results using these assays. Fish that responded through antibody production to early stages of infection were subjected to Western blotting analysis, which demonstrated a varied antibody response without a regular or recurring pattern of antigen recognition. However, strong naturally acquired immunity is evident in reinfected rainbow trout, as these fish resisted the penetration of even a large number of spores. The acquired resistance was only found among formerly actively infected rainbow trout with cartilage lesions [59][133].
5. Immune Modulation in WD-Resistant and Susceptible Fish
M. cerebralis can infect multiple species of salmonids [10][134][135]. Among the salmonids, brown trout is regarded as resistant, whereas rainbow trout is the most susceptible species and expresses serious disease consequences [10]. Coho salmon (Onchorynchus kisutch) is also considered a resistant strain, while the European Danube salmon (Hucho hucho) is highly vulnerable to WD [10]. It is not completely clear what causes the variable degrees of resistance shown in salmonids, and it seems that every species defends against the sickness through different mechanisms [15][84].
Severin et al. [66] evaluated the role of macrophages in the susceptibility of two different rainbow trout strains infected with M. cerebralis. The expression level of arginase-2 was noticeably more elevated in the susceptible strain TL than in the resistant strain Hofer (HO) at 2 h and 8 days post exposure. Moreover, the expression of iNOS was markedly induced at 24 h to 8 days post exposure in the susceptible American Trout lodge (TL) strain, and only at 8 days post exposure in the German strain HO. These findings suggested a low capability of the susceptible strain to regulate a successful immune response against infections with M. cerebralis [68]. Further, a study explored the dynamic transcriptional response of metallothionein and innate immune response genes to WD [136]. The obtained gene expression data elicited a more protective innate immune response of the Hofer strain than that of Trout lodge strain. The expressions of IFN-g, IL-1b, IRF1, and iNOS genes were higher in both susceptible and resistant rainbow trout after infection with M. cerebralis. In a different study, Nramp, as a candidate gene for resistance, was investigated in brown trout and rainbow trout after exposure to M. cerebralis. Reduced expression of Nramp α and β was evident in the Trout lodge strain compared to the resistant brown trout [28]. On the other hand, STAT3 was the only gene that showed significant upregulation in the German HO strain, while remaining consistent in the American TL strain [35].
In a preceding experiment, the gene expression profile was determined by microarray analysis and verified through qRT-PCR. Following exposure to M. cerebralis, the expression of ubiquitin-like protein 1 and interferon-regulating factor 1 was up-regulated 100-fold and 15-fold, respectively, in both rainbow trout strains. The expression of metallothionein B was increased over 5-fold in the resistant German HO strain compared to the susceptible American TL strain, wherein it remained unchanged. Metallothionein B is known to play a role in immune response and inflammation. On the other hand, the CC chemokine SCYA113 was increasingly expressed in the TL strain. The CC chemokine SCYA gene is a member of the CC chemokine family that directs leukocytes to areas of inflammation and infection [137]. The differential expression of these genes indicates that leukocyte migration to the infection site and their stimulation are crucial in determining fish’s vulnerability or resistance [136].
Flow cytometry-based research was conducted to investigate the dynamics of local and systemic immune cell responses in rainbow trout strains both susceptible and resistant to WD. A lower number of parasitic stages were noticed in the epidermis of the HO strain than in the TL strain at 12 h post exposure (Figure 4).
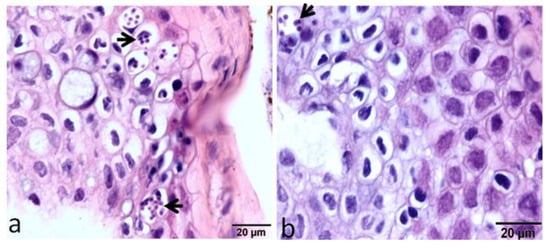
Figure 4. Large intracellular aggregates of the developmental stages of M. cerebralis appear in the epidermis of the susceptible host (a), while very few parasitic stages appear in the epidermis of the resistant strain (b) [29].
In caudal fins (CF), myeloid cells showed increased levels only at 24 h post exposure in HO fish, whereas TL myeloid cells exhibited increased levels at all time points post exposure to M. cerebralis. The number of IgM+ B cells also increased in both resistant and susceptible fish at various time points during WD. Likewise, CD8+ and CD8− T cells were also upregulated at multiple time points in both rainbow trout strains of M. cerebralis-exposed fish (Figure 5).
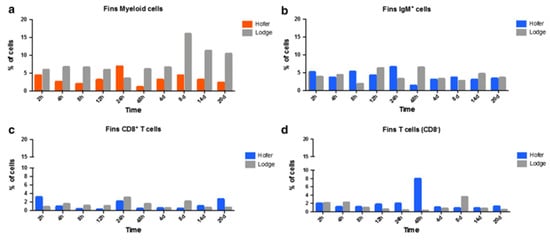
Figure 5. Flow cytometric analysis of HO and TL fish’s CF myeloid cells (a), IgM+ B cells (b), CD8+ T cells (c) and CD8− T cells (d) [29].
In the case of the head kidney (HK) and spleen of infected fish, the resistant HO strain elicited an increase in T cells and a decrease in myeloid cells compared to the susceptible TL strain. IgM+ B cells and CD8+ T cells were also markedly elevated in the HO strain compared to the TL strain. In the spleen, CD8+ and CD8− cells were upregulated at various time points in the German HO strain, and at day 14 in the American TL strain (Figure 6 and Figure 7) [29]. The TL susceptible strain expressed excessive immune responses at all time points. The uncontrolled and excessive immune response in TL fish triggered irreversible inflammatory responses and tissue damage, favouring parasite development and contributing to host susceptibility [29]. Although understanding of immune regulation has improved due to knowledge about immune response comparisons between susceptible and resistant strains, it would be useful to investigate the distribution and kinetics of regulatory and pro-inflammatory cells in both strains. Moreover, additional exploration of the cellular-based immune response implicated in WD would be helpful in disease exploration and ultimate disease prevention.
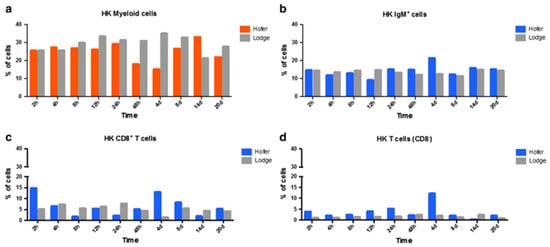
Figure 6. Flow cytometric analysis of HO and TL fish’s HK myeloid cells (a), IgM+ B cells (b), CD8+ T cells (c) and CD8− T cells (d) [29].
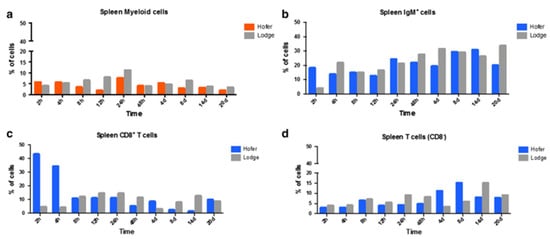
Figure 7. Flow cytometric analysis of HO and TL fish’s spleen myeloid cells (a), IgM+ B cells (b), CD8+ T cells (c) and CD8− T cells (d) [29].
6. Immune Modulation in Response to Co-Infection
Co-infection has detrimental effects on the host, notably influencing their vulnerability to other infectious agents, the duration of the infection, and clinical progression [138][139]. For instance, the immune modulation of rainbow trout exposed to M. cerebralis and Tetracapsuloides bryosalmonae was studied [140]. The host initially infected with M. cerebralis and then with T. bryosalmonae expressed greater numbers of parasites in both the posterior kidney and cranial cartilage, which are the target sites of T. bryosalmonae and M. cerebralis, respectively. The relative expression of the ribosomal protein L18 (RPL18) gene continued to rise, indicating parasitic activation. Moreover, the mortality rate was high, and upregulation of SOCS1 and SOCS3 was reported in both organs (Figure 8 and Figure 9).
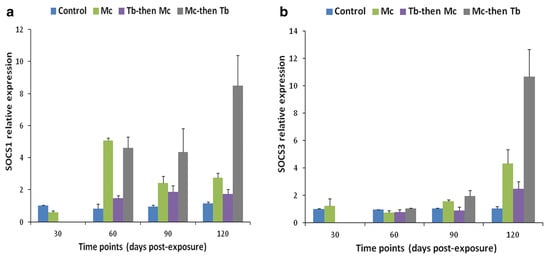
Figure 8. Relative gene expression of SOCS-1 (a) and SOCS-3 (b) in cranial cartilage during single infection and co-infections [140].
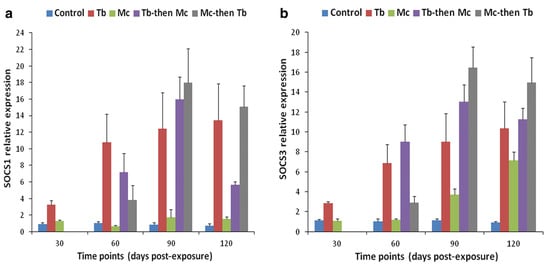
Figure 9. Relative gene expression of SOCS-1 (a) and SOCS-3 (b) in posterior kidneys during single and co-infections [140].
Likewise, elevated levels of JAK-1, and STAT-3 were also reported in both cranial cartilage and posterior kidneys (Figure 10 and Figure 11). The gene expression of SOCS1 and SOCS3 was much higher compared to JAK and STAT genes [140].
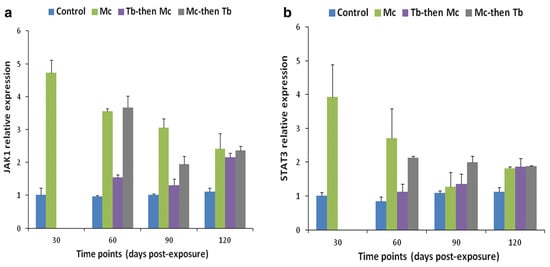
Figure 10. Relative gene expression of JAK-1 (a) and STAT-3 (b) in cranial cartilages during single and co-infections [140].
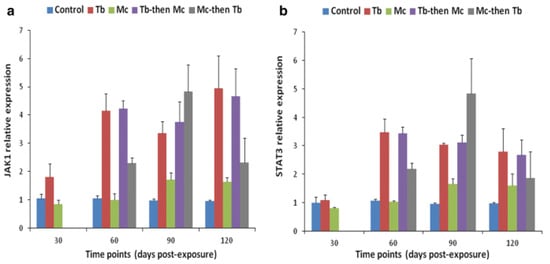
Figure 11. Relative gene expression of JAK-1 (a) and STAT-3 (b) in posterior kidneys during single and co-infections [140].
The synergistic effect established in the present case of co-infection was considered a result of T. bryosalmonae-mediated immunosuppression due to the downregulation of immune genes [140][141][142][143]. On the other hand, the fish group infected with T. bryosalmonae first and then co-infected with M. cerebralis, expressed a smaller number of both parasites. This was thought to occur due to the cross-reactivity between the sporogonic stages of both parasites, which led to cross immunity [144].
In another study, Densmore et al. (2004) reported the higher bactericidal activity of already M. cerebralis-infected rainbow trout against Y. ruckeri [145]. The greater bactericidal activity was due to the proliferative response of the immune system to M. cerebralis [146]. This represented an antagonistic interaction between the myxozoan parasite and bacterial pathogen. In the case of co-infection with primary infection of M. cerebralis followed by T. bryosalmonae, a synergistic effect was observed, resulting in more pronounced disease progression and mortality rate. However, in co-infection with T. bryosalmonae following M. cerebralis, less severe outcomes of the disease were noticed [41]. Hence, it was indicated that the consequences of co-infections depend upon the interaction between M. cerebralis and the co-infecting secondary pathogen.
7. Immune Modulation Due to Environmental Factors
As fish are poikilotherms, their physiology and body temperature are directly influenced by the ambient water temperature [147][148]. Immune response dynamics are linearly influenced by variations in water temperature due to changes in the season, salinity, microclimates, and fish migration [48]. Thermal stress can suppress the host immune system by altering the course of immune responses [48][149][150]. In a previous study, disease occurrence and the severity of lesions demonstrated a positive correlation with the increase in water temperature. This was due to the negative impact of temperature on fish immunity [14]. Another study investigated the contribution of bacterial pathogens, water temperature, and gas saturation in the mortality of rainbow trout fingerlings exposed to M. cerebralis. The increase in water temperature significantly increased mortality. Infection with Flavobacterium psychrophilum was only a significant issue when additional stressors were present, and the effect of gas supersaturation on mortality was negligible [151].
Furthermore, a study found a positive correlation between temperature and both the prevalence and mortality of M. cerebralis infection in rainbow trout [151]. Rainbow trout showed the highest prevalence of infection and the most serious lesions between 10 and 12 degrees Celsius. It is still not clear whether temperature-dependent illness variation in myxozoans, specifically M. cerebralis infection, is solely caused by adaptations in the immune system or by influences on the proliferation of myxozoans [152]. In addition, altitude can have an indirect impact on the immune mechanism in fish. A study was conducted to find a correlation between altitude and water temperature. A correlation between water temperature and altitude was identified. However, exceptions, such as low-altitude rivers fed by glacial water with consistently low temperatures, and high-altitude rivers with warmer temperatures due to surface water from shallow lakes, were observed. [153]. Based on the study mentioned earlier, it can be concluded that altering altitude influences water temperature, which can have effects on immune regulation. Makkula et al. (2007) observed that irradiated fish have decreased resistance to germs and parasites [154]. The impact of ultraviolet (UV) rays on the viability of the infective stage of M. cerebrealis in rainbow trout was evaluated. It was concluded that UV irradiation is effective in eliminating the infectivity of TAMs in fish [155]. Likewise, when juvenile rainbow trout were exposed to UV-treated TAMs, they did not inhibit epidermis attachment and penetration; however, they significantly hindered disease progression [156].
Stress is a further factor that has a major influence on the immune modulation of fish. Above all, corticosteroids and pro-inflammatory cytokines are potential factors causing such immunomodulation [157][158]. Increasing steroids in sea bream infected with Ceratomyxa diplodae [159] and T. bryosalmonae-infected rainbow trout caused increased susceptibility to their respective parasites [160]. In general, these studies suggested that stress has a negative impact on M. cerebralis-infected salmonids, but more research can help the understanding of the actual role of steroids in disease development.
Although studies have looked at the factors impacting morbidity and mortality, immune modulation in salmonids due to these effectors is still insufficiently understood. Hence, more research is needed to determine how stress, either external or internal, affects the immunological regulation of salmonids against M. cerebralis and other myxozoan parasites.
References
- Bianchi, M.C.G.; Camilleri, M.; Chopin, F.; Farme, T.; Franz, N.; Fuentevilla, C.; Garibaldi, L.; Grainger, R.; Hishamunda, N.; Jara, F.; et al. FAO: The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; pp. 1–230.
- Sudhagar, A.; Kumar, G.; El-Matbouli, M. The malacosporean myxozoan parasite Tetracapsuloides bryosalmonae: A threat to wild salmonids. Pathogens 2019, 9, 16.
- Bartholomew, J.L.; Reno, P.W. The history and dissemination of whirling disease. In American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 2002; pp. 3–24.
- Sitjà-Bobadilla, A. Fish immune response to Myxozoan parasites. Parasite 2008, 15, 420–425.
- Avila, B.W.; Winkelman, D.L.; Fetherman, E.R. Survival of Whirling-Disease-Resistant Rainbow Trout Fry in the Wild: A Comparison of Two Strains. J. Aquat. Anim. Health 2018, 30, 280–290.
- Blaylock, R.B.; Bullard, S.A. Counter-insurgents of the blue revolution? parasites and diseases affecting aquaculture and science. J. Parasitol. 2014, 100, 743–755.
- Elwell, L.C.S.; Stromberg, K.E.; Ryce, E.K.; Bartholomew, J.L. Whirling disease in the United States: A summary of progress in research and management. In Proceedings of the Wild Trout X Symposium, West Yellowstone, MT, USA, 28–39 September 2010; p. 203.
- Halliday, M.M. The biology of Myxosoma cerebralis: The causative organism of whirling disease of salmonids. J. Fish Biol. 1976, 9, 339–357.
- Turner, K.G.; Smith, M.J.; Ridenhour, B.J. Whirling disease dynamics: An analysis of intervention strategies. Prev. Vet. Med. 2014, 113, 457–468.
- O’Grodnick, J.J. Susceptibility of various salmonids to whirling disease (Myxosoma cerebralis). Trans. Am. Fish. Soc. 1979, 108, 187–190.
- O’Grodnick, J.J. Susceptibility studies of various salmonids in whirling disease: Histologic staining and spore concentration procedures. US Natl. Mar. Fish. Serv. Mar. Fish. Rev. 1978, 40, 30–31.
- Hoffmann, H.; Hipp, E.; Sedlmeier, U.A. Aerobic and anaerobic metabolism of the freshwater oligochaete Tubifex sp. Hydrobiologia 1987, 155, 157–158.
- Hedrick, R.P.; El-Matbouli, M. Recent advances with taxonomy, life cycle, and development of Myxobolus cerebralis in the fish and oligochaete hosts. In American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 2002; pp. 45–54.
- Baldwin, T.J.; Vincent, E.R.; Silflow, R.M.; Stanek, D. Myxobolus cerebralis infection in rainbow trout (Oncorhynchus mykiss) and brown trout (Salmo trutta) exposed under natural stream conditions. J. Vet. Diagn. Investig. 2000, 12, 312–321.
- Hedrick, R.P.; McDowell, T.S.; Mukkatira, K.; Georgiadis, M.P.; MacConnell, E. Susceptibility of selected inland salmonids to experimentally induced infections with Myxobolus cerebralis, the causative agent of whirling disease. J. Aquat. Anim. Health 1999, 11, 330–339.
- Sarker, S.; Kallert, D.; Hedrick, R.; El-Matbouli, M. Whirling disease revisited: Pathogenesis, parasite biology and disease intervention. Dis. Aquat. Org. 2015, 114, 155–175.
- Markiw, M. Portals of entry for salmonid whirling disease in rainbow trout. Dis. Aquat. Org. 1989, 6, 7–10.
- Gilbert, M.A.; Granath, W.O., Jr. Whirling disease of salmonid fish: Life cycle, biology, and disease. J. Parasitol. 2003, 89, 658–667.
- Markiw, M.E. Whirling disease: Earliest susceptible age of rainbow trout to the triactinomyxid of Myxobolus cerebralis. Aquaculture 1991, 92, 1–6.
- Gilbert, M.A.; Granath, W.O., Jr. Persistent infection of Myxobolus cerebralis, the causative agent of salmonid whirling disease, in Tubifex tubifex. J. Fish Biol. 2001, 87, 101–107.
- El-Matbouli, M.; Hoffmann, R.W.; Mandok, C. Light and electron microscopic observations on the route of the triactinomyxon-sporoplasm of Myxobolus cerebralis from epidermis into rainbow trout cartilage. J. Fish Biol. 1995, 46, 919–935.
- El-Matbouli, M.; Hoffmann, R.W.; Schoel, H.; McDowell, T.S.; Hedrick, R.P. Whirling disease:host specificity and interaction between the actinosporean stage of Myxobolus cerebralis and rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 1999, 35, 1–12.
- Silva, J.R.M.C.; Staines, N.A.; Hernandez-Blazquez, F.J.; Porto-Neto, L.R.; Borges, J.C.S. Phagocytosis and giant cell formation at 0 °C by macrophage (MØ) of Notothenia coriiceps. J. Fish Biol. 2002, 60, 466–478.
- Secombes, C.J.; Wang, T. The innate and adaptive immune system of fish. In Infectious Disease in Aquaculture; Woodhead Publishing: Cambridge, UK, 2012; pp. 3–68.
- Lom, J. Myxosporea: A new look at long-known parasites of fish. Parasitol. Today 1987, 3, 327–332.
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503.
- Sitjà-Bobadilla, A.; Alvarez-Pellitero, P. Pathologic effects of Sphaerospora dicentrarchi Sitjà-Bobadilla and Alvarez-Pellitero, 1992 and S. testicularis Sitjà-Bobadilla and Alvarez-Pellitero, 1990 (Myxosporea: Bivalvulida) parasitic in the Mediterranean sea bass Dicentrarchus labrax L. (Teleostei: Serranidae) and the cell-mediated immune reaction: A light and electron microscopy study. Parasitol. Res. 1993, 79, 119–129.
- Rucker, U.; El-Matbouli, M. Sequence analysis of OmNramp α and quantitative expression of Nramp homologues in different trout strains after infection with Myxobolus cerebralis. Dis. Aquat. Org. 2007, 76, 223–230.
- Saleh, M.; Montero, R.; Kumar, G.; Sudhagar, A.; Friedl, A.; Köllner, B.; El-Matbouli, M. Kinetics of local and systemic immune cell responses in whirling disease infection and resistance in rainbow trout. Parasites Vectors 2019, 12, 249.
- Saleh, M.; Friedl, A.; Srivastava, M.; Secombes, C.J.; El-Matbouli, M. Modulation of local and systemic immune responses in brown trout (Salmo trutta) following exposure to Myxobolus cerebralis. Fish Shellfish Immunol. 2020, 106, 844–851.
- Tovey, M.G.; Lallemand, C. Adjuvant activity of cytokines. In Vaccine Adjuvants: Methods and Protocols; Humana: Totowa, NJ, USA, 2010; pp. 287–309.
- Saleh, M.; Friedl, A.; Srivastava, M.; Soliman, H.; Secombes, C.J.; El-Matbouli, M. STAT3/SOCS3 axis contributes to the outcome of salmonid whirling disease. PLoS ONE 2020, 15, e0234479.
- Alvarez-Pellitero, P. Fish immunity and parasite infections: From innate immunity to immunoprophylactic prospects. Vet. Immunol. Immunopathol. 2008, 126, 171–198.
- Sitjà-Bobadilla, A. Living off a fish: A trade-off between parasites and the immune system. Fish Shellfish Immunol. 2008, 25, 358–372.
- Baerwald, M.R. Temporal expression patterns of rainbow trout immune-related genes in response to Myxobolus cerebralis exposure. Fish Shellfish Immunol. 2013, 35, 965–971.
- Sarker, S.; Menanteau-Ledouble, S.; Kotob, M.H.; El-Matbouli, M. A RNAi-based therapeutic proof of concept targets salmonid whirling disease in vivo. PLoS ONE 2017, 12, e0178687.
- Bailey, C.; Holland, J.W.; Secombes, C.J.; Tafalla, C. A portrait of the immune response to proliferative kidney disease (PKD) in rainbow trout. Parasite Immunol. 2020, 42, e12730.
- El-Matbouli, M.; Hoffmann, R. Light and electron microscopic studies on the chronological development of Myxobolus cerebralis to the actinosporean stage in Tubifex tubifex. Int. J. Parasitol. 1998, 28, 195–217.
- Rose, J.D.; Marrs, G.S.; Lewis, C.; Schisler, G. Whirling disease behavior and its relation to pathology of brain stem and spinal cord in rainbow trout. J. Aquat. Anim. Health 2000, 12, 107–118.
- Wolf, K. Salmonid Whirling Disease; US Department of the Interior, Fish and Wildlife Service, Division of Fishery Research: Lincoln, NE, USA, 1985; Volume 69.
- Kotob, M.H.; Gorgoglione, B.; Kumar, G.; Abdelzaher, M.; Saleh, M.; El-Matbouli, M. The impact of Tetracapsuloides bryosalmonae and Myxobolus cerebralis co-infections on pathology in rainbow trout. Parasites Vectors 2017, 10, 442.
- Platt, C. The peripheral vestibular system in fishes. In Fish Neurobiology; Northcutt, R.G., Davis, R.E., Eds.; University of Michigan Press: Ann Arbor, MI, USA, 1983; pp. 89–124.
- Mogensen, T.H. Pathogen Recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273.
- Whyte, S.K. The innate immune response of finfish–A review of current knowledge. Fish Shellfish Immunol. 2007, 23, 1127–1151.
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32.
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151.
- Ellis, A. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001, 25, 827–839.
- Magnadottir, B. Immunological control of fish diseases. Mar. Biotechnol. 2010, 12, 361–379.
- Esteban, M.Á. An Overview of the immunological defenses in fish skin. Int. Sch. Res. Not. 2012, 2012, 853470.
- Sveinbjornsson, B.; Olsen, R.; Paulsen, S. Immunocytochemical localization of lysozyme in intestinal eosinophilic granule cells (EGCs) of Atlantic salmon, Salmo salar L. J. Fish Dis. 1996, 19, 349–355.
- Fischer, U.; Utke, K.; Somamoto, T.; Köllner, B.; Ototake, M.; Nakanishi, T. Cytotoxic activities of fish leucocytes. Fish Shellfish Immunol. 2006, 20, 209–226.
- Kallert, D.; Eszterbauer, E.; Grabner, D.; El-Matbouli, M. In vivo exposure of susceptible and non-susceptible fish species to Myxobolus cerebralis actinospores reveals non-specific invasion behaviour. Dis. Aquat. Org. 2009, 84, 123–130.
- Sitjà-Bobadilla, A.; Schmidt-Posthaus, H.; Wahli, T.; Holland, J.W.; Secombes, C.J. Fish immune responses to Myxozoa. In Myxozoan Evolution, Ecology and Development; Springer: Cham, Switzerland, 2015; pp. 253–280.
- Estensoro, I.; Mulero, I.; Redondo, M.J.; Álvarez-Pellitero, P.; Mulero, V.; Sitjà-Bobadilla, A. Modulation of leukocytic populations of gilthead sea bream (Sparus aurata) by the intestinal parasite Enteromyxum leei (Myxozoa: Myxosporea). Parasitology 2014, 141, 425–440.
- Kania, P.W.; Evensen, O.; Larsen, T.B.; Buchmann, K. Molecular and immunohistochemical studies on epidermal responses in Atlantic salmon Salmo salar L. induced by Gyrodactylus salaris Malmberg, 1957. J. Helminthol. 2010, 84, 166–172.
- Yano, T. The nonspecific immune system: Humoral defense. In The Fish Immune System: Organism, Pathogen, Environment; Academic Press: San Diego, CA, USA, 1996; pp. 105–157.
- Kallert, D.M.; Borrelli, J.; Haas, W. Biostatic activity of piscine serum and mucus on myxozoan fish infective stages. Fish Shellfish Immunol. 2012, 33, 969–976.
- Jones, S.R.M. The occurrence and mechanisms of innate immunity against parasites in fish. Dev. Comp. Immunol. 2001, 25, 841–852.
- Hedrick, R.P.; Adkison, M.A.; El-Matbouli, M.; MacConnell, E. Whirling disease: Re-emergence among wild trout. Immunol. Rev. 1998, 166, 365–376.
- Kelley, G.O.; Adkison, M.A.; Leutenegger, C.M.; Hedrick, R.P. Myxobolus cerebralis: Identification of a cathepsin Z-like protease gene (MyxCP-1) expressed during parasite development in rainbow trout, Oncorhynchus mykiss. Exp. Parasitol. 2003, 105, 201–210.
- Kelley, G.; Zagmutt-Vergara, F.; Leutenegger, C.; Adkison, M.; Baxa, D.; Hedrick, R. Identification of a serine protease gene expressed by Myxobolus cerebralis during development in rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 2004, 59, 235–248.
- Dörfler, C.; El-Matbouli, M. Isolation of a subtilisin-like serine protease gene (MyxSubtSP) from spores of Myxobolus cerebralis, the causative agent of whirling disease. Dis. Aquat. Org. 2007, 73, 245–251.
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main components of fish immunity: An overview of the fish immune system. Fishes 2023, 8, 93.
- Secombes, C.; Fletcher, T. The role of phagocytes in the protective mechanisms of fish. Annu. Rev. Fish Dis. 1992, 2, 53–71.
- Secombes, C.; Wang, T.; Hong, S.; Peddie, S.; Crampe, M.; Laing, K.; Cunningham, C.; Zou, J. Cytokines and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 713–723.
- Severin, V.I.C.; Soliman, H.; El-Matbouli, M. Expression of immune-regulatory genes, arginase-2 and inducible nitric oxide synthase (iNOS), in two rainbow trout (Oncorhynchus mykiss) strains following exposure to Myxobolus cerebralis. Parasitol. Res. 2010, 106, 325–334.
- Joerink, M.; Savelkoul, H.F.; Wiegertjes, G.F. Evolutionary conservation of alternative activation of macrophages: Structural and functional characterization of arginase 1 and 2 in carp (Cyprinus carpio L.). Mol. Immunol. 2006, 43, 1116–1128.
- Jenkinson, C.P.; Grody, W.W.; Cederbaum, S.D. Comparative properties of arginases. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1996, 114, 107–132.
- Hedrick, R.P.; McDowell, T.S.; Marty, G.D.; Fosgate, G.T.; Mukkatira, K.; Myklebust, K.; El-Matbouli, M. Susceptibility of two strains of rainbow trout (one with suspected resistance to whirling disease) to Myxobolus cerebralis infection. Dis. Aquat. Org. 2003, 55, 37–44.
- Baker, S.T.; Barton, C.H.; E Biggs, T. A negative autoregulatory link between Nramp 1 function and expression. J. Leukoc. Biol. 2000, 67, 501–507.
- Grayson, T.H.; Cooper, L.F.; Wrathmell, A.B.; Roper, J.; Evenden, A.J.; Gilpin, M.L. Host responses to Renibacterium salmoninarum and specific components of the pathogen reveal the mechanisms of immune suppression and activation. Immunology 2002, 106, 273–283.
- Sigh, J.; Lindenstrøm, T.; Buchmann, K. Expression of pro-inflammatory cytokines in rainbow trout (Oncorhynchus mykiss) during an infection with Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2004, 17, 75–86.
- Overturf, K.; LaPatra, S. Quantitative expression (Walbaum) of immunological factors in rainbow trout, Oncorhynchus mykiss (Walbaum), after infection with either Flavobacterium psychrophilum, Aeromonas salmonicida, or infectious haematopoietic necrosis virus. J. Fish Dis. 2006, 29, 215–224.
- Manning, M.J.; Nakanishi, T. The specific immune system: Cellular defences. In The Fish Immune System; Academic Press: New York, NY, USA, 1996.
- Utke, K.; Bergmann, S.; Lorenzen, N.; Köllner, B.; Ototake, M.; Fischer, U. Cell-mediated cytotoxicity in rainbow trout, Oncorhynchus mykiss, infected with viral haemorrhagic septicaemia virus. Fish Shellfish Immunol. 2007, 22, 182–196.
- Densmore, C.L.; Blazer, V.S.; Waldrop, T.B.; Pooler, P.S. Effects of Whirling Disease on Selected Hematological Parameters in Rainbow Trout. J. Wildl. Dis. 2001, 37, 375–378. Available online: http://meridian.allenpress.com/jwd/article-pdf/37/2/375/2233840/0090-3558-37_2_375.pdf (accessed on 12 July 2023).
- Alvarez, F.; Razquin, B.; Villena, A.; Fierro, P.L.; Zapata, A. Alterations in the peripheral lymphoid organs and differential leukocyte counts in Saprolegnia-infected brown trout, Salmo trutta fario. Vet. Immunol. Immunopathol. 1988, 18, 181–193.
- Lamas, J.; Santos, Y.; Bruno, D.W.; Toranzo, A.E.; Anadon, R. Non-specific cellular responses of rainbow trout to Vibrio anguillarum and its extracellular products (ECPs). J. Fish Biol. 1994, 45, 839–854.
- Dick, P.T.; Dixon, D.G. Changes in circulating blood cell levels of rainbow trout, Salmo gairdneri Richardson, following acute and chronic exposure to copper. J. Fish Biol. 1985, 26, 475–481.
- McLeay, D. Effects of cortisol and dexamethasone on the pituitary-interrenal axis and abundance of white blood cell types in juvenile coho salmon, Oncorhynchus kisutch. Gen. Comp. Endocrinol. 1973, 21, 441–450.
- Pickering, A. Cortisol-induced lymphocytopenia in brown trout, Salmo trutta L. Gen. Comp. Endocrinol. 1984, 53, 252–259.
- Murad, A.; Houston, A. Leucocytes and leucopoietic capacity in goldfish, Carassius auratus, exposed to sublethal levels of cadmium. Aquat. Toxicol. 1988, 13, 141–154.
- Ainsworth, A. Fish granulocytes: Morphology, distribution, and function. Annu. Rev. Fish Dis. 1992, 2, 123–148.
- Hedrick, R.; McDowell, T.; Gay, M.; Marty, G.; Georgiadis, M.; MacConnell, E. Comparative susceptibility of rainbow trout Oncorhynchus mykiss and brown trout Salmo trutta to Myxobolus cerebralis, the cause of salmonid whirling disease. Dis. Aquat. Org. 1999, 37, 173–183.
- El-Matbouli, M.; Fischer-Scherl, T.; Hoffmann, R.W. Present knowledge on the life cycle, taxonomy, pathology, and therapy of some Myxosporea spp. important for freshwater fish. Annu. Rev. Fish Dis. 1992, 2, 367–402.
- Andree, K.B.; El-Matbouli, M.; Hoffman, R.W.; Hedrick, R.P. Comparison of 18S and ITS-1 rDNA sequences of selected geographic isolates of Myxobolus cerebralis. Int. J. Parasitol. 1999, 29, 771–775.
- Mokhtar, D.M. Fish Histology: From Cells to Organs; CRC Press: Boca Raton, FL, USA, 2021.
- Mokhtar, D.M. Characterization of the fish ovarian stroma during the spawning season: Cytochemical, immunohistochemical and ultrastructural studies. Fish Shellfish Immunol. 2019, 94, 566–579.
- Lauriano, E.R.; Calò, M.; Silvestri, G.; Zaccone, D.; Pergolizzi, S.; Cascio, P.L. Mast cells in the intestine and gills of the sea bream, Sparus aurata, exposed to a polychlorinated biphenyl, PCB 126. Acta Histochem. 2012, 114, 166–171.
- Hedrick, R.P.; McDowell, T.S.; Mukkatira, K.; Georgiadis, M.P.; MacConnell, E. Susceptibility of three species of anadromous salmonids to experimentally induced infections with Myxobolus cerebralis, the causative agent of whirling disease. J. Aquat. Anim. Health 2001, 13, 43–50.
- Reite, O.B. Mast cells/eosinophilic granule cells of teleostean fish: A review focusing on staining properties and functional responses. Fish Shellfish Immunol. 1998, 8, 489–513.
- Dezfuli, B.S.; Giari, L.; Konecny, R.; Jaeger, P.; Manera, M. Immunohistochemistry, ultrastructure and pathology of gills of Abramis brama from Lake Mondsee, Austria, infected with Ergasilus sieboldi (Copepoda). Dis. Aquat. Organ. 2003, 53, 257–262.
- Siderits, D.; Bielek, E. Rodlet cells in the thymus of the zebrafish Danio rerio (Hamilton, 1822). Fish Shellfish Immunol. 2009, 27, 539–548.
- Leino, R.L. Reaction of rodlet cells to a myxosporean infection in kidney of the bluegill, Lepomis macrochirus. Can. J. Zool. 1996, 74, 217–225.
- Reite, O.B. The rodlet cells of teleostean fish: Their potential role in host defence in relation to the role of mast cells/eosinophilic granule cells. Fish Shellfish Immunol. 2005, 19, 253–267.
- Munoz, P.; Sitjà-Bobadilla, A.; Alvarez-Pellitero, P. Cellular and humoral immune response of European sea bass (Dicentrarchus labrax L.) (Teleostei: Serranidae) immunized with Sphaerospora dicentrarchi (Myxosporea: Bivalvulida). Parasitology 2000, 120, 465–477.
- Reite, O.B. Mast cells/eosinophilic granule cells of salmonids: Staining properties and responses to noxious agents. Fish Shellfish Immunol. 1997, 7, 567–584.
- Muñoz, P.; Cuesta, A.; Athanassopoulou, F.; Golomazou, H.; Crespo, S.; Padrós, F.; Sitjà-Bobadilla, A.; Albiñana, G.; Esteban, M.; Alvarez-Pellitero, P.; et al. Sharpsnout sea bream (Diplodus puntazzo) humoral immune response against the parasite Enteromyxum leei (Myxozoa). Fish Shellfish Immunol. 2007, 23, 636–645.
- Wang, T.; Secombes, C.J. The cytokine networks of adaptive immunity in fish. Fish Shellfish Immunol. 2013, 35, 1703–1718.
- Chabalgoity, J.A.; Baz, A.; Rial, A.; Grille, S. The relevance of cytokines for development of protective immunity and rational design of vaccines. Cytokine Growth Factor Rev. 2007, 18, 195–207.
- Zou, J.; Carrington, A.; Collet, B.; Dijkstra, J.M.; Yoshiura, Y.; Bols, N.; Secombes, C. Identification and bioactivities of IFN-γ in rainbow trout Oncorhynchus mykiss: The first Th1-type cytokine characterized functionally in fish. J. Immunol. 2005, 175, 2484–2494.
- Zoua, J.; Grabowski, P.S.; Cunningham, C.; Secombes, C.J. Molecular cloning of interleukin 1β from rainbow trout Oncorhynchus mykiss reveals no evidence of an ice cut site. Cytokine 1999, 11, 552–560.
- Laing, K.J.; Secombes, C.J. Chemokines. Dev. Comp. Immunol. 2004, 28, 443–460.
- Laing, K.J.; Wang, T.; Zou, J.; Holland, J.; Hong, S.; Bols, N.; Hirono, I.; Aoki, T.; Secombes, C.J. Cloning and expression analysis of rainbow trout Oncorhynchus mykiss tumour necrosis factor-α. Eur. J. Biochem. 2001, 268, 1315–1322.
- Severin, V.I.C.; El-Matbouli, M. Relative quantification of immune-regulatory genes in two rainbow trout strains, Oncorhynchus mykiss, after exposure to Myxobolus cerebralis, the causative agent of whirling disease. Parasitol. Res. 2007, 101, 1019–1027.
- Cooper, M.D.; Alder, M.N. The evolution of adaptive immune systems. Cell 2006, 124, 815–822.
- Flajnik, M.F. A cold-blooded view of adaptive immunity. Nat. Rev. Immunol. 2018, 18, 438–453.
- Pauley, G.B. Fish Sporozoa: Extraction of Antigens from Myxosoma cerebralis Spores Which Mimic Tissue Antigens of Rainhow Trout (Salmo gairdneri). J. Fish. Res. Board Can. 1974, 31, 1481–1484.
- Halliday, M.M. Studies on Myxosoma cerebralis, a parasite of salmonids. 4. A preliminary immunofluorescent investigation of the spores of Myxosoma cerebralis. Nord. Vet. Med. 1974, 26, 173–179.
- Bartholomew, J.L.; Smith, C.E.; Rohovec, J.S.; Fryer, J.L. Characterization of a host response to the myxosporean parasite, Ceratomyxa shasta (Noble), by histology, scanning electron microscopy and immunological techniques. J. Fish Dis. 1989, 12, 509–522.
- Furuta, T.; Ogawa, K.; Wakabayashi, H. Humoral immune response of carp Cyprinus carpio to Myxobolus artus (Myxozoa: Myxobolidae) infection. J. Fish Biol. 1993, 43, 150–441.
- Saulnier, D.; de Kinkelin, P. Antigenic and biochemical study of PKX, the myxosporean causative agent of proliferative kidney disease of salmonid fish. Dis. Aquat. Org. 1996, 27, 103–114.
- Carmona, S.J.; Teichmann, S.A.; Ferreira, L.; Macaulay, I.C.; Stubbington, M.J.; Cvejic, A.; Gfeller, D. Single-cell transcriptome analysis of fish immune cells provides insight into the evolution of vertebrate immune cell types. Genome Res. 2017, 27, 451–461.
- Nakanishi, T.; Shibasaki, Y.; Matsuura, Y. T cells in fish. Biology 2015, 4, 640–663.
- Ashfaq, H.; Soliman, H.; Saleh, M.; El-Matbouli, M. CD4: A vital player in the teleost fish immune system. Vet. Res. 2019, 50, 1.
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23.
- Hu, Y.; Alnabulsi, A.; Alnabulsi, A.; Scott, C.; Tafalla, C.; Secombes, C.J.; Wang, T. Characterisation and analysis of IFN-gamma producing cells in rainbow trout Oncorhynchus mykiss. Fish Shellfish Immunol. 2021, 117, 328–338.
- Walker, J.A.; McKenzie, A.N.J. TH2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133.
- Braden, L.M.; Koop, B.F.; Jones, S.R. Signatures of resistance to Lepeophtheirus salmonis include a TH2-type response at the louse-salmon interface. Dev. Comp. Immunol. 2015, 48, 178–191.
- Gajewski, T.F.; Fitch, F.W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J. Immunol. 1988, 140, 4245–4252.
- Oriss, T.B.; A McCarthy, S.; Morel, B.F.; A Campana, M.; A Morel, P. Crossregulation between T helper cell (Th)1 and Th2: Inhibition of Th2 proliferation by IFN-gamma involves interference with IL-1. J. Immunol. 1997, 158, 3666–3672.
- Bottiglione, F.; Dee, C.T.; Lea, R.; Zeef, L.A.H.; Badrock, A.P.; Wane, M.; Bugeon, L.; Dallman, M.J.; Allen, J.E.; Hurlstone, A.F.L. Zebrafish IL-4–like cytokines and IL-10 suppress inflammation but only IL-10 is essential for gill homeostasis. J. Immunol. 2020, 205, 994–1008.
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532.
- Hu, X.; Ivashkiv, L.B. Cross-regulation of Signaling Pathways by Interferon-γ: Implications for Immune Responses and Autoimmune Diseases. Immunity 2009, 31, 539–550.
- Magadan, S.; Sunyer, O.J.; Boudinot, P. Unique features of fish immune repertoires: Particularities of adaptive immunity within the largest group of vertebrates. In Pathogen-Host Interactions: Antigenic Variation v. Somatic Adaptations; Springer: Cham, Switzerland, 2015; pp. 235–264.
- Mashoof, S.; Criscitiello, M.F. Fish immunoglobulins. Biology 2016, 5, 45.
- Wu, L.; Qin, Z.; Liu, H.; Lin, L.; Ye, J.; Li, J. Recent advances on phagocytic B cells in teleost fish. Front. Immunol. 2020, 11, 824.
- Zhang, N.; Zhang, X.-J.; Chen, D.-D.; Sunyer, J.O.; Zhang, Y.-A. Molecular characterization and expression analysis of three subclasses of IgT in rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 2017, 70, 94–105.
- Xu, Z.; Takizawa, F.; Parra, D.; Gómez, D.; Jørgensen, L.v.G.; LaPatra, S.E.; Sunyer, J.O. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat. Commun. 2016, 7, 10728.
- Castro, R.; Jouneau, L.; Pham, H.-P.; Bouchez, O.; Giudicelli, V.; Lefranc, M.-P.; Quillet, E.; Benmansour, A.; Cazals, F.; Six, A.; et al. Teleost fish mount complex clonal IgM and IgT responses in spleen upon systemic viral infection. PLoS Pathog. 2013, 9, e1003098.
- Piazzon, M.C.; Galindo-Villegas, J.; Pereiro, P.; Estensoro, I.; Calduch-Giner, J.A.; Gómez-Casado, E.; Novoa, B.; Mulero, V.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Differential Modulation of IgT and IgM upon Parasitic, Bacterial, Viral, and Dietary Challenges in a Perciform Fish. Front. Immunol. 2016, 7, 637.
- Ryce, E.K.N. Factors Affecting the Resistance of Juvenile Rainbow Trout to Whirling Disease; Montana State University: Bozeman, MT, USA, 2003.
- Griffin, B.R.; Davis, E.M. Myxosoma cerebralis: Detection of Circulating Antibodies in Infected Rainbow Trout (Salmo gairdneri). J. Fish. Res. Board Can. 1978, 35, 1186–1190.
- Hoffman, G.L. Myxobolus cerebralis, a worldwide cause of salmonid whirling disease. J. Aquat. Anim. Health 1990, 2, 30–37.
- MacConnell, E.; Vincent, E.R. The effects of Myxobolus cerebralis on the salmonid host. In American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 2002; pp. 95–108.
- Baerwald, M.R.; Welsh, A.B.; Hedrick, R.P.; May, B. Discovery of genes implicated in whirling disease infection and resistance in rainbow trout using genome-wide expression profiling. BMC Genom. 2008, 9, 37.
- Rossi, D.; Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000, 18, 217–242.
- Kotob, M.H.; Menanteau-Ledouble, S.; Kumar, G.; Abdelzaher, M.; El-Matbouli, M. The impact of co-infections on fish: A review. Vet. Res. 2017, 47, 98.
- Cox, F.E.G. Concomitant infections, parasites and immune responses. Parasitology 2001, 122, S23–S38.
- Kotob, M.H.; Kumar, G.; Saleh, M.; Gorgoglione, B.; Abdelzaher, M.; El-Matbouli, M. Differential modulation of host immune genes in the kidney and cranium of the rainbow trout (Oncorhynchus mykiss) in response to Tetracapsuloides bryosalmonae and Myxobolus cerebralis co-infections. Parasites Vectors 2018, 11, 326.
- Gorgoglione, B.; Wang, T.; Secombes, C.J.; Holland, J.W. Immune gene expression profiling of Proliferative Kidney Disease in rainbow trout Oncorhynchus mykiss reveals a dominance of anti-inflammatory, antibody and T helper cell-like activities. Vet. Res. 2013, 44, 55.
- Holland, J.W.; Gould, C.R.W.; Jones, C.S.; Noble, L.R.; Secombes, C.J. The expression of immune-regulatory genes in rainbow trout, Oncorhynchus mykiss, during a natural outbreak of proliferative kidney disease (PKD). Parasitology 2003, 126, S95–S102.
- Chilmonczyk, S.; Monge, D.; De Kinkelin, P. Proliferative kidney disease: Cellular aspects of the rainbow trout, Oncorhynchus mykiss (Walbaum), response to parasitic infection. J. Fish Dis. 2002, 25, 217–226.
- Morris, D.J.; Molnár, K.; Longshaw, M.; Adams, A. Immunostaining of spores and plasmodia of disparate myxozoan genera with comments on the properties of the sporular mucus envelope. Parasitology 2006, 132, 781–790.
- Densmore, C.L.; Ottinger, C.A.; Blazer, V.S.; Iwanowicz, L.R.; Smith, D.R. Immunomodulation and disease resistance in postyearling rainbow trout infected with Myxobolus cerebralis, the causative agent of whirling disease. J. Aquat. Anim. Health 2004, 16, 73–82.
- Woo, P.T. Immunological responses of fish to parasitic organisms. Annu. Rev. Fish Dis. 1992, 2, 339–366.
- van der Marel, M.; Adamek, M.; Gonzalez, S.F.; Frost, P.; Rombout, J.H.W.M.; Wiegertjes, G.F.; Savelkoul, H.F.J.; Steinhagen, D. Molecular cloning and expression of two β-defensin and two mucin genes in common carp (Cyprinus carpio L.) and their up-regulation after β-glucan feeding. Fish Shellfish Immunol. 2012, 32, 494–501.
- Malachowicz, M.; Wenne, R.; Burzynski, A. De novo assembly of the sea trout (Salmo trutta m. trutta) skin transcriptome to identify putative genes involved in the immune response and epidermal mucus secretion. PLoS ONE 2017, 12, e0172282.
- Sveen, L.R.; Grammes, F.T.; Ytteborg, E.; Takle, H.; Jørgensen, S.M. Genome-wide analysis of Atlantic salmon (Salmo salar) mucin genes and their role as biomarkers. PLoS ONE 2017, 12, e0189103.
- Marcos-López, M.; Calduch-Giner, J.A.; Mirimin, L.; MacCarthy, E.; Rodger, H.D.; O’Connor, I.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J.; Piazzon, M.C. Gene expression analysis of Atlantic salmon gills reveals mucin 5 and interleukin 4/13 as key molecules during amoebic gill disease. Sci. Rep. 2018, 8, 13689.
- Schisler, G.J.; Bergersen, E.P.; Walker, P.G. Effects of multiple stressors on morbidity and mortality of fingerling rainbow trout infected with Myxobolus cerebralis. Trans. Am. Fish. Soc. 2000, 129, 859–865.
- Vincent, E.R. The relationship of time, temperature, and fish life histories to whirling disease infections. In Proceedings of the Whirling Disease Symposium: Research in Progress, Fort Collins, CO, USA, 19–21 February 1998; pp. 81–83.
- Wahli, T.; Bernet, D.; Segner, H.; Schmidt-Posthaus, H. Role of altitude and water temperature as regulating factors for the geographical distribution of Tetracapsuloides bryosalmonae infected fishes in Switzerland. J. Fish Biol. 2008, 73, 2184–2197.
- Markkula, S.E.; Karvonen, A.; Salo, H.; Valtonen, E.T.; Jokinen, E.I. Ultraviolet B irradiation affects resistance of rainbow trout (Oncorhynchus mykiss) against bacterium yersinia ruckeri and trematode Diplostomum spathaceum. Photochem. Photobiol. 2007, 83, 1263–1269.
- Hedrick, R.; McDowell, T.; Marty, G.; Mukkatira, K.; Antonio, D.; Andree, K.; Bukhari, Z.; Clancy, T. Ultraviolet irradiation inactivates the waterborne infective stages of Myxobolus cerebralis: A treatment for hatchery water supplies. Dis. Aquat. Org. 2000, 42, 53–59.
- Hedrick, R.P.; McDowell, T.S.; Adkison, M.A.; Myklebust, K.A.; Mardones, F.O.; Petri, B. Invasion and initial replication of ultraviolet irradiated waterborne infective stages of Myxobolus cerebralis results in immunity to whirling disease in rainbow trout. Int. J. Parasitol. 2012, 42, 657–666.
- Stolte, E.H.; Nabuurs, S.B.; Bury, N.R.; Sturm, A.; Flik, G.; Savelkoul, H.F.; Kemenade, B.L.V.-V. Stress and innate immunity in carp: Corticosteroid receptors and pro-inflammatory cytokines. Mol. Immunol. 2008, 46, 70–79.
- Kemenade, B.V.-V.; Ribeiro, C.; Chadzinska, M. Neuroendocrine–immune interaction in fish: Differential regulation of phagocyte activity by neuroendocrine factors. Gen. Comp. Endocrinol. 2011, 172, 31–38.
- Katharios, P.; Garaffo, M.; Sarter, K.; Athanassopoulou, F.; Mylonas, C.C. A case of high mortality due to heavy infestation of Ceratomyxa diplodae in sharpsnout sea bream (Diplodus puntazzo) treated with reproductive steroids. Bull.-Eur. Assoc. Fish Pathol. 2007, 27, 43.
- Kent, M.L.; Hedrick, R.P. Effects of cortisol implants on the PKX myxosporean causing proliferative kidney disease in rainbow trout, salmo gairdneri. J. Parasitol. 1987, 73, 455–461.
More
Information
Subjects:
Fisheries
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
435
Revisions:
2 times
(View History)
Update Date:
01 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




