Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alisa Gisina | -- | 4196 | 2023-12-25 19:31:58 | | | |
| 2 | Lindsay Dong | -436 word(s) | 3760 | 2023-12-27 02:03:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gisina, A.; Kim, Y.; Yarygin, K.; Lupatov, A. CD133 as a Prognostic Biomarker in Oncology. Encyclopedia. Available online: https://encyclopedia.pub/entry/53120 (accessed on 01 March 2026).
Gisina A, Kim Y, Yarygin K, Lupatov A. CD133 as a Prognostic Biomarker in Oncology. Encyclopedia. Available at: https://encyclopedia.pub/entry/53120. Accessed March 01, 2026.
Gisina, Alisa, Yan Kim, Konstantin Yarygin, Alexey Lupatov. "CD133 as a Prognostic Biomarker in Oncology" Encyclopedia, https://encyclopedia.pub/entry/53120 (accessed March 01, 2026).
Gisina, A., Kim, Y., Yarygin, K., & Lupatov, A. (2023, December 25). CD133 as a Prognostic Biomarker in Oncology. In Encyclopedia. https://encyclopedia.pub/entry/53120
Gisina, Alisa, et al. "CD133 as a Prognostic Biomarker in Oncology." Encyclopedia. Web. 25 December, 2023.
Copy Citation
The CD133 cell membrane glycoprotein, also termed prominin-1, is expressed on some of the tumor cells of both solid and blood malignancies. The CD133-positive tumor cells were shown to exhibit higher proliferative activity, greater chemo- and radioresistance, and enhanced tumorigenicity compared to their CD133-negative counterparts. The CD133-positive cells are related to the cancer stem cell subpopulation in many types of cancer. For this reason, CD133 is regarded as a potential prognostic biomarker in oncology.
CD133
prominin-1
cancer biomarker
cancer
1. Introduction
The availability of reliable biomarkers for cancer screening and prognosis is essential for the early start of treatment and the achievement of more favorable outcomes. CD133, also termed prominin-1, is considered a promising prognostic biomarker for a wide range of tumor types. CD133 is an integral plasma membrane protein also detected inside the cell [1], including the endoplasmic reticulum and Golgi apparatus, where its maturation and glycosylation occurs [2]. The glycosylated CD133 has a molecular weight of ≈115–120 kDa [3][4], and in the plasma membrane, it is located predominantly at the membrane protrusions, such as microvilli and primary cilia [5]. Due to this, the name “prominin” comes from the Latin word “prominere”, which means “prominent”. The N-terminus and two large loops of this molecule are located outside the cell, and the C-terminus and two small loops are in the cytoplasm [6][7] (Figure 1A). It has been established that the CD133 glycoprotein on the plasma membrane resides in specialized lipid microdomains rich in cholesterol and sphingolipids, i.e., lipid rafts [8]. Recent studies have also revealed nuclear localization of CD133 [9][10][11]. Figure 1B shows all sites of the CD133 cellular localization.
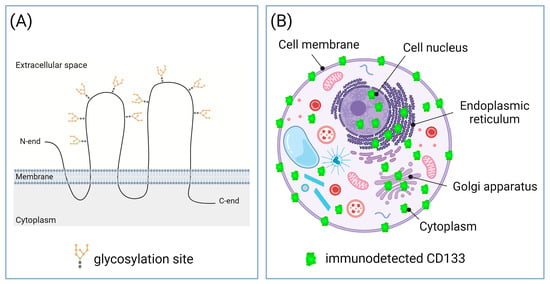
Figure 1. Structure and cellular localization of CD133. (A) Topological model of CD133 molecule at the plasma membrane. CD133 molecule has nine N-linked glycosylation sites [12]. (B) Subcellular localization of CD133. CD133 was detected in cell membrane; cytoplasm, including endoplasmic reticulum and Golgi apparatus; and nucleus. Created with BioRender.com.
CD133 is found in various tissues. In early embryos, CD133 is observed in trophoblast cells but not in the inner cell mass [13]. In developing embryos, CD133 is expressed in the epithelia of the three germinal layers [5][14]. In the postnatal period, CD133 expression occurs in stem and progenitor cells, such as hematopoietic stem cells [4], endothelial progenitor cells [15], prostate epithelial stem cells [16], brain stem/progenitor cells [17], myogenic cells [18], and hepatic progenitor cells [19]. CD133 is also expressed in differentiated cells of the adult body, and in particular, on various types of glandular epithelium and the epithelium of kidney proximal tubules [1][5][20][21]. CD133 is present on photoreceptor cells throughout human life, and mutations in the PROM1 gene are associated with retinal degeneration leading to blindness [22][23].
In addition, CD133 is detected in many malignant tumors, such as prostate [24], lung [25][26], ovarian [27], breast [28], endometrial [29], renal [30], thyroid [31], esophageal [32], gastric [33][34], colorectal [35], pancreatic [1], hepatocellular [36][37], and gallbladder [38] carcinoma, as well as in glioma [39], meningioma [40], osteosarcoma [41], melanoma [42], cutaneous squamous cell carcinoma [43], and other solid malignant neoplasms. CD133 is also expressed on myeloid [44] and lymphoblastic leukemia cells [45]. Comparison of normal and tumor tissues revealed that the CD133 expression level in the vast majority of cases is significantly higher in the latter [25][46][47][48][49][50][51]. In addition, there is evidence that CD133 expression is higher in samples from patients with metastases and at the advanced stages of cancer [52]. There is an abundance of evidence of the existence of a relationship between the level of the CD133 protein or mRNA expression and the disease severity. However, there are also reports of the absence of such an association and even of a correlation with a favorable course of the disease. Thus, there is no final opinion with respect to the prognostic significance of CD133. For this reason, CD133 has not yet become a conventional prognostic cancer biomarker despite more than 20 years of intensive studies.
2. Putative Explanation of the Cause-and-Effect Relationship between CD133 Expression and More Malignant Phenotype of Tumor Cells
The strong correlation between high CD133 levels in tumor cells and negative cancer prognosis complies with the association of CD133 expression with the cancer stem cell (CSC) phenotype. Initially, CSCs were identified as a subpopulation of tumor cells with an exceptional or increased ability to form tumors when transplanted into immunodeficient mice [53][54]. Later, it was shown that in addition to high tumorigenicity, CSCs are also characterized by increased invasiveness and metastasis, increased chemo- and radioresistance, higher proliferative activity, and the ability to form tumorspheres in vitro [55]. The role of CD133 as a CSC marker has been demonstrated in a wide range of human tumors. CD133 turned out to be effective for identifying CSCs in carcinomas of lung [25][26][56], ovary [57], stomach [58], colon [46][59], gallbladder [60], head and neck [61], pancreas [47], and liver [36], as well as glioblastoma [62], lymphoma [45], Ewing’s sarcoma [63], and in other human neoplasms [64][65].
2.1. Increased Proliferation
There is evidence of enhanced proliferation of tumor cells expressing CD133. For example, populations of CD133-positive cells from samples of oral squamous cell carcinoma [66] and cells of the hepatocellular carcinoma line [67] demonstrate a significantly higher proliferation rate than CD133-negative cells. This is consistent with the data obtained in colorectal adenocarcinoma cell lines, where the percentage of proliferating cells measured via BrdU incorporation, the number of visualized mitoses, expression of the proliferation marker Ki67, and the ability to form colonies in vitro were higher in populations with high CD133 expression compared with cells of the same lines with low or lacking CD133 expression [68][69]. There is also evidence that CD133-positive cells of osteosarcoma cell lines were mainly in the G2/M phase, expressed Ki67, and demonstrated higher proliferative activity compared to the CD133-negative cells, which were mainly in the G0/G1 phase and did not express Ki67 [70].
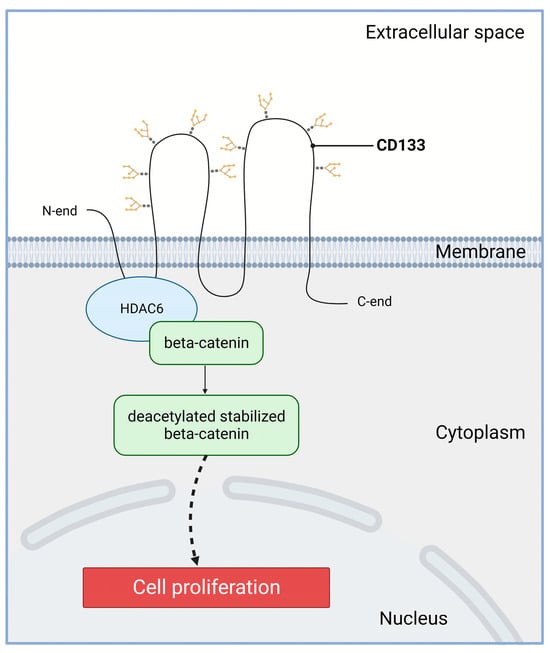
However, it is important to note that the populations of proliferating cells and the populations of CD133-positive cells were never completely identical. Therefore, though CD133 is not a marker of proliferation, it is likely involved in this process. The molecular mechanism of the CD133-dependent stimulation of proliferation may be based on the activation of the Wnt/beta-catenin signaling pathway (Figure 2). Histone deacetylase 6 (HDAC6) is able to physically bind to both the first intracellular loop domain of CD133 and β-catenin stabilizing β-catenin in a ternary complex [71]. The CD133/HDAC6 complex induces deacetylation and inhibits proteasomal degradation of beta-catenin. Next, stabilized beta-catenin enters the nucleus and activates target genes activating cell proliferation and, accordingly, accelerating tumor growth. Reduced expression of CD133 or HDAC6 leads to increased degradation of beta-catenin, which correlates with a decrease in the proliferative activity of tumor cells [71].

Figure 2. Involvement of CD133 in the Wnt/β-catenin signaling in cancer cells. Wnt—wingless-related integration site; HDAC6—histone deacetylase 6. The first intracellular loop domain of CD133 is able to physically bind to HDAC6 and β-catenin forming a ternary complex ensuring β-catenin stabilization. Created with BioRender.com.
2.2. Increased Chemo- and Radioresistance
Multiple studies revealed correlation between the CD133 expression in primary carcinomas and the resistance to chemotherapeutic drugs [72]. It was shown that CD133-positive cells of oral squamous cell carcinoma were more resistant to cisplatin [66]. Silencing of CD133 in colorectal cancer cells lead to their high sensitivity to oxaliplatin [73]. CD133-positive glioblastoma cells have been reported to be more resistant to various drugs, including temozolomide, carboplatin, paclitaxel, and etoposide [74]. In in vitro experiments, scholars found a correlation between the chemoresistance of colorectal cancer cells HT-29 and the expression of CD133. The HT-29 subline, enriched by FACS by the cells with high CD133 expression level, showed higher resistance to the protein kinase inhibitors sorafenib, sunitinib, temsirolimus, and everolimus, compared with the subline that did not contain CD133-expressing cells. A change in the sensitivity of tumor cells to mTOR inhibitors, temsirolimus and everolimus, was also found in HT-29 cells with a complete knockout of the PROM1 gene [75]. It was also shown that in tumor cells isolated from primary glioblastomas, there was an enrichment of the CD133-positive cells after exposure to ionizing radiation in vitro and in vivo, which was combined with enhanced DNA repair and reduced sensitivity to radiation-induced apoptosis [39].
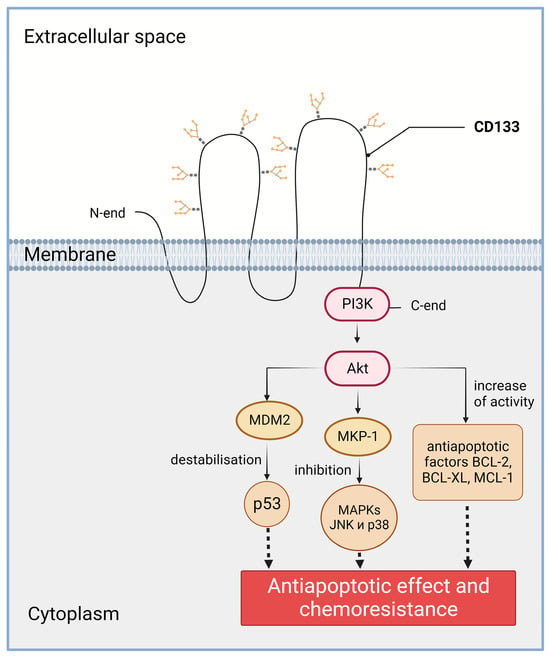
The role of CD133 in the chemo- and radioresistance of tumor cells may be related to the ability of CD133 to activate the PI3K/Akt signaling pathway (Figure 3). CD133 has a short C-terminal cytoplasmic domain with five tyrosine residues, including a tyrosine phosphorylation site. When studying glioma cells, it was found that phosphorylation of the tyrosine-828 residue of CD133 leads to activation of the PI3K (phosphoinositide 3-kinase) pathway through direct interaction of CD133 and the 85kDa subunit of PI3K [76]. Specifically, in CD133-positive cancer cells, Src kinase phosphorylates tyrosine-828 in the C-terminal cytoplasmic domain of CD133. The phosphotyrosine-828 residue interacts with p85, leading to the activation of the p110 catalytic subunit of PI3K. Activated PI3K converts PIP2 (phosphatidylinositol 4,5-bisphosphate) to PIP3 (phosphatidylinositol 3,4,5-trisphosphate). PIP3 then induces the phosphorylation and activation of Akt (also known as protein kinase B) [76]. There is also evidence that CD133 knockdown inhibits PI3K/Akt activity and increases the survival of mice in tumor cell xenotransplantation tests. CD133 activates the PI3K signaling pathway and, as a result, Akt is also activated. Activation of Akt, in turn, leads to increased activity of the BCL-2, BCL-XL, and MCL-1 anti-apoptotic factors [76].

Figure 3. Involvement of CD133 in the PI3K/Akt signaling pathway in cancer cells. PI3K—phosphoinositide 3-kinase; Akt—RAC-alpha serine/threonine-protein kinase (a.k.a. protein kinase B alpha); MDM2—E3 ubiquitin-protein ligase Mdm2; p53—transformation-related protein 53; MKP-1—protein kinase phosphatase-1; MAPKs—mitogen-activated protein kinases; JNK—c-Jun-NH(2)-terminal kinase; BCL-2—B-cell lymphoma 2 protein, apoptosis regulator; BCL-XL—anti-apoptotic protein, BCL2 family member; MCL-1—apoptosis regulator, BCL2 family member. The C-terminal cytoplasmic domain of CD133 is able to physically bind to PI3K. Created with BioRender.com.
2.3. Increased Invasion and Metastasis
Epithelial–mesenchymal transition (EMT) is the process of acquiring the mesenchymal cell phenotype by the epithelial cells. It can occur under normal physiological conditions, for example, during embryogenesis, organ formation, and wound healing [77], or during carcinogenesis [78]. Since after EMT tumor cells become more malignant, i.e., prone to tissue invasion and metastasizing [79], the role of EMT in carcinogenesis has been extensively studied, including the assessment of the involved molecules, such as E-cadherin, N-cadherin, vimentin, and others [78].
In the experiments with HT-29 cells, it has been shown that the knockdown of the epithelial marker E-cadherin, downregulated during EMT, enhances cell migration and invasiveness, upregulates EMT-associated proteins, promotes morphological transition towards mesenchymal phenotype, and boosts the expression of CSC markers CD44 and CD133 [80]. There is also evidence that CD133 itself can influence the EMT process. Thus, its knockout in LoVo colorectal cancer cells leads to a decrease in cell migration ability and invasiveness, as well as loss of the expression of vimentin, usually upregulated during EMT [81].
The correlation between the CD133 level, EMT markers, and the clinicopathological characteristics, such as invasiveness and metastases, was also established in surgical biopsies taken from patients. In isolated breast cancer cells, CD133 expression directly correlated with that of N-cadherin typically expressed in the mesenchymal cells [82]. Notably, in metastases, the expression of N-cadherin was higher compared to the primary lesions. In line with this, CD133 expression was found to inversely correlate with the epithelial E-cadherin in the specimens of ovarian cancer [82], intrahepatic cholangiocarcinoma [83], colorectal cancer [84], and lung cancer [85].
2.4. CD133 as a Target of Therapies Aimed to Eliminate the Most Malignant Cells
The role of CD133 in tumor progression is further evidenced by the results of the preclinical and clinical studies of antitumor drugs targeting the CD133 molecule (for more information, see reviews [86][87]). Such approaches include delivery systems for conventional chemotherapy drugs, as well as drugs that activate the cytotoxic mechanisms of the immune system. In particular, a chimeric drug that is a CD133-targeted DNA aptamer combined with doxorubicin showed selective killing effect in human colorectal cancer HCT116 cells expressing CD133. The in vitro and in vivo results demonstrated the high therapeutic efficacy and low toxicity of this chimera [88]. Also, recent in vitro study has shown the effective use of Fc-optimized antibodies against CD133 to induce natural killer (NK) cell reactivity [89]. In the work, engineered mAbs with Fc parts that display enhanced affinity to the CD16 Fc receptor expressed on NK cells were used as drugs in order to improve antibody-dependent cellular cytotoxicity against CD133-expressing cells.
3. Association of CD133 with Cancer Progression and Poor Prognosis
The direct correlation between CD133 expression on one hand and various clinicopathological parameters, such as overall survival, tumor stage and differentiation level, metastasis, and recurrence rate on the other, has been demonstrated for many types of malignancies. Moreover, a number of the meta-analyses have shown correlation of CD133 expression levels with high stage, tumor progression or severe course of cancer in the case of osteosarcoma [90], glioma [91], colorectal cancer [92], head and neck squamous cell carcinoma [93], hepatocellular carcinoma [94], non-small cell lung cancer [95], ovarian cancer [96], pancreatic ductal adenocarcinoma [97], gastric cancer [98], and breast cancer [99].
Despite the convincing evidence of the association between CD133 expression and the unfavorable cancer progression, the opposite was also reported. There are data indicating the absence of such a correlation or even an association of CD133 expression with a favorable disease prognosis. For illustration, in the immunohistochemical analysis of 88 glioblastoma samples, the presence of CD133 was detected in 52 cases, but a comparative analysis of the studied glioblastoma cases did not reveal a statistically significant association between the presence of CD133 and patient survival [100].
A recently published study analyzed CD133 mRNA expression in 60 colon cancer samples and found no correlation of its expression with the aggressive phenotype of primary and metastatic tumors. In primary tumors, CD133 mRNA expression did not correlate with aggressive phenotypes, and in liver metastases, it was significantly lower compared with primary tumors [101]. In another colorectal cancer study, the immunohistochemical analysis of the CD133 expression in 142 primary and 75 peritoneal lesions identified CD133 in 55% and 40% of the tumor samples, respectively. CD133 expression was not found to be associated with overall patient survival. Moreover, the disease-free survival of patients was higher in the CD133-positive group compared with the CD133-negative group. Interestingly, in this study the patient benefit from systemic chemotherapy was significantly greater in the CD133-negative group [48]. Since, as noted above, CD133 may contribute to cell chemoresistance, this result might be expected. However, in another study, Mia-Jan et al. obtained opposite results [102]. They examined 271 samples of stage II and III colorectal cancer, including 171 samples from patients who underwent adjuvant chemotherapy after surgery and 100 samples from patients without adjuvant therapy. Surprisingly, they found that among patients who received adjuvant therapy, CD133-positive tumors were associated with longer overall survival [102].
For some tumors, including renal cell carcinoma and esophageal cancer, a correlation between CD133 expression and the unfavorable disease course was not found even in meta-analyses [103][104].
4. Possible Reasons for the Discrepancies in the Data on the Association of CD133 with the Disease Severity
4.1. Different CD133 Immunodetection Techniques
The results of the CD133 detection in different studies may vary due to the variations in the methods of tumor tissue preparation for immunohistochemical staining. CD133 is known to bind to cholesterol [105] and possibly to gangliosides within lipid rafts on a plasma membrane [106][107][108]. Most raft proteins, such as glycosylphosphatidylinositol (GPI)-anchored proteins, are insoluble in Triton X-100 detergent used for membrane permeabilization due to their tight packing with cholesterol and glycolipids [109][110]. However, it was found that CD133-containing rafts could be dissolved in Triton X-100 [111]. Thus, the use of this detergent, for example, in intracellular staining, can wash CD133 away from the cell membrane, reducing its level. An alternative may be Lubrol WX, which does not dissolve the CD133-containing rafts [111].
It is known that cross-links in proteins formed as a result of exposure to formaldehyde can lead to changes in their topology and difficulty in recognizing certain epitopes by antibodies [112]. In particular, this previously impeded accurate detection of the proliferation marker Ki-67, commonly used in the clinic, in paraffin sections [113][114]. There is also an assumption that xylene, used in the deparaffinization of archival tumor tissue samples, can alter the spatial structure the membrane proteins concealing certain epitopes [115].
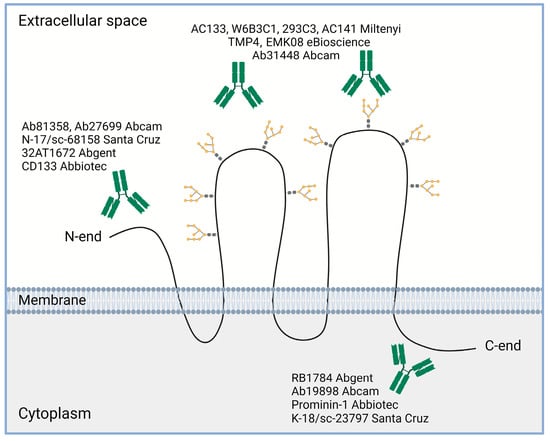
Importantly, since the initial identification of the CD133 molecule using the mouse monoclonal antibody AC133 [4], a large panel of commercial and custom antibodies was created that recognize various parts of the CD133 molecule [72] (Figure 4). Among them, the AC133, W6B3C1, 293C3, AC141 (Miltenyi, Bergisch-Gladbach, Germany), TMP4 and EMK08 (eBioscience, San Diego, CA, USA), and Ab31448 (Abcam, Cambridge, UK) antibodies are suitable for detecting the extracellular loops of the CD133 molecule; the CD133 (Abbiotec, Escondido, CA, USA), Ab81358 and Ab27699 (Abcam), 32AT1672 (Abgent, San Diego, CA, USA), and N-17/sc-68158 (Santa Cruz, Santa Cruz, CA, USA) antibodies are used to reveal the N-terminal domain of the CD133 molecule; and the Prominin-1 (Abbiotec), RB1784 (Abgent), K-18/sc-23797 (Santa Cruz), and Ab19898 (Abcam) antibodies bind to the C-terminal domain. A more complete list of existing anti-CD133 antibodies is presented in the review by Grosse-Gehling et al. [72].

Figure 4. Some examples of commercial antibodies used to recognize different domains of CD133 molecule (based on review [72]). Created with BioRender.com.
To make things more complicated, the PROM1 has five different promoters that are regulated in a tissue-dependent manner, leading to the expression of alternative CD133 splice variants [116], including a splice variant with truncated C-terminal domain [117]. Alternative splice variants may have a varying topology within the plasma membrane, also impairing their recognition by some antibodies. Apparently, the truncated splice variant will not be recognized by antibodies targeting the C-terminal domain of CD133.
4.2. Some CD133-Positive Cells Present within Tumors Are Normal, Benign Cells
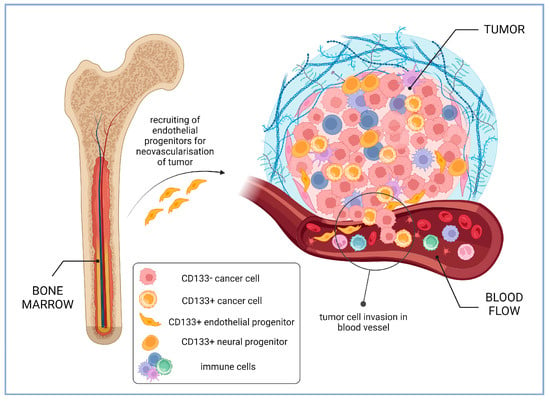
Since endothelial progenitors and other stem and progenitor cells express CD133, their integration in tumor tissue can be wrongly interpreted as the presence of CSCs. For instance, in study of glioma samples, it was revealed that CD133-positive cells present in low-grade gliomas were predominantly endothelial cells expressing CD31, while in glioblastoma samples, the majority of CD133-positive cells were of tumor origin, although CD31+CD133+ cells were also detected. It was found that the CD133 level on the surface of CD45-CD31- tumor cells inversely correlated with the patient’s survival time [118]. Perivascular niches are the predominant site of the CD133-positive cells’ localization within gliomas, and those of them enriched with CD133-positive cells are several times more numerous in the higher stage tumors [119] (Figure 5).

Figure 5. Intra-tumoral and circulating CD133-positive cells in cancer patients (based on the results of glioma studies). Higher percentage of CD133-positive endothelial progenitors recruited from bone marrow for tumor neovascularization is associated with lower patient survival [120]. Perivascular niches enriched with CD133-positive cells are several times more numerous in the higher stage gliomas [119]. CD133-positive neural progenitors in glioblastomas may contribute to a more favorable course of cancer disease [121]. Created with BioRender.com.
The benign, non-tumor CD133-positive cells can contribute to tumor progression. It was reported that, in vitro, the CD133-positive hematopoietic progenitor cells from the umbilical cord blood can invoke additional malignancy in breast cancer cells, blocking spontaneous apoptosis and stimulating the process of the epithelial–mesenchymal transition [122]. The tumor-activating effect of the CD133-positive endothelial progenitors may also explain communications stating that the VEGF-stimulated CD133-positive cancer cells induce the relapse of the hepatocellular carcinoma [123] or the migration/invasion of salivary adenoid cystic carcinoma cells [124] by inducing the vasculogenic mimicry formation. It has also been shown that CD133-positive cells have a higher ability to migrate and invade and also demonstrate higher levels of endothelial cell markers VE-cadherin, MMP-2, and MMP-9 [125].
4.3. Subcellular Localization of CD133 May Vary at Different Stages of Carcinogenesis
It has long been known that CD133 is not only associated with the cell membrane; it can also be found in the cytoplasm, including the endoplasmic reticulum and the Golgi apparatus, where its maturation occurs [2][126]. Nuclear localization of CD133 has also been demonstrated, for example, in the immunohistochemical studies of hepatocellular carcinoma samples [127] and samples of triple-negative breast cancer [10]. Nunukova et al. found atypical nuclear localization of CD133 in five rhabdomyosarcoma cell lines [9]. Moreover, a small subpopulation of cells exhibiting exclusively nuclear localization was identified. The authors verified the nuclear localization of CD133 using different types of antibodies to the extracellular loops and the C- and N-termini, and confocal as well as transmission electron microscopy. Additionally, they performed cellular fractionation followed by immunoblotting. All obtained data confirmed the presence of CD133 in the nucleus. Also, the authors noted that in addition to cells with typical membranous and exclusively nuclear localization of CD133, there were also sporadic clusters of positive signaling in the cytoplasm near the cell nucleus or very close to the nuclear envelope [9]. In a study of samples of tumor tissue and corresponding adjacent normal tissue from 239 patients with non-small cell lung cancer, nuclear localization of CD133 was also detected in addition to the cytoplasmic localization [49]. Both cytoplasmic and nuclear CD133 protein expression levels were significantly higher in tumor tissue compared to the corresponding peritumoral tissue. The expression of CD133 in the nuclei of tumor cells correlated with tumor diameter, degree of tumor differentiation, and the stage of the disease. Moreover, it was found that high nuclear CD133 expression and high cytoplasmic CD133 expression were prognostic factors for poor outcome of non-small cell lung cancer [49]. Pietrus et al. investigated the association of subcellular expression of CD133 with the clinical manifestations and outcomes of the endometrial cancer [11]. They performed an immunohistochemical study of CD133 expression in the plasma membrane, nucleus, and cytoplasm in a group of 64 patients with endometrial cancer and found that CD133 nuclear expression is directly associated with the stage of disease. Specifically, CD133 nuclear expression was increased in stages IB-IV compared to stage IA [11].
5. Conclusions
When CD133 was first suggested as a CSC marker, little was known about its function and properties. However, recent studies proved the involvement of CD133 in the regulation of proliferation, autophagy, apoptosis, and epithelial–mesenchymal transition in cancer cells. This new evidence suggests that CD133 can be a good prognostic tool for some cancer patients, since it is involved in defining the CSC phenotype of cancer cells.
Despite the abundance of data concerning the prognostic significance of CD133, this molecule has not yet found its place in clinical oncology as a biomarker. Probably, translation of the research results into clinical practice is complicated by the following reasons. First, not all CSCs express CD133. Some tumor cells maintain CSC phenotype utilizing alternative regulatory mechanisms, not involving CD133. Second, CD133 is expressed by some normal, not malignant cells present within the tumor lesion. Third, CD133 expression by CSC can be transient and depends upon the cellular microenvironment (niche) [128] or other internal and external factors like hypoxia [129]. Fourth, CD133 expression and its impact on the tumor progression may differ at the different stages of tumor growth.
References
- Immervoll, H.; Hoem, D.; Sakariassen, P.Ø.; Steffensen, O.J.; Molven, A. Expression of the “Stem Cell Marker” CD133 in Pancreas and Pancreatic Ductal Adenocarcinomas. BMC Cancer 2008, 8, 48.
- Mak, A.B.; Pehar, M.; Nixon, A.M.L.; Williams, R.A.; Uetrecht, A.C.; Puglielli, L.; Moffat, J. Post-Translational Regulation of CD133 by ATase1/ATase2-Mediated Lysine Acetylation. J. Mol. Biol. 2014, 426, 2175–2182.
- Miraglia, S.; Godfrey, W.; Yin, A.H.; Atkins, K.; Warnke, R.; Holden, J.T.; Bray, R.A.; Waller, E.K.; Buck, D.W. A Novel Five-Transmembrane Hematopoietic Stem Cell Antigen: Isolation, Characterization, and Molecular Cloning. Blood 1997, 90, 5013–5021.
- Yin, A.H.; Miraglia, S.; Zanjani, E.D.; Almeida-Porada, G.; Ogawa, M.; Leary, A.G.; Olweus, J.; Kearney, J.; Buck, D.W. AC133, a Novel Marker for Human Hematopoietic Stem and Progenitor Cells. Blood 1997, 90, 5002–5012.
- Weigmann, A.; Corbeil, D.; Hellwig, A.; Huttner, W.B. Prominin, a Novel Microvilli-Specific Polytopic Membrane Protein of the Apical Surface of Epithelial Cells, Is Targeted to Plasmalemmal Protrusions of Non-Epithelial Cells. Proc. Natl. Acad. Sci. USA 1997, 94, 12425–12430.
- Fargeas, C.A.; Florek, M.; Huttner, W.B.; Corbeil, D. Characterization of Prominin-2, a New Member of the Prominin Family of Pentaspan Membrane Glycoproteins. J. Biol. Chem. 2003, 278, 8586–8596.
- Shmelkov, S.V.; St Clair, R.; Lyden, D.; Rafii, S. AC133/CD133/Prominin-1. Int. J. Biochem. Cell Biol. 2005, 37, 715–719.
- Karbanová, J.; Lorico, A.; Bornhäuser, M.; Corbeil, D.; Fargeas, C.A. Prominin-1/CD133: Lipid Raft Association, Detergent Resistance, and Immunodetection. Stem Cells Transl. Med. 2017, 7, 155–160.
- Nunukova, A.; Neradil, J.; Skoda, J.; Jaros, J.; Hampl, A.; Sterba, J.; Veselska, R. Atypical Nuclear Localization of CD133 Plasma Membrane Glycoprotein in Rhabdomyosarcoma Cell Lines. Int. J. Mol. Med. 2015, 36, 65–72.
- Cantile, M.; Collina, F.; D’Aiuto, M.; Rinaldo, M.; Pirozzi, G.; Borsellino, C.; Franco, R.; Botti, G.; Di Bonito, M. Nuclear Localization of Cancer Stem Cell Marker CD133 in Triple-Negative Breast Cancer: A Case Report. Tumori 2013, 99, e245–e250.
- Pietrus, M.; Pitynski, K.; Waligora, M.; Milian-Ciesielska, K.; Bialon, M.; Ludwin, A.; Skrzypek, K. CD133 Expression in the Nucleus Is Associated with Endometrial Carcinoma Staging and Tumor Angioinvasion. J. Clin. Med. 2021, 10, 2144.
- Liu, Y.; Ren, S.; Xie, L.; Cui, C.; Xing, Y.; Liu, C.; Cao, B.; Yang, F.; Li, Y.; Chen, X.; et al. Mutation of N-Linked Glycosylation at Asn548 in CD133 Decreases Its Ability to Promote Hepatoma Cell Growth. Oncotarget 2015, 6, 20650–20660.
- Kania, G.; Corbeil, D.; Fuchs, J.; Tarasov, K.V.; Blyszczuk, P.; Huttner, W.B.; Boheler, K.R.; Wobus, A.M. Somatic Stem Cell Marker Prominin-1/CD133 Is Expressed in Embryonic Stem Cell-Derived Progenitors. Stem Cells Dayt. Ohio 2005, 23, 791–804.
- Corbeil, D.; Röper, K.; Hellwig, A.; Tavian, M.; Miraglia, S.; Watt, S.M.; Simmons, P.J.; Peault, B.; Buck, D.W.; Huttner, W.B. The Human AC133 Hematopoietic Stem Cell Antigen Is Also Expressed in Epithelial Cells and Targeted to Plasma Membrane Protrusions. J. Biol. Chem. 2000, 275, 5512–5520.
- Peichev, M.; Naiyer, A.J.; Pereira, D.; Zhu, Z.; Lane, W.J.; Williams, M.; Oz, M.C.; Hicklin, D.J.; Witte, L.; Moore, M.A.; et al. Expression of VEGFR-2 and AC133 by Circulating Human CD34(+) Cells Identifies a Population of Functional Endothelial Precursors. Blood 2000, 95, 952–958.
- Richardson, G.D.; Robson, C.N.; Lang, S.H.; Neal, D.E.; Maitland, N.J.; Collins, A.T. CD133, a Novel Marker for Human Prostatic Epithelial Stem Cells. J. Cell Sci. 2004, 117, 3539–3545.
- Uchida, N.; Buck, D.W.; He, D.; Reitsma, M.J.; Masek, M.; Phan, T.V.; Tsukamoto, A.S.; Gage, F.H.; Weissman, I.L. Direct Isolation of Human Central Nervous System Stem Cells. Proc. Natl. Acad. Sci. USA 2000, 97, 14720–14725.
- Torrente, Y.; Belicchi, M.; Sampaolesi, M.; Pisati, F.; Meregalli, M.; D’Antona, G.; Tonlorenzi, R.; Porretti, L.; Gavina, M.; Mamchaoui, K.; et al. Human Circulating AC133(+) Stem Cells Restore Dystrophin Expression and Ameliorate Function in Dystrophic Skeletal Muscle. J. Clin. Investig. 2004, 114, 182–195.
- Kordes, C.; Sawitza, I.; Müller-Marbach, A.; Ale-Agha, N.; Keitel, V.; Klonowski-Stumpe, H.; Häussinger, D. CD133+ Hepatic Stellate Cells Are Progenitor Cells. Biochem. Biophys. Res. Commun. 2007, 352, 410–417.
- Karbanová, J.; Missol-Kolka, E.; Fonseca, A.-V.; Lorra, C.; Janich, P.; Hollerová, H.; Jászai, J.; Ehrmann, J.; Kolář, Z.; Liebers, C.; et al. The Stem Cell Marker CD133 (Prominin-1) Is Expressed in Various Human Glandular Epithelia. J. Histochem. Cytochem. 2008, 56, 977–993.
- Karbanová, J.; Laco, J.; Marzesco, A.-M.; Janich, P.; Voborníková, M.; Mokrý, J.; Fargeas, C.A.; Huttner, W.B.; Corbeil, D. Human Prominin-1 (CD133) Is Detected in Both Neoplastic and Non-Neoplastic Salivary Gland Diseases and Released into Saliva in a Ubiquitinated Form. PLoS ONE 2014, 9, e98927.
- Maw, M.A.; Corbeil, D.; Koch, J.; Hellwig, A.; Wilson-Wheeler, J.C.; Bridges, R.J.; Kumaramanickavel, G.; John, S.; Nancarrow, D.; Röper, K.; et al. A Frameshift Mutation in Prominin (Mouse)-like 1 Causes Human Retinal Degeneration. Hum. Mol. Genet. 2000, 9, 27–34.
- Jászai, J.; Fargeas, C.A.; Florek, M.; Huttner, W.B.; Corbeil, D. Focus on Molecules: Prominin-1 (CD133). Exp. Eye Res. 2007, 85, 585–586.
- Pellacani, D.; Oldridge, E.E.; Collins, A.T.; Maitland, N.J. Prominin-1 (CD133) Expression in the Prostate and Prostate Cancer: A Marker for Quiescent Stem Cells. Adv. Exp. Med. Biol. 2013, 777, 167–184.
- Bertolini, G.; Roz, L.; Perego, P.; Tortoreto, M.; Fontanella, E.; Gatti, L.; Pratesi, G.; Fabbri, A.; Andriani, F.; Tinelli, S.; et al. Highly Tumorigenic Lung Cancer CD133+ Cells Display Stem-like Features and Are Spared by Cisplatin Treatment. Proc. Natl. Acad. Sci. USA 2009, 106, 16281–16286.
- Eramo, A.; Lotti, F.; Sette, G.; Pilozzi, E.; Biffoni, M.; Di Virgilio, A.; Conticello, C.; Ruco, L.; Peschle, C.; De Maria, R. Identification and Expansion of the Tumorigenic Lung Cancer Stem Cell Population. Cell Death Differ. 2008, 15, 504–514.
- Bellio, C.; DiGloria, C.; Foster, R.; James, K.; Konstantinopoulos, P.A.; Growdon, W.B.; Rueda, B.R. PARP Inhibition Induces Enrichment of DNA Repair-Proficient CD133 and CD117 Positive Ovarian Cancer Stem Cells. Mol. Cancer Res. MCR 2019, 17, 431–445.
- Joseph, C.; Arshad, M.; Kurozomi, S.; Althobiti, M.; Miligy, I.M.; Al-Izzi, S.; Toss, M.S.; Goh, F.Q.; Johnston, S.J.; Martin, S.G.; et al. Overexpression of the Cancer Stem Cell Marker CD133 Confers a Poor Prognosis in Invasive Breast Cancer. Breast Cancer Res. Treat. 2019, 174, 387–399.
- Shang, C.; Lang, B.; Meng, L.-R. Blocking NOTCH Pathway Can Enhance the Effect of EGFR Inhibitor through Targeting CD133+ Endometrial Cancer Cells. Cancer Biol. Ther. 2018, 19, 113–119.
- Saeednejad Zanjani, L.; Madjd, Z.; Abolhasani, M.; Andersson, Y.; Rasti, A.; Shariftabrizi, A.; Asgari, M. Cytoplasmic Expression of CD133 Stemness Marker Is Associated with Tumor Aggressiveness in Clear Cell Renal Cell Carcinoma. Exp. Mol. Pathol. 2017, 103, 218–228.
- Wang, C.; Wang, Z.; Liu, W.; Ai, Z. CD133 Promotes the Self-Renewal Capacity of Thyroid Cancer Stem Cells through Activation of Glutamate Aspartate Transporter SLC1A3 Expression. Biochem. Biophys. Res. Commun. 2019, 511, 87–91.
- Gupta, P.; Rizvi, S.Z.; Lal, N.; Gupta, V.; Srivastav, A.N.; Musa, O. Expression of CD44 and CD133 Stem Cell Markers in Squamous Cell Carcinoma of Esophagus. Indian J. Pathol. Microbiol. 2021, 64, 472–478.
- Feitosa, N.P.P.; Pereira, V.B.M.; Silva, B.G.B.; Queroz, A.V.F.; Rodrigues, B.J.; Costa, M.L.V.; Alencar, C.H.; Lima-Júnior, R.C.P.; Wong, D.V.T.; Frota, C.C.; et al. Cancerous and Non-Neoplastic Stem Cells in the Stomach Similarly Express CD44 and CD133. Acta Histochem. 2021, 123, 151787.
- Wattanawongdon, W.; Bathpho, T.S.; Tongtawee, T. Co-Expression of LGR5 and CD133 Cancer Stem Cell Predicts a Poor Prognosis in Patients With Gastric Cancer. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2021, 32, 261–268.
- Ren, F.; Sheng, W.-Q.; Du, X. CD133: A Cancer Stem Cells Marker, Is Used in Colorectal Cancers. World J. Gastroenterol. 2013, 19, 2603–2611.
- Yin, S.; Li, J.; Hu, C.; Chen, X.; Yao, M.; Yan, M.; Jiang, G.; Ge, C.; Xie, H.; Wan, D.; et al. CD133 Positive Hepatocellular Carcinoma Cells Possess High Capacity for Tumorigenicity. Int. J. Cancer 2007, 120, 1444–1450.
- Piao, L.S.; Hur, W.; Kim, T.-K.; Hong, S.W.; Kim, S.W.; Choi, J.E.; Sung, P.S.; Song, M.J.; Lee, B.-C.; Hwang, D.; et al. CD133+ Liver Cancer Stem Cells Modulate Radioresistance in Human Hepatocellular Carcinoma. Cancer Lett. 2012, 315, 129–137.
- Li, C.; Wang, C.; Xing, Y.; Zhen, J.; Ai, Z. CD133 Promotes Gallbladder Carcinoma Cell Migration through Activating Akt Phosphorylation. Oncotarget 2016, 7, 17751–17759.
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature 2006, 444, 756–760.
- Maier, A.D.; Mirian, C.; Bartek, J.; Juhler, M.; Bartkova, J.; Broholm, H.; Mathiesen, T.I. Expression of the Stem Cell Marker CD133 in Malignant Meningioma. Clin. Neuropathol. 2021, 40, 151–159.
- Xie, Y.; Huang, J.; Wu, M.; Zhou, Y. Expression of CD133 Protein in Osteosarcoma and Its Relationship with the Clinicopathological Features and Prognosis. J. Cancer Res. Ther. 2018, 14, 892–895.
- Madjd, Z.; Erfani, E.; Gheytanchi, E.; Moradi-Lakeh, M.; Shariftabrizi, A.; Asadi-Lari, M. Expression of CD133 Cancer Stem Cell Marker in Melanoma: A Systematic Review and Meta-Analysis. Int. J. Biol. Markers 2016, 31, e118–e125.
- Quan, X.X.; Hawk, N.V.; Chen, W.; Coupar, J.; Lee, S.K.; Petersen, D.W.; Meltzer, P.S.; Montemarano, A.; Braun, M.; Chen, Z.; et al. Targeting Notch1 and IKKα Enhanced NF-κB Activation in CD133+ Skin Cancer Stem Cells. Mol. Cancer Ther. 2018, 17, 2034–2048.
- Taussig, D.C.; Pearce, D.J.; Simpson, C.; Rohatiner, A.Z.; Lister, T.A.; Kelly, G.; Luongo, J.L.; Danet-Desnoyers, G.-A.H.; Bonnet, D. Hematopoietic Stem Cells Express Multiple Myeloid Markers: Implications for the Origin and Targeted Therapy of Acute Myeloid Leukemia. Blood 2005, 106, 4086–4092.
- Medina, D.J.; Abass-Shereef, J.; Walton, K.; Goodell, L.; Aviv, H.; Strair, R.K.; Budak-Alpdogan, T. Cobblestone-Area Forming Cells Derived from Patients with Mantle Cell Lymphoma Are Enriched for CD133+ Tumor-Initiating Cells. PLoS ONE 2014, 9, e91042.
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A Human Colon Cancer Cell Capable of Initiating Tumour Growth in Immunodeficient Mice. Nature 2007, 445, 106–110.
- Hermann, P.C.; Huber, S.L.; Herrler, T.; Aicher, A.; Ellwart, J.W.; Guba, M.; Bruns, C.J.; Heeschen, C. Distinct Populations of Cancer Stem Cells Determine Tumor Growth and Metastatic Activity in Human Pancreatic Cancer. Cell Stem Cell 2007, 1, 313–323.
- Nagata, H.; Ishihara, S.; Kishikawa, J.; Sonoda, H.; Murono, K.; Emoto, S.; Kaneko, M.; Sasaki, K.; Otani, K.; Nishikawa, T.; et al. CD133 Expression Predicts Post-Operative Recurrence in Patients with Colon Cancer with Peritoneal Metastasis. Int. J. Oncol. 2018, 52, 721–732.
- Huang, M.; Zhu, H.; Feng, J.; Ni, S.; Huang, J. High CD133 Expression in the Nucleus and Cytoplasm Predicts Poor Prognosis in Non-Small Cell Lung Cancer. Dis. Markers 2015, 2015, 986095.
- Park, E.K.; Lee, J.C.; Park, J.W.; Bang, S.Y.; Yi, S.A.; Kim, B.K.; Park, J.H.; Kwon, S.H.; You, J.S.; Nam, S.W.; et al. Transcriptional Repression of Cancer Stem Cell Marker CD133 by Tumor Suppressor P53. Cell Death Dis. 2015, 6, e1964.
- Liu, T.T.; Li, X.F.; Wang, L.; Yang, J.L. CD133 Expressionand Clinicopathologic Significance in Benign and Malignant Breast Lesions. Cancer Biomark. Sect. Dis. Markers 2020, 28, 293–299.
- Kostovski, O.; Antovic, S.; Trajkovski, G.; Kostovska, I.; Jovanovic, R.; Jankulovski, N. High Expression of CD133—Stem Cell Marker for Prediction of Clinically Agressive Type of Colorectal Cancer. Pol. Przegl. Chir. 2020, 92, 9–14.
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a Cancer Stem Cell in Human Brain Tumors. Cancer Res. 2003, 63, 5821–5828.
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of Human Brain Tumour Initiating Cells. Nature 2004, 432, 396–401.
- Atashzar, M.R.; Baharlou, R.; Karami, J.; Abdollahi, H.; Rezaei, R.; Pourramezan, F.; Zoljalali Moghaddam, S.H. Cancer Stem Cells: A Review from Origin to Therapeutic Implications. J. Cell. Physiol. 2020, 235, 790–803.
- Hsu, H.-S.; Huang, P.-I.; Chang, Y.-L.; Tzao, C.; Chen, Y.-W.; Shih, H.-C.; Hung, S.-C.; Chen, Y.-C.; Tseng, L.-M.; Chiou, S.-H. Cucurbitacin I Inhibits Tumorigenic Ability and Enhances Radiochemosensitivity in Nonsmall Cell Lung Cancer-Derived CD133-Positive Cells. Cancer 2011, 117, 2970–2985.
- Curley, M.D.; Therrien, V.A.; Cummings, C.L.; Sergent, P.A.; Koulouris, C.R.; Friel, A.M.; Roberts, D.J.; Seiden, M.V.; Scadden, D.T.; Rueda, B.R.; et al. CD133 Expression Defines a Tumor Initiating Cell Population in Primary Human Ovarian Cancer. Stem Cells Dayt. Ohio 2009, 27, 2875–2883.
- Attia, S.; Atwan, N.; Arafa, M.; Shahin, R.A. Expression of CD133 as a Cancer Stem Cell Marker in Invasive Gastric Carcinoma. Pathologica 2019, 111, 18–23.
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and Expansion of Human Colon-Cancer-Initiating Cells. Nature 2007, 445, 111–115.
- Shi, C.-J.; Gao, J.; Wang, M.; Wang, X.; Tian, R.; Zhu, F.; Shen, M.; Qin, R.-Y. CD133+ Gallbladder Carcinoma Cells Exhibit Self-Renewal Ability and Tumorigenicity. World J. Gastroenterol. WJG 2011, 17, 2965–2971.
- Chu, P.-Y.; Hu, F.-W.; Yu, C.-C.; Tsai, L.-L.; Yu, C.-H.; Wu, B.-C.; Chen, Y.-W.; Huang, P.-I.; Lo, W.-L. Epithelial-Mesenchymal Transition Transcription Factor ZEB1/ZEB2 Co-Expression Predicts Poor Prognosis and Maintains Tumor-Initiating Properties in Head and Neck Cancer. Oral Oncol. 2013, 49, 34–41.
- Bayin, N.S.; Modrek, A.S.; Dietrich, A.; Lebowitz, J.; Abel, T.; Song, H.-R.; Schober, M.; Zagzag, D.; Buchholz, C.J.; Chao, M.V.; et al. Selective Lentiviral Gene Delivery to CD133-Expressing Human Glioblastoma Stem Cells. PLoS ONE 2014, 9, e116114.
- Suvà, M.-L.; Riggi, N.; Stehle, J.-C.; Baumer, K.; Tercier, S.; Joseph, J.-M.; Suvà, D.; Clément, V.; Provero, P.; Cironi, L.; et al. Identification of Cancer Stem Cells in Ewing’s Sarcoma. Cancer Res. 2009, 69, 1776–1781.
- Kim, Y.S.; Kaidina, A.M.; Chiang, J.-H.; Yarygin, K.N.; Lupatov, A.Y. Cancer Stem Cell Molecular Markers Verified in Vivo. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. 2017, 11, 43–54.
- Suvorov, R.E.; Kim, Y.S.; Gisina, A.M.; Chiang, J.H.; Yarygin, K.N.; Lupatov, A.Y. Surface Molecular Markers of Cancer Stem Cells: Computation Analysis of Full-Text Scientific Articles. Bull. Exp. Biol. Med. 2018, 166, 135–140.
- Ma, Z.; Zhang, C.; Liu, X.; Fang, F.; Liu, S.; Liao, X.; Tao, S.; Mai, H. Characterisation of a Subpopulation of CD133+ Cancer Stem Cells from Chinese Patients with Oral Squamous Cell Carcinoma. Sci. Rep. 2020, 10, 8875.
- Hur, W.; Ryu, J.Y.; Kim, H.U.; Hong, S.W.; Lee, E.B.; Lee, S.Y.; Yoon, S.K. Systems Approach to Characterize the Metabolism of Liver Cancer Stem Cells Expressing CD133. Sci. Rep. 2017, 7, 45557.
- Gisina, A.M.; Kim, Y.S.; Potashnikova, D.M.; Tvorogova, A.V.; Yarygin, K.N.; Lupatov, A.Y. Proliferative Activity of Colorectal Cancer Cells with Different Levels of CD133 Expression. Bull. Exp. Biol. Med. 2019, 167, 541–545.
- Kim, Y.S.; Potashnikova, D.M.; Gisina, A.M.; Kholodenko, I.V.; Kopylov, A.T.; Tikhonova, O.V.; Kurbatov, L.K.; Saidova, A.A.; Tvorogova, A.V.; Kholodenko, R.V.; et al. TRIM28 Is a Novel Regulator of CD133 Expression Associated with Cancer Stem Cell Phenotype. Int. J. Mol. Sci. 2022, 23, 9874.
- Tirino, V.; Desiderio, V.; d’Aquino, R.; Francesco, F.D.; Pirozzi, G.; Galderisi, U.; Cavaliere, C.; Rosa, A.D.; Papaccio, G. Detection and Characterization of CD133+ Cancer Stem Cells in Human Solid Tumours. PLoS ONE 2008, 3, e3469.
- Mak, A.B.; Nixon, A.M.L.; Kittanakom, S.; Stewart, J.M.; Chen, G.I.; Curak, J.; Gingras, A.-C.; Mazitschek, R.; Neel, B.G.; Stagljar, I.; et al. Regulation of CD133 by HDAC6 Promotes β-Catenin Signaling to Suppress Cancer Cell Differentiation. Cell Rep. 2012, 2, 951–963.
- Grosse-Gehling, P.; Fargeas, C.A.; Dittfeld, C.; Garbe, Y.; Alison, M.R.; Corbeil, D.; Kunz-Schughart, L.A. CD133 as a Biomarker for Putative Cancer Stem Cells in Solid Tumours: Limitations, Problems and Challenges. J. Pathol. 2013, 229, 355–378.
- Asadzadeh, Z.; Mansoori, B.; Mohammadi, A.; Kazemi, T.; Mokhtarzadeh, A.; Shanehbandi, D.; Hemmat, N.; Derakhshani, A.; Brunetti, O.; Safaei, S.; et al. The Combination Effect of Prominin1 (CD133) Suppression and Oxaliplatin Treatment in Colorectal Cancer Therapy. Biomed. Pharmacother. 2021, 137, 111364.
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of Gene Expression and Chemoresistance of CD133+ Cancer Stem Cells in Glioblastoma. Mol. Cancer 2006, 5, 67.
- Kholodenko, I.V.; Kim, Y.S.; Gisina, A.M.; Lupatov, A.Y.; Kholodenko, R.V.; Yarygin, K.N. Analysis of the Correlation between CD133 Expression on Human Colorectal Adenocarcinoma Cells HT-29 and Their Resistance to Chemotherapeutic Drugs. Bull. Exp. Biol. Med. 2021, 171, 156–163.
- Wei, Y.; Jiang, Y.; Zou, F.; Liu, Y.; Wang, S.; Xu, N.; Xu, W.; Cui, C.; Xing, Y.; Liu, Y.; et al. Activation of PI3K/Akt Pathway by CD133-P85 Interaction Promotes Tumorigenic Capacity of Glioma Stem Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 6829–6834.
- Kim, D.H.; Xing, T.; Yang, Z.; Dudek, R.; Lu, Q.; Chen, Y.-H. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J. Clin. Med. 2017, 7, 1.
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773.
- Pearson, G.W. Control of Invasion by Epithelial-to-Mesenchymal Transition Programs during Metastasis. J. Clin. Med. 2019, 8, 646.
- Saltanatpour, Z.; Johari, B.; Alizadeh, A.; Lotfinia, M.; Majidzadeh-A, K.; Nikbin, B.; Kadivar, M. Enrichment of Cancer Stem-like Cells by the Induction of Epithelial-Mesenchymal Transition Using Lentiviral Vector Carrying E-Cadherin shRNA in HT29 Cell Line. J. Cell. Physiol. 2019, 234, 22935–22946.
- Li, W.; Cho, M.-Y.; Lee, S.; Jang, M.; Park, J.; Park, R. CRISPR-Cas9 Mediated CD133 Knockout Inhibits Colon Cancer Invasion through Reduced Epithelial-Mesenchymal Transition. PLoS ONE 2019, 14, e0220860.
- Yu, L.; Zhou, L.; Wu, S.; Song, W.; Cheng, Z.; Guo, B. Expressions of CD133, E-cadherin, and Snail in epithelial ovarian cancer and their clinicopathologic and prognostic implications. Nan Fang Yi Ke Da Xue Xue Bao 2015, 35, 1297–1302.
- Cai, X.; Li, J.; Yuan, X.; Xiao, J.; Dooley, S.; Wan, X.; Weng, H.; Lu, L. CD133 Expression in Cancer Cells Predicts Poor Prognosis of Non-Mucin Producing Intrahepatic Cholangiocarcinoma. J. Transl. Med. 2018, 16, 50.
- Sun, W.; Dou, J.; Zhang, L.; Qiao, L.; Shen, N.; Gao, W. Expression of CD133, E-Cadherin and WWOX in Colorectal Cancer and Related Analysis. Pak. J. Med. Sci. 2017, 33, 425–429.
- Su, Y.-J.; Chang, Y.-W.; Lin, W.-H.; Liang, C.-L.; Lee, J.-L. An Aberrant Nuclear Localization of E-Cadherin Is a Potent Inhibitor of Wnt/β-Catenin-Elicited Promotion of the Cancer Stem Cell Phenotype. Oncogenesis 2015, 4, e157.
- Pospieszna, J.; Dams-Kozlowska, H.; Udomsak, W.; Murias, M.; Kucinska, M. Unmasking the Deceptive Nature of Cancer Stem Cells: The Role of CD133 in Revealing Their Secrets. Int. J. Mol. Sci. 2023, 24, 10910.
- Ullah, M.; Pocard, M.; Mirshahi, M. CD133 Clinical Trials: Safety and Efficacy. J. Regen. Med. 2019, 8, 2.
- Li, W.; Wang, Z.; Gao, T.; Sun, S.; Xu, M.; Pei, R. Selection of CD133-Targeted DNA Aptamers for the Efficient and Specific Therapy of Colorectal Cancer. J. Mater. Chem. B 2022, 10, 2057–2066.
- Riegg, F.; Lutz, M.S.; Schmied, B.J.; Heitmann, J.S.; Queudeville, M.; Lang, P.; Jung, G.; Salih, H.R.; Märklin, M. An Fc-Optimized CD133 Antibody for Induction of NK Cell Reactivity against B Cell Acute Lymphoblastic Leukemia. Cancers 2021, 13, 1632.
- Xu, N.; Kang, Y.; Wang, W.; Zhou, J. The Prognostic Role of CD133 Expression in Patients with Osteosarcoma. Clin. Exp. Med. 2020, 20, 261–267, doi:10.1007/s10238-020-00607-6.
- Han, M.; Guo, L.; Zhang, Y.; Huang, B.; Chen, A.; Chen, W.; Liu, X.; Sun, S.; Wang, K.; Liu, A.; et al. Clinicopathological and Prognostic Significance of CD133 in Glioma Patients: A Meta-Analysis. Mol. Neurobiol. 2016, 53, 720–727, doi:10.1007/s12035-014-9018-9.
- Zhao, Y.; Peng, J.; Zhang, E.; Jiang, N.; Li, J.; Zhang, Q.; Zhang, X.; Niu, Y. CD133 Expression May Be Useful as a Prognostic Indicator in Colorectal Cancer, a Tool for Optimizing Therapy and Supportive Evidence for the Cancer Stem Cell Hypothesis: A Meta-Analysis. Oncotarget 2016, 7, 10023–10036, doi:10.18632/oncotarget.7054.
- Fan, Z.; Li, M.; Chen, X.; Wang, J.; Liang, X.; Wang, H.; Wang, Z.; Cheng, B.; Xia, J. Prognostic Value of Cancer Stem Cell Markers in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Sci. Rep. 2017, 7, 43008, doi:10.1038/srep43008.
- Zhong, C.; Wu, J.-D.; Fang, M.-M.; Pu, L.-Y. Clinicopathological Significance and Prognostic Value of the Expression of the Cancer Stem Cell Marker CD133 in Hepatocellular Carcinoma: A Meta-Analysis. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2015, 36, 7623–7630, doi:10.1007/s13277-015-3487-y.
- Chen, E.; Zeng, Z.; Bai, B.; Zhu, J.; Song, Z. The Prognostic Value of CSCs Biomarker CD133 in NSCLC: A Meta-Analysis. Oncotarget 2016, 7, 56526–56539, doi:10.18632/oncotarget.10964.
- Tao, Y.; Li, H.; Huang, R.; Mo, D.; Zeng, T.; Fang, M.; Li, M. Clinicopathological and Prognostic Significance of Cancer Stem Cell Markers in Ovarian Cancer Patients: Evidence from 52 Studies. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 1716–1726, doi:10.1159/000489586.
- Li, X.; Zhao, H.; Gu, J.; Zheng, L. Prognostic Value of Cancer Stem Cell Marker CD133 Expression in Pancreatic Ductal Adenocarcinoma (PDAC): A Systematic Review and Meta-Analysis. Int. J. Clin. Exp. Pathol. 2015, 8, 12084–12092.
- Yiming, L.; Yunshan, G.; Bo, M.; Yu, Z.; Tao, W.; Gengfang, L.; Dexian, F.; Shiqian, C.; Jianli, J.; Juan, T.; et al. CD133 Overexpression Correlates with Clinicopathological Features of Gastric Cancer Patients and Its Impact on Survival: A Systematic Review and Meta-Analysis. Oncotarget 2015, 6, 42019–42027.
- Li, Z.; Yin, S.; Zhang, L.; Liu, W.; Chen, B.; Xing, H. Clinicopathological Characteristics and Prognostic Value of Cancer Stem Cell Marker CD133 in Breast Cancer: A Meta-Analysis. OncoTargets Ther. 2017, 10, 859–870, doi:10.2147/OTT.S124733.
- Kim, K.-J.; Lee, K.-H.; Kim, H.-S.; Moon, K.-S.; Jung, T.-Y.; Jung, S.; Lee, M.-C. The Presence of Stem Cell Marker-Expressing Cells Is Not Prognostically Significant in Glioblastomas. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2011, 31, 494–502.
- Kaida, T.; Fujiyama, Y.; Soeno, T.; Yokota, M.; Nakamoto, S.; Goto, T.; Watanabe, A.; Okuno, K.; Nie, Y.; Fujino, S.; et al. Less Demand on Stem Cell Marker-Positive Cancer Cells May Characterize Metastasis of Colon Cancer. PLoS ONE 2023, 18, e0277395.
- Mia-Jan, K.; Jung, S.Y.; Kim, I.-Y.; Oh, S.S.; Choi, E.; Chang, S.J.; Kang, T.Y.; Cho, M.-Y. CD133 Expression Is Not an Independent Prognostic Factor in Stage II and III Colorectal Cancer but May Predict the Better Outcome in Patients with Adjuvant Therapy. BMC Cancer 2013, 13, 166.
- Cheng, B.; Yang, G.; Jiang, R.; Cheng, Y.; Yang, H.; Pei, L.; Qiu, X. Cancer Stem Cell Markers Predict a Poor Prognosis in Renal Cell Carcinoma: A Meta-Analysis. Oncotarget 2016, 7, 65862–65875, doi:10.18632/oncotarget.11672.
- Kamil Mohammed Al-Mosawi, A.; Cheshomi, H.; Hosseinzadeh, A.; M Matin, M. Prognostic and Clinical Value of CD44 and CD133 in Esophageal Cancer: A Systematic Review and Meta-Analysis. Iran. J. Allergy Asthma Immunol. 2020, 19, 105–116, doi:10.18502/ijaai.v19i2.2756.
- Marzesco, A.-M.; Janich, P.; Wilsch-Bräuninger, M.; Dubreuil, V.; Langenfeld, K.; Corbeil, D.; Huttner, W.B. Release of Extracellular Membrane Particles Carrying the Stem Cell Marker Prominin-1 (CD133) from Neural Progenitors and Other Epithelial Cells. J. Cell Sci. 2005, 118, 2849–2858.
- Janich, P.; Corbeil, D. GM1 and GM3 Gangliosides Highlight Distinct Lipid Microdomains within the Apical Domain of Epithelial Cells. FEBS Lett. 2007, 581, 1783–1787.
- Taïeb, N.; Maresca, M.; Guo, X.-J.; Garmy, N.; Fantini, J.; Yahi, N. The First Extracellular Domain of the Tumour Stem Cell Marker CD133 Contains an Antigenic Ganglioside-Binding Motif. Cancer Lett. 2009, 278, 164–173.
- Fargeas, C.A.; Karbanová, J.; Jászai, J.; Corbeil, D. CD133 and Membrane Microdomains: Old Facets for Future Hypotheses. World J. Gastroenterol. 2011, 17, 4149–4152.
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The Mystery of Membrane Organization: Composition, Regulation and Roles of Lipid Rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374.
- Lingwood, D.; Binnington, B.; Róg, T.; Vattulainen, I.; Grzybek, M.; Coskun, U.; Lingwood, C.A.; Simons, K. Cholesterol Modulates Glycolipid Conformation and Receptor Activity. Nat. Chem. Biol. 2011, 7, 260–262.
- Röper, K.; Corbeil, D.; Huttner, W.B. Retention of Prominin in Microvilli Reveals Distinct Cholesterol-Based Lipid Micro-Domains in the Apical Plasma Membrane. Nat. Cell Biol. 2000, 2, 582–592.
- Krenacs, L.; Krenacs, T.; Stelkovics, E.; Raffeld, M. Heat-Induced Antigen Retrieval for Immunohistochemical Reactions in Routinely Processed Paraffin Sections. Methods Mol. Biol. Clifton NJ 2010, 588, 103–119.
- Yerushalmi, R.; Woods, R.; Ravdin, P.M.; Hayes, M.M.; Gelmon, K.A. Ki67 in Breast Cancer: Prognostic and Predictive Potential. Lancet Oncol. 2010, 11, 174–183.
- Royce, M.; Osgood, C.; Mulkey, F.; Bloomquist, E.; Pierce, W.F.; Roy, A.; Kalavar, S.; Ghosh, S.; Philip, R.; Rizvi, F.; et al. FDA Approval Summary: Abemaciclib With Endocrine Therapy for High-Risk Early Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1155–1162.
- Kandyala, R.; Raghavendra, S.P.C.; Rajasekharan, S.T. Xylene: An Overview of Its Health Hazards and Preventive Measures. J. Oral Maxillofac. Pathol. JOMFP 2010, 14, 1–5.
- Shmelkov, S.V.; Jun, L.; St Clair, R.; McGarrigle, D.; Derderian, C.A.; Usenko, J.K.; Costa, C.; Zhang, F.; Guo, X.; Rafii, S. Alternative Promoters Regulate Transcription of the Gene That Encodes Stem Cell Surface Protein AC133. Blood 2004, 103, 2055–2061.
- Fargeas, C.A.; Joester, A.; Missol-Kolka, E.; Hellwig, A.; Huttner, W.B.; Corbeil, D. Identification of Novel Prominin-1/CD133 Splice Variants with Alternative C-Termini and Their Expression in Epididymis and Testis. J. Cell Sci. 2004, 117, 4301–4311.
- Rebetz, J.; Tian, D.; Persson, A.; Widegren, B.; Salford, L.G.; Englund, E.; Gisselsson, D.; Fan, X. Glial Progenitor-like Phenotype in Low-Grade Glioma and Enhanced CD133-Expression and Neuronal Lineage Differentiation Potential in High-Grade Glioma. PLoS ONE 2008, 3, e1936.
- Christensen, K.; Schrøder, H.D.; Kristensen, B.W. CD133 Identifies Perivascular Niches in Grade II–IV Astrocytomas. J. Neurooncol. 2008, 90, 157–170.
- Greenfield, J.P.; Jin, D.K.; Young, L.M.; Christos, P.J.; Abrey, L.; Rafii, S.; Gutin, P.H. Surrogate Markers Predict Angiogenic Potential and Survival in Patients with Glioblastoma Multiforme. Neurosurgery 2009, 64, 819–826; discussion 826–827.
- Pallini, R.; Ricci-Vitiani, L.; Montano, N.; Mollinari, C.; Biffoni, M.; Cenci, T.; Pierconti, F.; Martini, M.; De Maria, R.; Larocca, L.M. Expression of the Stem Cell Marker CD133 in Recurrent Glioblastoma and Its Value for Prognosis. Cancer 2011, 117, 162–174.
- Zhang, Z.; Zheng, Q.; Liu, Y.; Sun, L.; Han, P.; Wang, R.; Zhao, J.; Hu, S.; Zhao, X. Human CD133-Positive Hematopoietic Progenitor Cells Enhance the Malignancy of Breast Cancer Cells. BMC Cancer 2020, 20, 1158.
- Liu, K.; Hao, M.; Ouyang, Y.; Zheng, J.; Chen, D. CD133+ Cancer Stem Cells Promoted by VEGF Accelerate the Recurrence of Hepatocellular Carcinoma. Sci. Rep. 2017, 7, 41499.
- Wang, D.; Guo, Y.; Li, Y.; Li, W.; Zheng, X.; Xia, H.; Mao, Q. Detection of CD133 Expression in U87 Glioblastoma Cells Using a Novel Anti-CD133 Monoclonal Antibody. Oncol. Lett. 2015, 9, 2603–2608.
- Wang, S.-S.; Gao, X.-L.; Liu, X.; Gao, S.-Y.; Fan, Y.-L.; Jiang, Y.-P.; Ma, X.-R.; Jiang, J.; Feng, H.; Chen, Q.-M.; et al. CD133+ Cancer Stem-like Cells Promote Migration and Invasion of Salivary Adenoid Cystic Carcinoma by Inducing Vasculogenic Mimicry Formation. Oncotarget 2016, 7, 29051–29062.
- Florek, M.; Haase, M.; Marzesco, A.-M.; Freund, D.; Ehninger, G.; Huttner, W.B.; Corbeil, D. Prominin-1/CD133, a Neural and Hematopoietic Stem Cell Marker, Is Expressed in Adult Human Differentiated Cells and Certain Types of Kidney Cancer. Cell Tissue Res. 2005, 319, 15–26.
- Zenali, M.J.; Tan, D.; Li, W.; Dhingra, S.; Brown, R.E. Stemness Characteristics of Fibrolamellar Hepatocellular Carcinoma: Immunohistochemical Analysis with Comparisons to Conventional Hepatocellular Carcinoma. Ann. Clin. Lab. Sci. 2010, 40, 126–134.
- Calabrese, C.; Poppleton, H.; Kocak, M.; Hogg, T.L.; Fuller, C.; Hamner, B.; Oh, E.Y.; Gaber, M.W.; Finklestein, D.; Allen, M.; et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell 2007, 11, 69–82.
- Iida, H.; Suzuki, M.; Goitsuka, R.; Ueno, H. Hypoxia Induces CD133 Expression in Human Lung Cancer Cells by Up-Regulation of OCT3/4 and SOX2. Int. J. Oncol. 2012, 40, 71–79.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
532
Revisions:
2 times
(View History)
Update Date:
27 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





