Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paula Tilley | -- | 2779 | 2023-12-22 03:37:37 | | | |
| 2 | Lindsay Dong | Meta information modification | 2779 | 2023-12-22 03:53:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Simões, J.; Tilley, P. Decision Making in Severe Equine Asthma. Encyclopedia. Available online: https://encyclopedia.pub/entry/53053 (accessed on 07 February 2026).
Simões J, Tilley P. Decision Making in Severe Equine Asthma. Encyclopedia. Available at: https://encyclopedia.pub/entry/53053. Accessed February 07, 2026.
Simões, Joana, Paula Tilley. "Decision Making in Severe Equine Asthma" Encyclopedia, https://encyclopedia.pub/entry/53053 (accessed February 07, 2026).
Simões, J., & Tilley, P. (2023, December 22). Decision Making in Severe Equine Asthma. In Encyclopedia. https://encyclopedia.pub/entry/53053
Simões, Joana and Paula Tilley. "Decision Making in Severe Equine Asthma." Encyclopedia. Web. 22 December, 2023.
Copy Citation
Decision making consists of gathering quality data in order to correctly assess a situation and determine the best course of action. This process is a fundamental part of medicine and is what enables practitioners to accurately diagnose diseases and select appropriate treatment protocols. Despite severe equine asthma (SEA) being a highly prevalent lower respiratory disease amongst equids, clinicians still struggle with the optimization of routine diagnostic procedures. The use of several ancillary diagnostic tests has been reported for disease identification and monitoring, but many are only suitable for research purposes or lack practicality for everyday use.
equine
severe equine asthma
diagnosis
clinical history
1. Introduction
Severe equine asthma (SEA) is a chronic insidious respiratory disease which commonly affects mature adult horses [1][2]. Its estimated prevalence in the northern hemisphere is 20%, but the number of affected individuals continues to rise [3][4][5].
The precise immunological pathways of this multifactorial disease are complex and not yet fully understood [6][7][8][9][10][11][12], but it is known that when susceptible individuals are exposed to high concentrations of respirable particles they develop inflammation, bronchospasm and airway hyperreactivity [13][14][15][16]. Consequently these animals develop increased respiratory effort at rest, cough and nasal discharge which, depending on inflammation severity, may impact athletic performance and the horse’s well-being [17][18].
According to the type of inflammatory triggers associated with SEA exacerbation, two major disease phenotypes have been described [1]. The stable-associated SEA mainly involves exposure to organic respirable particles found indoors during the colder months, such as in bedding materials, hay and straw [19][20][21], whilst the pasture-associated SEA occurs in animals kept at pasture during the warmer season due to exposure to pollen [22][23][24].
However, inflammation can be triggered by a large number of molecules with a synergistic effect, such as LPS, pollen, mites, fungi spores, or even plastic particles, which can be found in the horses’ habitat [19][20][21][25][26][27][28]. Because antigen avoidance can be extremely difficult to achieve, affected animals tend to present recurrent episodes of disease exacerbation [29].
2. Diagnosis
In evidence-based medicine, reaching a correct diagnosis is paramount to produce individualized patient care and optimize clinical outcome [30]. Every patient is unique, as their medical conditions can vary in severity, underlying causes, and treatment response. Thus, by accurately identifying the patient’s condition, veterinarians can tailor their treatment plans to address the specific needs of the animal and their owner, taking into account risks and potential benefits, ultimately maximizing the chances of therapeutic success and animal well-being [29][31]. Therefore, diagnosis must be sought using the most up-to-date research and clinical guidelines in the decision-making process. This approach ensures that patients are subjected to the most relevant clinical interventions and receive treatments that have been proven effective [30][32]. Also, regarding the practitioner, evidence-based medicine is also the best way to efficiently allocate resources and contribute to continuous learning and improvement in healthcare practice.
2.1. History, Clinical Signs and Clinical Scores
Severely asthmatic horses will have a history of recurrent respiratory disease with clinical signs being triggered by exposure to specific environmental factors [1]. Often these signs will be seasonal with coughing or labored breathing occurring during spring or autumn. Some horses will also develop clinical signs when performing physical exercise in dusty arenas or when moved to stables with poor air quality [33][34] which is frequently associated with dusty bedding materials, hay or straw and poor ventilation [35][36][37]. Thus, it is important to carefully examine the horses’ environment but also to inquire about any recently made changes, since some owners may have decided to alter the animals’ environment but have forgotten to report it during the initial consultation. Most commonly, owners will report that in response to being fed poor quality hay the horse will exhibit bouts of cough [38][39]. Two different phenotypes have been reported in SEA which means that asthmatic horses can develop clinical signs when exposed to stable environmental conditions (fungal spores, organic dust, mites, endotoxins, among others) or when exposed to a pasture environment (pollen and fungal spores) [4][19][40][41]. Identifying the horses’ phenotype will determine the best treatment protocol for these animals, and intradermal testing can be useful for constructing an individualized allergen eviction protocol [42][43].
The clinical signs associated with SEA are quite distinctive [39][44], which can lead to the temptation of making a presumptive diagnosis based only on the patient’s clinical history and physical examination. Nonetheless, this should be discouraged since it can lead to an erroneous diagnosis especially in horses examined during disease remission or with low grade airway hyperreactivity.
Severely asthmatic horses have a history of chronic and persistent cough, which can be seasonal, associated with stabling and hay feeding or pasture [45]. Close inspection of the affected horses’ living quarters and feed will normally reveal the triggering factor.
Airway inflammation, hyperreactivity and obstruction result in cough, exercise intolerance, increased respiratory effort at rest and nasal discharge [17][43][46], and the severity of the exhibited clinical signs tend to correlate with the degree and persistence of the inflammatory process [18][47][48].
Cough is usually the first clinical sign reported by owners and although the onset of exercise intolerance precedes it [49], the latter is not always reported early on. This could be related to the expected athletic level of the affected horse, since low grade inflammation may not impair the performance of less strenuous physical activities [50]. With disease progression the severity of cough increases and paroxysmal bouts of cough are typically observed [39][51].
The associated airway obstruction results in overt respiratory distress with consequent modification of the respiratory pattern, characterized by a short inspiration followed by prolonged exhalation and abdominal lift [52]. Affected animals present increased abdominal effort, with consequent hypertrophy of the external oblique muscles (‘heave line’) and nasal flaring as an attempt to reduce upper airway resistance [17][18][47][53].
Changes in mucus rheology along with its decreased clearance result in the accumulation of secretions in the tracheobronchial tree [54][55]. However, due to the regular swallowing of these secretions, horses only exhibit nasal discharge occasionally [56].
Thoracic auscultation of affected animals will reveal increased bronchovesicular sounds, end-expiratory wheezes due to airway narrowing, and inspiratory crackles caused by the opening of collapsed airways. An expanded pulmonary field can also be auscultated due to lung hyperinflation, secondary to air entrapment in the alveoli [1][2][57]. In some cases, lung auscultation will be unusually silent despite the horse exhibiting substantial respiratory effort due to a remarkable compromise of airflow associated with significant airway obstruction [57].
Loss of body mass and cachexia can also occur in horses with extreme respiratory distress due to a combination of reduced food intake and increased energy expenditure to overcome the expiratory obstruction [50][58][59].
However, these scores alone can be insufficient, since mild or subclinical cases of severe equine asthma have little or no clinical signs despite maintaining some degree of airway inflammation and hyperreactivity [39][44][45][60]. Although scores based on nasal flaring and abdominal effort correlate with airway dysfunction, exercise intolerance does not, and cough may not occur during disease remission [47][53][61].
The main limitation of clinical sign scores is therefore the correct diagnosis of severely asthmatic horses when in disease remission, making other ancillary diagnostic tests crucial for accurate disease characterization and exclusion of other alternative diagnoses [44][46][53]. Nonetheless, they can be useful in the initial triage and for the continuous assessment of treatment response, without resorting to more invasive diagnostic methods.
Furthermore, methods like the Horse Owner Assessed Respiratory Signs Index (HOARSI) [39][46], and the Visual Analog Scale (VAS) [62], can be particularly useful for triage since they rely on information reported by horse owners to help identify the presence of respiratory disease. The HOARSI questionnaire scoring system is based on clinical history and on cough frequency, nasal discharge, breathing at rest and the horse’s performance [15].
2.2. Diagnostic Imaging
Radiography and ultrasonography are routinely used ancillary diagnostic tests in equine ambulatory practice, since both are non-invasive and well accepted by horses [63][64].
In SEA, the radiographic findings have been found to correlate with the clinical signs and disease severity [17]. SEA-affected horses exhibit lung pattern changes, such as increased bronchovascular, interstitial and bronchial interstitial lung patterns, along with thickening of tracheal and bronchial walls [17][65]. In extreme cases, when disease progression results in lung remodeling, interstitial infiltration, increased lung radiopacity and bronchiectasis can also be observed [66][67].
Ultrasound examination allows the clinician to attain more detailed information about the surface of the lung, including the assessment of effusion and its characteristics. However the most common ultrasonographic finding in severely asthmatic horses is the presence of comet tails which are non-specific artifacts associated with inflammation [68][69][70].
2.3. Endoscopy
Respiratory endoscopy alone or when combined with other sampling techniques can provide insight about the equine upper and lower airways [54][71][72]. Thus it can contribute to the characterization of SEA and to the exclusion of other differentials [1][17][55][72].
Endoscopic examination of severely asthmatic horses will reveal the presence of mucus in the trachea and bronchi and thickening of the carina (tracheal septum). The presence of bronchospasm and mucosal hyperemia associated with inflammation can also be assessed [17][55][73][74][75][76][77].
Tracheal secretions will usually form a small pool at the lowest point of the trachea. However, decreased mucus clearance and altered rheology increases the amount of mucus observed and mucus strings can be observed in the lateral and dorsal aspects of the trachea [55][78].
Most endoscopic scoring systems assess the quantity and quality of tracheal mucus [17][73][74][75][77], and mucus accumulation was found to correlate with clinical scores [79], cough frequency [51] and cytological indicators of airway inflammation [55].
2.4. Lung Function Tests
Pulmonary function testing has become an important tool in the evaluation of the respiratory system since it provides information about ventilation and the dynamics of this system [63][80]. They are considered the gold standard for the diagnosis of asthma both in human and equine medicine [81]. In SEA they contribute to disease diagnosis and assessment of disease severity as well as treatment response. Nevertheless, no single test can be considered perfect, as they all have their strengths and weaknesses, and their selection will be determined by the clinician’s needs, their practicality and availability.
Arterial blood gas analysis assesses lung gas exchange and can be used in an ambulatory setting [82]. Severely asthmatic horses commonly present only hypoxemia, but lower values of pH and increased values of PaCO2 have also been reported [18][83][84][85][86]. Its main disadvantage is that it lacks sensitivity for recognizing animals in remission [86].
The change in pleural pressure (ΔPpl), assessed by an oesophageal balloon catheter, is considered the gold standard for the diagnosis of SEA. The measured values can be interpreted on their own, with ΔPpl > 15 cm H2O being considered the cut off value for disease diagnosis [1][46]. This method can easily be used in an ambulatory setting, but in the authors’ experience some owners may be reticent to allow this test to be performed on their horses due to its invasive nature. When combined with a pneumotachograph, standard lung mechanics can be assessed, namely dynamic compliance (Cdyn), pulmonary resistance (RL), and work of breathing (W). Airway obstruction results in increased RL, W and ΔPpl, and decreased Cdyn [1][53][87].
Flowmetrics is based in boxless plethysmography and it combines respiratory inductance plethysmography (RIP) with pneumotachography [88]. This system, which has been specifically developed for equines and was suited for field testing [89], is no longer commercially available. It had a sensitivity similar to that of pleural pressure but, when combined with histamine bronchoprovocation, allowed the detection of horses in disease remission [18][46][90][91].
Lung function testing can be associated with either a histamine or bronchodilator challenge [80]. Airway hyperreactivity, the reversible narrowing of airways in response to a bronchoconstrictor stimulus, such as histamine, occurs in all asthmatic horses, especially during disease exacerbation but is also present in cases of mild obstruction without apparent clinical signs [1][18][46][89][90][92][93][94].
When asthmatic horses present with a severe compromise of baseline pulmonary function, it is recommended to perform a bronchodilator challenge instead [2][59]. A bronchodilator is administered (e.g., albuterol 450–900 μg) after a base-line reading and 15 min later pulmonary function is re-assessed [1][80]. Within 10 min, a 50% improvement of airway resistance should occur in SEA-affected horses in exacerbation [80]. Furthermore, both the maximum value and the magnitude of the observed bronchodilation can also predict, to some degree, the future therapeutic response.
The use of spirometry [44][95][96][97], electrical impedance tomography [98], and impulse oscillation system (IOS) for assessing the dynamics of equine lower airways have shown promising results, particularly IOS, which has been reported to differentiate severely asthmatic horses in remission from healthy controls [99][100][101][102].
2.5. Cytology
In equine ambulatory medicine, airway cytology remains a fundamental technique for diagnosing and monitoring SEA. It provides insight into the inflammatory status of airways and although it is not considered the gold standard for the diagnosis of this disease, its practicality in an ambulatory context has rendered this ancillary diagnostic test popular. Cytological samples can be collected using a wide variety of methods, including brush cytology, tracheal wash (TW), bronchoalveolar lavage fluid (BALF) or even bronchial biopsies [103][104][105][106].
Of these methods, BALF cytology is considered to be the one which most accurately reflects the cellular populations of the bronchi and alveoli and the consequent degree of inflammation found in the horses’ lungs [105][107][108]. Sampling can easily be performed transendoscopicaly or ‘blindly’, via a balloon catheter, by instilling a volume of 250 to 500 mL of saline and a minimum of 400 cells should be counted [2][18][105][109][110].
BALF samples of healthy animals have <400 cells/μL and a superficial foam layer, indicating the presence of pulmonary surfactant. Alveolar macrophages (40–70%) and lymphocytes (30–60%) are the most commonly observed immune cells followed by neutrophils (<5%), mast cells (<2%) and eosinophils (<1%) [1]. The cytological profile of the severely asthmatic horse is usually characterized by neutrophilia (>20% neutrophils) and a reduction in macrophage and lymphocyte percentages [1][17][18][111][112][113]. An increased amount of mucus is also observed which can form Curschmann’s spirals [114][115].
BALF differential cell counts correlate well with airway obstruction and hyperresponsiveness, and a higher percentage of neutrophils is associated with greater disease severity, coughing and worse mucus scores [17][47][51][115][116]. During clinical remission, affected horses maintain a slightly elevated neutrophil percentage [117], and despite corticosteroid treatment, BALF neutrophilia can persist when severely asthmatic horses continue to be exposed to the offending respirable particles [40][118][119][120].
Furthermore, it has been reported that the percentage of neutrophils in BALF can be used to classify the severity of SEA, as it correlates well with the clinical signs exhibited by asthmatic horses, changes observed in thoracic x-rays, mucus scores determined through endoscopy and airway remodeling [17][112].
2.6. SEA Staging
One of the main challenges of managing a severely asthmatic patient is continuously monitoring disease severity and response to treatment. An initial detailed characterization of the disease may help select the best therapeutic protocol and optimize environmental management catering to the horses’ and owners’ individualized needs [81].
Staging methods were developed to help clinicians gather information about disease severity by combining history, physical examination and a variety of ancillary diagnostic tests.
A relatively complete SEA clinical staging method has been published, which encompasses clinical history reported by the owners and clinical signs observed during clinical examination, namely cough frequency, nasal flare and abdominal lift. It also uses ancillary diagnostic tests to quantify airway inflammation and remodeling, such as thoracic x-ray, endoscopy and BALF cytology [17]. This staging method has to be carried out in a hospital and only evaluates the present condition of the horse and does not take into account reported history
An alternative staging system for ambulatory practice that included lung function assessment was later developed [18]. This method included data of the horses’ physical examination (clinical score), BALF cytology (neutrophil percentage), arterial blood oxygen pressure (PaO2), pleural pressure (ΔPpl) and histamine bronchoprovocation (maximum tolerated concentration). All the diagnostic procedures could easily be performed in the field, since all the equipment used was portable.
2.7. SEA Characterization
Although the role of immunoglobulin E (IgE) in the pathophysiology of SEA remains controversial, differences in allergen-specific IgE concentrations in the sera and BALF between healthy and SEA-affected horses have been reported [26][121][122][123][124]. The measurement of allergen-specific IgE concentrations has helped ascertain the association between SEA and sensitization to fungi and mites [26][121][125].
Intradermal tests (IDT) are used to assess the patient’s reaction to an allergen. It requires the injection of specific allergens intradermally and if the horse is sensitized, a local allergic reaction occurs (papule). In SEA-affected horses the use of IDT allowed the identification of allergen sensitization [43][122]. Lo Feudo and colleagues reported that insects, the mite Dermatophagoides spp. and dog epithelium were the major allergen profiles associated with SEA [43].
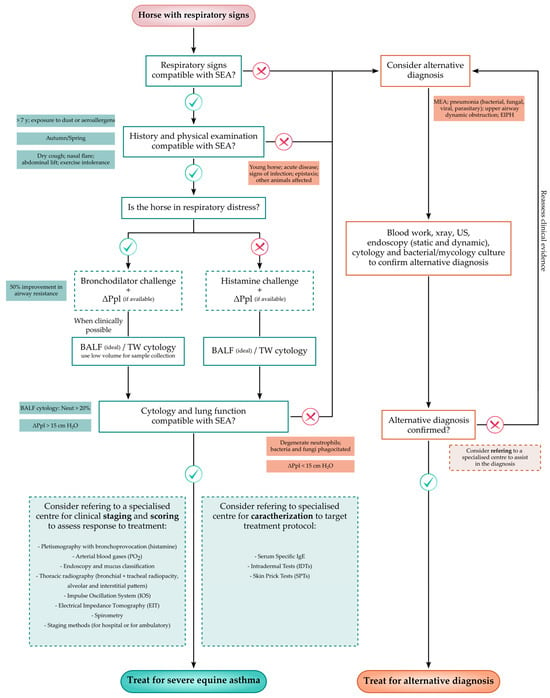
3. Diagnostic Flow-Chart
Because horses affected by severe equine asthma may not exhibit clinical signs during disease remission, disease diagnosis can be challenging, particularly to the less experienced practitioner. Thus, a flow-chart which aims to assist in the recognition of severely asthmatic horses by including the main steps required to correctly diagnose the disease, is proposed in Figure 1.

Figure 1. Flow-chart proposal for the diagnosis of severe equine asthma to be used in everyday veterinary practice [17][18][42][43][55][63][102]. MEA—Mild/Moderate Equine Asthma; EIPH—Exercise Induced Pulmonary Hemorrhage; BALF—Bronchoalveolar Lavage Fluid; TW—Tracheal Wash; ΔPpl—Change in pleural Pressure (indirect); SEA—Severe Equine Asthma; and Neut—Neutrophils.
References
- Couëtil, L.L.; Cardwell, J.M.; Gerber, V.; Lavoie, J.P.; Léguillette, R.; Richard, E.A. Inflammatory Airway Disease of Horses-Revised Consensus Statement. J. Vet. Intern. Med. 2016, 30, 503–515.
- Couetil, L.; Cardwell, J.M.; Leguillette, R.; Mazan, M.; Richard, E.; Bienzle, D.; Bullone, M.; Gerber, V.; Ivester, K.; Lavoie, J.P.; et al. Equine Asthma: Current Understanding and Future Directions. Front. Vet. Sci. 2020, 7, 450.
- Hotchkiss, J.W.; Reid, S.W.J.; Christley, R.M. A survey of horse owners in Great Britain regarding horses in their care. Part 1: Horse demographic characteristics and management. Equine Vet. J. 2007, 39, 294–300.
- Hotchkiss, J.W.; Reid, S.W.J.; Christley, R.M. A survey of horse owners in Great Britain regarding horses in their care. Part 2: Risk factors for recurrent airway obstruction. Equine Vet. J. 2007, 39, 301–308.
- Ireland, J.L.; Christley, R.M.; McGowan, C.M.; Clegg, P.D.; Pinchbeck, G.L.; Martynski, P.D.; Payne, R.J.; Wylie, C.E.; Smith, S.; Marr, C.M.; et al. Prevalence of and Risk Factors for Recurrent Airway Obstruction in Geriatric Horses and Ponies. Equine Vet. J. 2015, 47, 25.
- Giguère, S.; Viel, L.; Lee, E.; MacKay, R.J.; Hernandez, J.; Franchini, M. Cytokine induction in pulmonary airways of horses with heaves and effect of therapy with inhaled fluticasone propionate. Vet. Immunol. Immunopathol. 2002, 85, 147–158.
- Horohov, D.W.; Beadle, R.E.; Mouch, S.; Pourciau, S.S. Temporal regulation of cytokine mRNA expression in equine recurrent airway obstruction. Vet. Immunol. Immunopathol. 2005, 108, 237–245.
- Hulliger, M.F.; Pacholewska, A.; Vargas, A.; Lavoie, J.P.; Leeb, T.; Gerber, V.; Jagannathan, V. An integrative mirna-mrna expression analysis reveals striking transcriptomic similarities between severe equine asthma and specific asthma endotypes in humans. Genes 2020, 11, 1143.
- Pacholewska, A.; Kraft, M.F.; Gerber, V.; Jagannathan, V. Differential expression of serum MicroRNAs supports CD4+ t cell differentiation into Th2/Th17 cells in severe equine asthma. Genes 2017, 8, 383.
- Padoan, E.; Ferraresso, S.; Pegolo, S.; Castagnaro, M.; Barnini, C.; Bargelloni, L. Real time RT-PCR analysis of inflammatory mediator expression in recurrent airway obstruction-affected horses. Vet. Immunol. Immunopathol. 2013, 156, 190–199.
- Ainsworth, D.M.; Wagner, B.; Franchini, M.; Grünig, G.; Erb, H.N.; Tan, J.Y. Time-dependent alterations in gene expression of interleukin-8 in the bronchial epithelium of horses with recurrent airway obstruction. Am. J. Vet. Res. 2006, 67, 669–677.
- Simões, J.; Batista, M.; Tilley, P. The Immune Mechanisms of Severe Equine Asthma—Current Understanding and What Is Missing. Animals 2022, 12, 744.
- Marti, E.; Gerber, H.; Essich, G.; Oulehla, J.; Lazary, S. The genetic basis of equine allergic diseases 1. Chronic hypersensitivity bronchitis. Equine Vet. J. 1991, 23, 457–460.
- Gerber, V.; Baleri, D.; Klukowska-Rötzler, J.; Swinburne, J.E.; Dolf, G. Mixed inheritance of equine recurrent airway obstruction. J. Vet. Intern. Med. 2009, 23, 626–630.
- Ramseyer, A.; Gaillard, C.; Burger, D.; Straub, R.; Jost, U.; Boog, C.; Marti, E.; Gerber, V. Effects of Genetic and Environmental Factors on Chronic Lower Airway Disease in Horses. J. Vet. Intern. Med. 2007, 21, 149–156.
- Schnider, D.; Rieder, S.; Leeb, T.; Gerber, V.; Neuditschko, M. A genome-wide association study for equine recurrent airway obstruction in European Warmblood horses reveals a suggestive new quantitative trait locus on chromosome 13. Anim. Genet. 2017, 48, 691–693.
- Tilley, P.; Sales Luis, J.P.; Branco Ferreira, M. Correlation and discriminant analysis between clinical, endoscopic, thoracic X-ray and bronchoalveolar lavage fluid cytology scores, for staging horses with recurrent airway obstruction (RAO). Res. Vet. Sci. 2012, 93, 1006–1014.
- Simões, J.; Sales Luís, J.; Tilley, P. Contribution of lung function tests to the staging of severe equine asthma syndrome in the field. Res. Vet. Sci. 2019, 123, 112–117.
- Moore-Colyer, M.J.S.; Taylor, J.L.E.; James, R. The Effect of Steaming and Soaking on the Respirable Particle, Bacteria, Mould, and Nutrient Content in Hay for Horses. J. Equine Vet. Sci. 2016, 39, 62–68.
- White, S.J.; Moore-Colyer, M.; Marti, E.; Hannant, D.; Gerber, V.; Coüetil, L.; Richard, E.A.; Alcocer, M. Antigen array for serological diagnosis and novel allergen identification in severe equine asthma. Sci. Rep. 2019, 9, 15171.
- Pirie, R.S.; Collie, D.D.S.; Dixon, P.M.; McGorum, B.C. Inhaled endotoxin and organic dust particulates have synergistic proinflammatory effects in equine heaves (organic dust-induced asthma). Clin. Exp. Allergy 2003, 33, 676–683.
- Costa, L.R.R.; Johnson, J.R.; Baur, M.E.; Beadle, R.E. Temporal clinical exacerbation of summer pasture-associated recurrent airway obstruction and relationship with climate and aeroallergens in horses. Am. J. Vet. Res. 2006, 67, 1635–1642.
- Bullone, M.; Murcia, R.Y.; Lavoie, J.P. Environmental heat and airborne pollen concentration are associated with increased asthma severity in horses. Equine Vet. J. 2016, 48, 479–484.
- Ferrari, C.R.; Cooley, J.; Mujahid, N.; Costa, L.R.; Wills, R.W.; Johnson, M.E.; Swiderski, C.E. Horses With Pasture Asthma Have Airway Remodeling That Is Characteristic of Human Asthma. Vet. Pathol. 2018, 55, 144–158.
- McGorum, B.C.; Ellison, J.; Cullen, R.T. Total and respirable airborne dust endotoxin concentrations in three equine management systems. Equine Vet. J. 1998, 30, 430–434.
- Niedzwiedz, A.; Jaworski, Z.; Kubiak, K. Serum concentrations of allergen-specific IgE in horses with equine recurrent airway obstruction and healthy controls assessed by ELISA. Vet. Clin. Pathol. 2015, 44, 391–396.
- Pacholewska, A.; Jagannathan, V.; Drögemüller, M.; Klukowska-Rötzler, J.; Lanz, S.; Hamza, E.; Dermitzakis, E.T.; Marti, E.; Leeb, T.; Gerber, V. Impaired cell cycle regulation in a natural equine model of asthma. PLoS ONE 2015, 10, e0136103.
- Klier, J.; Geis, S.; Steuer, J.; Geh, K.; Reese, S.; Fuchs, S.; Mueller, R.S.; Winter, G.; Gehlen, H. A comparison of nanoparticullate CpG immunotherapy with and without allergens in spontaneously equine asthma-affected horses, an animal model. Immun. Inflamm. Dis. 2018, 6, 81–96.
- Simões, J.; Sales Luís, J.P.; Tilley, P. Owner Compliance to an Environmental Management Protocol for Severe Equine Asthma Syndrome. J. Equine Vet. Sci. 2020, 87, 102937.
- Masic, I.; Miokovic, M.; Muhamedagic, B. Evidence Based Medicine—New Approaches and Challenges. Acta Inform. Medica 2008, 16, 219.
- Guilleminault, L.; Ouksel, H.; Belleguic, C.; Le Guen, Y.; Germaud, P.; Desfleurs, E.; Leroyer, C.; Magnan, A. Personalised medicine in asthma: From curative to preventive medicine. Eur. Respir. Rev. 2017, 26, 160010.
- Kavanagh, J.; Jackson, D.J.; Kent, B.D. Over-and under-diagnosis in asthma. Breathe 2019, 15, e20–e27.
- Claußen, G.; Hessel, E.F. Particulate Matter in Equestrian Stables and Riding Arenas. J. Equine Vet. Sci. 2017, 55, 60–70.
- Lühe, T.; Volkmann, N.; Probst, J.; Dreyer-Rendelsmann, C.; Schulz, J.; Kemper, N. Bacterial Burden in the Air of Indoor Riding Arenas. Agriculture 2022, 12, 2111.
- Riihimäki, M.; Raine, A.; Elfman, L.; Pringle, J. Markers of respiratory inflammation in horses in relation to seasonal changes in air quality in a conventional racing stable. Can. J. Vet. Res. 2008, 72, 432–439.
- Mönki, J.; Saastamoinen, M.; Karikoski, N.; Norring, M.; Rajamäki, M.; Mykkänen, A. Effects of Bedding Material on Equine Lower Airway Inflammation: A Comparison of Two Peat Beddings, Wood Pellet, and Straw Pellet. Front. Vet. Sci. 2021, 8, 289.
- Orard, M.; Hue, E.; Couroucé, A.; Bizon-Mercier, C.; Toquet, M.P.; Moore-Colyer, M.; Couëtil, L.; Pronost, S.; Paillot, R.; Demoor, M.; et al. The influence of hay steaming on clinical signs and airway immune response in severe asthmatic horses. BMC Vet. Res. 2018, 14, 345.
- Wasko, A.J.; Barkema, H.W.; Nicol, J.; Fernandez, N.; Logie, N.; Léguillette, R. Evaluation of a risk-screening questionnaire to detect equine lung inflammation: Results of a large field study. Equine Vet. J. 2011, 43, 145–152.
- Laumen, E.; Doherr, M.G.; Gerber, V. Relationship of horse owner assessed respiratory signs index to characteristics of recurrent airway obstruction in two Warmblood families. Equine Vet. J. 2010, 42, 142–148.
- Holcombe, S.J.; Jackson, C.; Gerber, V.; Jefcoat, A.; Berney, C.; Eberhardt, S.; Robinson, N.E. Stabling is associated with airway inflammation in young Arabian horses. Equine Vet. J. 2001, 33, 244–249.
- Klier, J.; Lindner, D.; Reese, S.; Mueller, R.S.; Gehlen, H. Comparison of Four Different Allergy Tests in Equine Asthma Affected Horses and Allergen Inhalation Provocation Test. J. Equine Vet. Sci. 2021, 102, 103433.
- Tilley, P.; Sales Luis, J.P.; Branco Ferreira, M. Comparison of Skin Prick Tests with In Vitro Allergy Tests in the Characterization of Horses with Recurrent Airway Obstruction. J. Equine Vet. Sci. 2012, 32, 719–727.
- Lo Feudo, C.M.; Stucchi, L.; Alberti, E.; Conturba, B.; Zucca, E.; Ferrucci, F. Intradermal testing results in horses affected by mild-moderate and severe equine asthma. Animals 2021, 11, 2086.
- Couëtil, L.L.; Rosenthal, F.S.; DeNicola, D.B.; Chilcoat, C.D. Clinical signs, evaluation of bronchoalveolar lavage fluid, and assessment of pulmonary function in horses with inflammatory respiratory disease. Am. J. Vet. Res. 2001, 62, 538–546.
- Bosshard, S.; Gerber, V. Evaluation of coughing and nasal discharge as early indicators for an increased risk to develop equine recurrent airway obstruction (RAO). J. Vet. Intern. Med. 2014, 28, 618–623.
- Rettmer, H.; Hoffman, A.M.; Lanz, S.; Oertly, M.; Gerber, V. Owner-reported coughing and nasal discharge are associated with clinical findings, arterial oxygen tension, mucus score and bronchoprovocation in horses with recurrent airway obstruction in a field setting. Equine Vet. J. 2015, 47, 291–295.
- Bedenice, D.; Mazan, M.R.; Hoffman, A.M. Association between cough and cytology of bronchoalveolar lavage fluid and pulmonary function in horses diagnosed with inflammatory airway disease. J. Vet. Intern. Med. 2008, 22, 1022–1028.
- Janssen, P.; Tosi, I.; Hego, A.; Maréchal, P.; Marichal, T.; Radermecker, C. Neutrophil Extracellular Traps Are Found in Bronchoalveolar Lavage Fluids of Horses With Severe Asthma and Correlate With Asthma Severity. Front. Immunol. 2022, 13, 921077.
- Lo Feudo, C.M.; Stucchi, L.; Conturba, B.; Stancari, G.; Ferrucci, F. Impact of Lower Airway Inflammation on Fitness Parameters in Standardbred Racehorses. Animals 2022, 12, 3228.
- Robinson, N.E.; Derksen, F.J.; Jackson, C.A.; Peroni, D.; Gerber, V. Management of heaves. Equine Vet. Educ. 2001, 13, 247–259.
- Robinson, N.E.; Berney, C.; deFeijter-Rupp, H.L.; Jefcoat, A.M.; Cornelisse, C.J.; Gerber, V.M.; Derksen, F.J. Coughing, mucus accumulation, airway obstruction, and airway inflammation in control horses and horses affected with recurrent airway obstruction. Am. J. Vet. Res. 2003, 64, 550–557.
- Robinson, N.E.; Derksen, F.J.; Olszewski, M.A.; Buechner-Maxwell, V.A. The pathogenesis of chronic obstructive pulmonary disease of horses. Br. Vet. J. 1996, 152, 283–306.
- Robinson, N.E.; Olszewski, M.A.; Boehler, D.; Berney, C.; Hakala, J.; Matson, C.; Derksen, F.J. Relationship between clinical signs and lung function in horses with recurrent airway obstruction (heaves) during a bronchodilator trial. Equine Vet. J. 2000, 32, 393–400.
- Gerber, V.; King, M.; Schneider, D.A.; Robinson, N.E. Tracheobronchial mucus viscoelasticity during environmental challenge in horses with recurrent airway obstruction. Equine Vet. J. 2000, 32, 411–417.
- Gerber, V.; Straub, R.; Marti, E.; Hauptman, J.; Herholz, C.; King, M.; Imhof, A.; Tahon, L.; Robinson, N.E. Endoscopic scoring of mucus quantity and quality: Observer and horse variance and relationship to inflammation, mucus viscoelasticity and volume. Equine Vet. J. 2004, 36, 576–582.
- Pirie, R.S. Recurrent airway obstruction: A review. Equine Vet. J. 2014, 46, 276–288.
- Naylor, J.M.; Clark, E.G.; Clayton, H.M. Chronic obstructive pulmonary disease: Usefulness of clinical signs, bronchoalveolar lavage, and lung biopsy as diagnostic and prognostic aids. Can. Vet. J. 1992, 33, 591–598.
- Couëtil, L.L.; Ward, M.P. Analysis of risk factors for recurrent airway obstruction in North American horses: 1444 Cases (1990–1999). J. Am. Vet. Med. Assoc. 2003, 223, 1645–1650.
- Mazan, M.R.; Deveney, E.F.; DeWitt, S.; Bedenice, D.; Hoffman, A. Energetic cost of breathing, body composition, and pulmonary function in horses with recurrent airway obstruction. J. Appl. Physiol. 2004, 97, 91–97.
- Gerber, V.; Robinson, N.E.; Venta, P.J.; Rawson, J.; Jefcoat, A.M.; Hotchkiss, J.A. Mucin genes in horse airways: MUC5AC, but not MUC2, may play a role in recurrent airway obstruction. Equine Vet. J. 2003, 35, 252–257.
- Rush, B.R.; Raub, E.S.; Rhoads, W.S.; Flaminio, M.J.B.F.; Matson, C.J.; Hakala, J.E.; Gillespie, J.R. Pulmonary function in horses with recurrent airway obstruction after aerosol and parenteral administration of beclomethasone dipropionate and dexamethasone, respectively. Am. J. Vet. Res. 1998, 59, 1039–1043.
- Gerber, V.; Schott, H.C.; Robinson, N.E. Owner assessment in judging the efficacy of airway disease treatment. Equine Vet. J. 2011, 43, 153–158.
- Kozłowska, N.; Wierzbicka, M.; Jasiński, T.; Domino, M. Advances in the Diagnosis of Equine Respiratory Diseases: A Review of Novel Imaging and Functional Techniques. Animals 2022, 12, 381.
- Ribonnet, C.; Palmers, K.; Saegerman, C.; Vanderperren, K.; van Galen, G. Perioperative lung ultrasonography in healthy horses undergoing general anesthesia for elective surgery. J. Vet. Intern. Med. 2022, 36, 1160–1172.
- Bakos, Z. Digital luminescence thoracic radiography in horses with recurrent airway obstruction. Vet. Rec. 2008, 162, 122–124.
- Lavoie, J.-P.; Dalle, S.; Breton, L.; Hélie, P. Bronchiectasis in Three Adult Horses with Heaves. J. Vet. Intern. Med. 2004, 18, 757–760.
- Allen, K.; Franklin, S. RAO and IAD: Respiratory disease in horses revisited. Practice 2007, 29, 76–82.
- Bakos, Z.; Vörös, K.; Kellokoski, H.; Reiczigel, J. Comparison of the caudal lung borders determined by percussion and ultrasonography in horses with recurrent airway obstruction. Acta Vet. Hung. 2003, 51, 249–258.
- Siwinska, N.; Zak, A.; Slowikowska, M.; Krupinska, P.; Niedzwiedz, A. Prevalence and severity of ultrasonographic pulmonary findings in horses with asthma—A preliminary study. Pol. J. Vet. Sci. 2019, 22, 653–659.
- Lo Feudo, C.M.; Stucchi, L.; Alberti, E.; Stancari, G.; Conturba, B.; Zucca, E.; Ferrucci, F. The role of thoracic ultrasonography and airway endoscopy in the diagnosis of equine asthma and exercise-induced pulmonary hemorrhage. Vet. Sci. 2021, 8, 276.
- Wysocka, B.; Kluciński, W. Cytological evaluation of tracheal aspirate and broncho-alveolar lavage fluid in comparison to endoscopic assessment of lower airways in horses with recurrent airways obstruction or inflammatory airway disease. Pol. J. Vet. Sci. 2015, 18, 587–597.
- Feudo, C.M.L.; Stancari, G.; Collavo, F.; Stucchi, L.; Conturba, B.; Zucca, E.; Ferrucci, F. Upper and Lower Airways Evaluation and Its Relationship with Dynamic Upper Airway Obstruction in Racehorses. Animals 2022, 12, 1563.
- Dixon, P.M.; Railton, D.I.; McGorum, B.C. Equine pulmonary disease: A case control study of 300 referred cases. Part 2: Details of animals and of historical and clinical findings. Equine Vet. J. 1995, 27, 422–427.
- Hare, J.E.; Viel, L. Pulmonary eosinophilia associated with increased airway responsiveness in young racing horses. J. Vet. Intern. Med. 1998, 12, 163–170.
- Koch, C.; Straub, R.; Ramseyer, A.; Widmer, A.; Robinson, N.E.; Gerber, V. Endoscopic scoring of the tracheal septum in horses and its clinical relevance for the evaluation of lower airway health in horses. Equine Vet. J. 2007, 39, 107–112.
- Kutasi, O.; Balogh, N.; Lajos, Z.; Nagy, K.; Szenci, O. Diagnostic Approaches for the Assessment of Equine Chronic Pulmonary Disorders. J. Equine Vet. Sci. 2011, 31, 400–410.
- Koblinger, K.; Nicol, J.; Mcdonald, K.; Wasko, A.; Logie, N.; Weiss, M.; Léguillette, R. Endoscopic Assessment of Airway Inflammation in Horses. J. Vet. Intern. Med. 2011, 25, 1118–1126.
- Gerber, V.; Lindberg, Å.; Berney, C.; Robinson, N.E. Airway Mucus in Recurrent Airway Obstruction-Short-Term Response to Environmental Challenge. J. Vet. Intern. Med. 2004, 18, 92–97.
- Rodrigues Costa, L.R.; Seahorn, T.L.; Moore, R.M.; Taylor, H.W.; Gaunt, S.D.; Beadle, R.E. Correlation of clinical score, intrapleural pressure, cytologic findings of bronchoalveolar fluid, and histopathologic lesions of pulmonary tissue in horses with summer pasture-associated obstructive pulmonary disease. Am. J. Vet. Res. 2000, 61, 167–173.
- Mazan, M.R.; Hoffman, A.M. Clinical techniques for diagnosis of inflammatory airway disease in the horse. Clin. Tech. Equine Pract. 2003, 2, 238–257.
- GINA. Global Strategy for Asthma Management and Prevention; GINA: Fontana, WI, USA, 2023.
- Chevalier, H.; Divers, T.J. Pulmonary dysfunction in adult horses in the intensive care unit. Clin. Tech. Equine Pract. 2003, 2, 165–177.
- Nuytten, J.; Deprez, P.; Picavet, T.; Van Den Hende, C.; Muylle, E. Comparison of different pulmonary function tests and their prognostic value in horses affected with COPD. J. Equine Vet. Sci. 1988, 8, 361–364.
- Couëtil, L.L.; Denicola, D.B. Blood gas, plasma lactate and bronchoalveolar lavage cytology analyses in racehorses with respiratory disease. Equine Vet. J. Suppl. 1999, 30, 77–82.
- Sánchez, A.; Couëtil, L.L.; Ward, M.P.; Clark, S.P. Effect of Airway Disease on Blood Gas Exchange in Racehorses. J. Vet. Intern. Med. 2005, 19, 87–92.
- Stopyra, A.; Sobiech, P.; Wacławska-Matyjasik, A. Acid-base indicators in the venous and arterial blood of horses affected by recurrent airway obstruction (RAO). Pol. J. Vet. Sci. 2012, 15, 463–467.
- Lavoie, J.P.; Leclere, M.; Rodrigues, N.; Lemos, K.R.; Bourzac, C.; Lefebvre-Lavoie, J.; Beauchamp, G.; Albrecht, B. Efficacy of inhaled budesonide for the treatment of severe equine asthma. Equine Vet. J. 2019, 51, 401–407.
- Hoffman, A.M.; Oura, T.J.; Riedelberger, K.J.; Mazan, M.R. Plethysmographic comparison of breathing pattern in heaves (recurrent airway obstruction) versus experimental bronchoconstriction or hyperpnea in horses. J. Vet. Intern. Med. 2007, 21, 184–192.
- Nolen-Walston, R.D.; Kuehn, H.; Boston, R.C.; Mazan, M.R.; Wilkins, P.A.; Bruns, S.; Hoffman, A.M. Reproducibility of airway responsiveness in horses using flowmetric plethysmography and histamine bronchoprovocation. J. Vet. Intern. Med. 2009, 23, 631–635.
- Wichtel, M.; Gomez, D.; Burton, S.; Wichtel, J.; Hoffman, A. Relationships between equine airway reactivity measured by flowmetric plethysmography and specific indicators of airway inflammation in horses with suspected inflammatory airway disease. Equine Vet. J. 2016, 48, 466–471.
- Dixon, C.E.; Bedenice, D.; Mazan, M.R. Comparison of Flowmetric Plethysmography and Forced Oscillatory Mechanics to Measure Airway Hyperresponsiveness in Horses. Front. Vet. Sci. 2021, 7, 511023.
- Derksen, F.J.; Robinson, N.E.; Armstrong, P.J.; Stick, J.A.; Slocombe, R.F. Airway reactivity in ponies with recurrent airway obstruction (heaves). J. Appl. Physiol. 1985, 58, 598–604.
- Vandenput, S.; Votion, D.; Duvivier, D.H.; Van Erck, E.; Anciaux, N.; Art, T.; Lekeux, P. Effect of a set stabled environmental control on pulmonary function and airway reactivity of COPD affected horses. Vet. J. 1998, 155, 189–195.
- Mazan, M.R.; Huffman, A.M.; Manjerovic, N. Comparison of forced oscillation with the conventional method for histamine bronchoprovocation testing in horses. Am. J. Vet. Res. 1999, 60, 174–180.
- Burnheim, K.; Hughes, K.J.; Evans, D.L.; Raidal, S.L. Reliability of breath by breath spirometry and relative flow-time indices for pulmonary function testing in horses. BMC Vet. Res. 2016, 12, 268.
- Herholz, C.; Straub, R.; Braendlin, C.; Imhof, A.; Lüthi, S.; Busato, A. Measurement of tidal breathing flow-volume loop indices in horses used for different sporting purposes with and without recurrent airway obstruction. Vet. Rec. 2003, 152, 288–292.
- Raidal, S.L.; Burnheim, K.; Evans, D.; Hughes, K.J. Effects of sedation and salbutamol administration on hyperpnoea and tidal breathing spirometry in healthy horses. Vet. J. 2017, 222, 22–28.
- Secombe, C.; Adler, A.; Hosgood, G.; Raisis, A.; Mosing, M. Can bronchoconstriction and bronchodilatation in horses be detected using electrical impedance tomography? J. Vet. Intern. Med. 2021, 35, 2035–2044.
- Van Erck, E.; Votion, D.; Art, T.; Lekeux, P. Measurement of respiratory function by impulse oscillometry in horses. Equine Vet. J. 2004, 36, 21–28.
- Van Erck, E.; Votion, D.; Kirschvink, N.; Genicot, B.; Lindsey, J.; Art, T.; Lekeux, P. Influence of breathing pattern and lung inflation on impulse oscillometry measurements in horses. Vet. J. 2004, 168, 259–269.
- Richard, E.A.; Fortier, G.D.; Denoix, J.M.; Art, T.; Lekeux, P.M.; van Erck, E. Influence of subclinical inflammatory airway disease on equine respiratory function evaluated by impulse oscillometry. Equine Vet. J. 2009, 41, 384–389.
- Stucchi, L.; Ferrucci, F.; Bullone, M.; Dellacà, R.L.; Lavoie, J.P. Within-breath oscillatory mechanics in horses affected by severe equine asthma in exacerbation and in remission of the disease. Animals 2022, 12, 4.
- Lee, G.K.C.; Beeler-Marfisi, J.; Viel, L.; Piché, É.; Kang, H.; Sears, W.; Bienzle, D. Bronchial brush cytology, endobronchial biopsy, and SALSA immunohistochemistry in severe equine asthma. Vet. Pathol. 2022, 59, 100–111.
- Niedzwiedz, A.; Mordak, R.; Jaworski, Z.; Nicpon, J. Utility of the Histological Examination of the Bronchial Mucosa in the Diagnosis of Severe Equine Asthma Syndrome in Horses. J. Equine Vet. Sci. 2018, 67, 44–49.
- Hoffman, A.M. Bronchoalveolar lavage technique and cytological diagnosis of small airway inflammatory disease. Equine Vet. Educ. 1999, 11, 330–336.
- Dauvillier, J.; ter Woort, F.; van Erck-Westergren, E. Fungi in respiratory samples of horses with inflammatory airway disease. J. Vet. Intern. Med. 2019, 33, 968–975.
- Hoffman, A.M.; Mazan, M.R. Programme of lung function testing horses suspected with small airway disease. Equine Vet. Educ. 1999, 11, 322–328.
- Malikides, N.; Hughes, K.J.; Hodgson, D.R.; Hodgson, J.L. Comparison of tracheal aspirates and bronchoalveolar lavage in racehorses 2. Evaluation of the diagnostic significance of neutrophil percentage. Aust. Vet. J. 2003, 81, 685–687.
- Hermange, T.; Le Corre, S.; Bizon, C.; Richard, E.A.; Couroucé, A. Bronchoalveolar lavage fluid from both lungs in horses: Diagnostic reliability of cytology from pooled samples. Vet. J. 2019, 244, 28–33.
- Fernandez, N.J.; Hecker, K.G.; Gilroy, C.V.; Warren, A.L.; Léguillette, R. Reliability of 400-cell and 5-field leukocyte differential counts for equine bronchoalveolar lavage fluid. Vet. Clin. Pathol. 2013, 42, 92–98.
- Fairbairn, S.M.; Page, C.P.; Lees, P.; Cunningham, F.M. Early neutrophil but not eosinophil or platelet recruitment to the lungs of allergic horses following antigen exposure. Clin. Exp. Allergy 1993, 23, 821–828.
- Bullone, M.; Joubert, P.; Gagné, A.; Lavoie, J.P.; Hélie, P. Bronchoalveolar lavage fluid neutrophilia is associated with the severity of pulmonary lesions during equine asthma exacerbations. Equine Vet. J. 2018, 50, 609–615.
- Couetil, L.L.; Thompson, C.A. Airway Diagnostics: Bronchoalveolar Lavage, Tracheal Wash, and Pleural Fluid. Vet. Clin. N. Am. Equine Pract. 2020, 36, 87–103.
- Couëtil, L.L.; Hawkins, J.F. Respiratory Diseases of the Horse: A Problem-Oriented Approach to Diagnosis & Management; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781840766479.
- Hoffman, A.M. Bronchoalveolar Lavage: Sampling Technique and Guidelines for Cytologic Preparation and Interpretation. Vet. Clin. N. Am. Equine Pract. 2008, 24, 423–435.
- Moran, G.; Araya, O.; Ortloff, A.; Folch, H. Cytologic broncheoalveolar lavage findings and humoral immune response against Aspergillus fumigatus in Chilote horses with recurrent airway obstructions “heaves”. Arch. Med. Vet. 2009, 41, 83–88.
- Miskovic, M.; Couëtil, L.L.L.; Thompson, C.A.A. Lung function and airway cytologic profiles in horses with recurrent airway obstruction maintained in low-dust environments. J. Vet. Intern. Med. 2007, 21, 1060–1066.
- Fairbairn, S.M.; Lees, P.; Page, C.P.; Cunningham, F.M. Duration of antigen-induced hyperresponsiveness in horses with allergic respiratory disease and possible links with early airway obstruction. J. Vet. Pharmacol. Ther. 1993, 16, 469–476.
- Couëtil, L.L.; Art, T.; De Moffarts, B.; Becker, M.; Mélotte, D.; Jaspar, F.; Bureau, F.; Lekeux, P. Effect of beclomethasone dipropionate and dexamethasone isonicotinate on lung function, bronchoalveolar lavage fluid cytology, and transcription factor expression in airways of horses with recurrent airway obstruction. J. Vet. Intern. Med. 2006, 20, 399–406.
- Leclere, M.; Lavoie-Lamoureux, A.; Joubert, P.; Relave, F.; Setlakwe, E.L.; Beauchamp, G.; Couture, C.; Martin, J.G.; Lavoie, J.P. Corticosteroids and antigen avoidance decrease airway smooth muscle mass in an equine asthma model. Am. J. Respir. Cell Mol. Biol. 2012, 47, 589–596.
- Künzle, F.; Gerber, V.; Van Der Haegen, A.; Wampfler, B.; Straub, R.; Marti, E. IgE-bearing cells in bronchoalveolar lavage fluid and allergen-specific IgE levels in sera from RAO-affected horses. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2007, 54, 40–47.
- Tahon, L.; Baselgia, S.; Gerber, V.; Doherr, M.G.; Straub, R.; Robinson, N.E.; Marti, E. In vitro allergy tests compared to intradermal testing in horses with recurrent airway obstruction. Vet. Immunol. Immunopathol. 2009, 127, 85–93.
- Hansen, S.; Otten, N.D.; Birch, K.; Skovgaard, K.; Hopster-Iversen, C.; Fjeldborg, J. Bronchoalveolar lavage fluid cytokine, cytology and IgE allergen in horses with equine asthma. Vet. Immunol. Immunopathol. 2020, 220, 109976.
- Verdon, M.; Lanz, S.; Rhyner, C.; Gerber, V.; Marti, E. Allergen-specific immunoglobulin E in sera of horses affected with insect bite hypersensitivity, severe equine asthma or both conditions. J. Vet. Intern. Med. 2019, 33, 266–274.
- Eder, C.; Crameri, R.; Mayer, C.; Eicher, R.; Straub, R.; Gerber, H.; Lazary, S.; Marti, E. Allergen-specific IgE levels against crude mould and storage mite extracts and recombinant mould allergens in sera from horses affected with chronic bronchitis. Vet. Immunol. Immunopathol. 2000, 73, 241–253.
More
Information
Subjects:
Veterinary Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
797
Revisions:
2 times
(View History)
Update Date:
22 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




