
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giacomo Visioli | -- | 2461 | 2023-12-21 00:59:38 | | | |
| 2 | Giacomo Visioli | Meta information modification | 2461 | 2023-12-21 01:03:58 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2461 | 2023-12-22 03:03:19 | | | | |
| 4 | Lindsay Dong | Meta information modification | 2461 | 2023-12-22 03:04:00 | | |
Video Upload Options
Examining the ocular fundus demonstrates significant clinical relevance in systemic infections among inpatients. The necessity of such evaluations is not universal for all infectious cases but critical when systemic infections, notably those demonstrating a potential for ocular involvement. The utilization of a collaborative decision tree is proposed to guide clinicians in identifying patients who may derive substantial benefit from fundus examinations, thereby enhancing diagnostic accuracy and tailoring therapeutic interventions. A well-structured, interdisciplinary approach, combining systemic and ocular assessments, is crucial to establish diagnostic clarity and refine therapeutic approaches, especially in the complex clinical scenarios often presented by inpatients with systemic infections. Ultimately, adopting this strategic framework aims to promote better patient outcomes through informed and timely intervention strategies.
1. Introduction
2. Ocular Fundus Examination
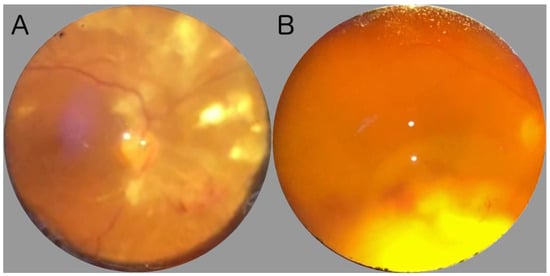
3. Principal Systemic Infections at Bedside
3.1. Systemic Bacterial Infections
3.2. Systemic Viral Infections
3.3. Systemic Fungal Infections
3.4. Systemic Parasitic Infections
4. When Assessing Ocular Fundus in Systemic Infections
4.1. The Evidence about Ophthalmoscopy in Systemic Fungal Infections
4.2. A Decision Tree for Clinicians

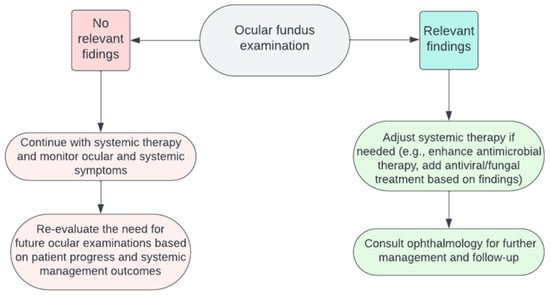
5. Patient Management after Ocular Fundus Examination
References
- Pirani, V.; Pelliccioni, P.; De Turris, S.; Rosati, A.; Franceschi, A.; Cesari, C.; Nicolai, M.; Mariotti, C. The Eye as a Window to Systemic Infectious Diseases: Old Enemies, New Imaging. J. Clin. Med. 2019, 8, 1392.
- Yannuzzi, L.A.; Ober, M.D.; Slakter, J.S.; Spaide, R.F.; Fisher, Y.L.; Flower, R.W.; Rosen, R. Ophthalmic Fundus Imaging: Today and Beyond. Am. J. Ophthalmol. 2004, 137, 511–524.
- Salvetat, M.L.; Musa, M.; Pellegrini, F.; Salati, C.; Spadea, L.; Zeppieri, M. Considerations of COVID-19 in Ophthalmology. Microorganisms 2023, 11, 2220.
- Ong, J.; Zarnegar, A.; Corradetti, G.; Singh, S.R.; Chhablani, J. Advances in Optical Coherence Tomography Imaging Technology and Techniques for Choroidal and Retinal Disorders. J. Clin. Med. 2022, 11, 5139.
- Bypareddy, R.; Sujatha Rathod, B.L.; Shilpa, Y.D.; Hithashree, H.R.; Nagaraj, K.B.; Hemalatha, B.C.; Basumatary, J.; Bekal, D.; Niranjan, R.; Anusha, P.G. Fundus Evaluation in COVID-19 Positives with Non-Severe Disease. Indian. J. Ophthalmol. 2021, 69, 1271.
- Narayana, S.; McGee, S. Bedside Diagnosis of the ‘Red Eye’: A Systematic Review. Am. J. Med. 2015, 128, 1220–1224.e1.
- Pirraglia, M.P.; Ceccarelli, G.; Cerini, A.; Visioli, G.; d’Ettorre, G.; Mastroianni, C.M.; Pugliese, F.; Lambiase, A.; Gharbiya, M. Retinal Involvement and Ocular Findings in COVID-19 Pneumonia Patients. Sci. Rep. 2020, 10, 17419.
- Mark, H.H. On the Evolution of Binocular Ophthalmoscopy. Arch. Ophthalmol. 2007, 125, 830–833.
- Lynn, W.A.; Lightman, P.S. The Eye in Systemic Infection. Lancet 2004, 364, 1439–1450.
- Uchio, E.; Ohno, S. Ocular Manifestations of Systemic Infections. Curr. Opin. Ophthalmol. 1999, 10, 452–457.
- Madu, A.A.; Mayers, M. Ocular Manifestation of Systemic Infections. Curr. Opin. Ophthalmol. 1996, 7, 85–90.
- 20 Surprising Health Problems an Eye Exam Can Catch—American Academy of Ophthalmology. Available online: https://www.aao.org/eye-health/tips-prevention/surprising-health-conditions-eye-exam-detects (accessed on 12 October 2023).
- Keeler, C.R. The Ophthalmoscope in the Lifetime of Hermann von Helmholtz. Arch. Ophthalmol. 2002, 120, 194–201.
- Benbassat, J.; Polak, B.C.P.; Javitt, J.C. Objectives of Teaching Direct Ophthalmoscopy to Medical Students. Acta Ophthalmol. 2012, 90, 503–507.
- Rodenbeck, S.J.; MacKay, D.D. Examining the Ocular Fundus in Neurology. Curr. Opin. Neurol. 2019, 32, 105–110.
- Sun, M.; Ma, A.; Li, F.; Cheng, K.; Zhang, M.; Yang, H.; Nie, W.; Zhao, B. Sensitivity and Specificity of Red Reflex Test in Newborn Eye Screening. J. Pediatr. 2016, 179, 192–196.e4.
- Cordero, I. Understanding and Caring for an Indirect Ophthalmoscope. Community Eye Health 2016, 29, 57.
- Coburn, P.S.; Wiskur, B.J.; Astley, R.A.; Callegan, M.C. Blood–Retinal Barrier Compromise and Endogenous Staphylococcus aureus Endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7303–7311.
- Yang, X.; Yu, X.W.; Zhang, D.D.; Fan, Z.G.; Wei, P.F. Blood-Retinal Barrier as a Converging Pivot in Understanding the Initiation and Development of Retinal Diseases. Chin. Med. J. 2020, 133, 2586.
- Coburn, P.S.; Wiskur, B.J.; Miller, F.C.; Lagrow, A.L.; Astley, R.A.; Elliott, M.H.; Callegan, M.C. Bloodstream-To-Eye Infections Are Facilitated by Outer Blood-Retinal Barrier Dysfunction. PLoS ONE 2016, 11, e0154560.
- Jackson, T.L.; Paraskevopoulos, T.; Georgalas, I. Systematic Review of 342 Cases of Endogenous Bacterial Endophthalmitis. Surv. Ophthalmol. 2014, 59, 627–635.
- Ho, V.; Ho, L.Y.; Ranchod, T.M.; Drenser, K.A.; Williams, G.A.; Garretson, B.R. Endogenous Methicillin-Resistant Staphylococcus aureus Endophthalmitis. Retina 2011, 31, 596–601.
- Levison, M.E.; Levison, J.H. Pharmacokinetics and Pharmacodynamics of Antibacterial Agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815.
- Sallam, A.B.; Kirkland, K.A.; Barry, R.; Soliman, M.K.; Ali, T.K.; Lightman, S. A Review of Antimicrobial Therapy for Infectious Uveitis of the Posterior Segment. Med. Hypothesis Discov. Innov. Ophthalmol. 2018, 7, 140.
- Restivo, L.; Abbouda, A.; Nardella, C.; Bruscolini, A.; Pirraglia, M.P.; Pezzi, P.P. Uveitis Heralding Previously Unknown Luetic and HIV Infection. Syphilitic Uveitis in an Italian Referral Center. Ann. Ist. Super. Sanita 2013, 49, 133–137.
- Wathek, C.; Rannen, R.; Wathek, C.; Rannen, R. Ocular Manifestations of Endocarditis. In Contemporary Challenges in Endocarditis; IntechOpen: London, UK, 2016.
- Arora, N.; Dhibar, D.P.; Bashyal, B.; Agarwal, A. Roth’s Spots, a Clinical Diagnostic Clue for Infective Endocarditis. Perm. J. 2020, 24, 20.038.
- Ganatra, J.B.; Chandler, D.; Santos, C.; Kuppermann, B.; Margolis, T.P. Viral Causes of the Acute Retinal Necrosis Syndrome. Am. J. Ophthalmol. 2000, 129, 166–172.
- Wu, X.N.; Lightman, S.; Tomkins-Netzer, O. Viral Retinitis: Diagnosis and Management in the Era of Biologic Immunosuppression: A Review. Clin. Exp. Ophthalmol. 2019, 47, 381–395.
- Pathai, S.; Deshpande, A.; Gilbert, C.; Lawn, S.D. Prevalence of HIV-Associated Ophthalmic Disease among Patients Enrolling for Antiretroviral Treatment in India: A Cross-Sectional Study. BMC Infect. Dis. 2009, 9, 158.
- Sudharshan, S.; Nair, N.; Curi, A.; Banker, A.; Kempen, J. Human Immunodeficiency Virus and Intraocular Inflammation in the Era of Highly Active Anti Retroviral Therapy—An Update. Indian. J. Ophthalmol. 2020, 68, 1787.
- Carmichael, A. Cytomegalovirus and the Eye. Eye 2012, 26, 237.
- Accorinti, M.; Abbouda, A.; Gilardi, M.; Zito, R.; Iannetti, L. Cytomegalovirus-Related Scleritis. Ocul. Immunol. Inflamm. 2013, 21, 413–415.
- Loubsens, E.; Adam, R.; Debard, A.; Barioulet, L.; Varenne, F.; Fournié, P.; Sales de Gauzy, T.; Ollé, P.; Martin-Blondel, G.; Soler, V. First-Line Management of Necrotizing Herpetic Retinitis by Prioritizing the Investigation of Immune Status and Prognostic Factors for Poor Visual Outcomes. Int. Ophthalmol. 2023, 43, 2545.
- Jabs, D.A.; Belfort, R.; Bodaghi, B.; Graham, E.; Holland, G.N.; Lightman, S.L.; Oden, N.; Palestine, A.G.; Smith, J.R.; Thorne, J.E.; et al. Classification Criteria for Cytomegalovirus Retinitis. Am. J. Ophthalmol. 2021, 228, 245.
- Ho, M.; Invernizzi, A.; Zagora, S.; Tsui, J.; Oldani, M.; Lui, G.; McCluskey, P.; Young, A.L. Presenting Features, Treatment and Clinical Outcomes of Cytomegalovirus Retinitis: Non-HIV Patients Vs HIV Patients. Ocul. Immunol. Inflamm. 2020, 28, 651–658.
- Vasudevan, A.; Rojas-Moreno, C.; Tarun, T. Acute Retinal Necrosis Secondary to Varicella Zoster Virus. IDCases 2019, 18, e00585.
- Venkatesh, A.; Patel, R.; Goyal, S.; Rajaratnam, T.; Sharma, A.; Hossain, P. Ocular Manifestations of Emerging Viral Diseases. Eye 2021, 35, 1117–1139.
- El-Abiary, M.; Jones, B.; Williams, G.; Lockington, D. Fundoscopy Screening for Intraocular Candida in Patients with Positive Blood Cultures—Is It Justified? Eye 2018, 32, 1697.
- Vinikoor, M.J.; Zoghby, J.; Cohen, K.L.; Tucker, J.D. Do All Candidemic Patients Need an Ophthalmic Examination? Int. J. Infect. Dis. 2013, 17, e146–e148.
- Klastersky, J. Empirical Antifungal Therapy. Int. J. Antimicrob. Agents 2004, 23, 105–112.
- Gauthier, G.M.; Nork, T.M.; Prince, R.; Andes, D. Subtherapeutic Ocular Penetration of Caspofungin and Associated Treatment Failure in Candida Albicans Endophthalmitis. Clin. Infect. Dis. 2005, 41, e27–e28.
- Zhang, M.K.; Rao, Z.G.; Ma, T.; Tang, M.; Xu, T.Q.; He, X.X.; Li, Z.P.; Liu, Y.; Xu, Q.J.; Yang, K.Y.; et al. Appropriate Empirical Antifungal Therapy Is Associated with a Reduced Mortality Rate in Intensive Care Unit Patients with Invasive Fungal Infection: A Real-World Retrospective Study Based on the MIMIC-IV Database. Front. Med. 2022, 9, 952611.
- Danielescu, C.; Stanca, H.T.; Iorga, R.E.; Darabus, D.M.; Potop, V. The Diagnosis and Treatment of Fungal Endophthalmitis: An Update. Diagnostics 2022, 12, 679.
- Das, D.; Ramachandra, V.; Islam, S.; Bhattacharjee, H.; Biswas, J.; Koul, A.; Deka, P.; Deka, A. Update on Pathology of Ocular Parasitic Disease. Indian. J. Ophthalmol. 2016, 64, 794.
- El-Sayed, N.M.; Safar, E.H. Characterization of the Parasite-Induced Lesions in the Posterior Segment of the Eye. Indian. J. Ophthalmol. 2015, 63, 881.
- Song, H.B.; Jun, H.O.; Kim, J.H.; Lee, Y.H.; Choi, M.H.; Kim, J.H. Disruption of Outer Blood-Retinal Barrier by Toxoplasma gondii-Infected Monocytes Is Mediated by Paracrinely Activated FAK Signaling. PLoS ONE 2017, 12, e0175159.
- Pleyer, U.; Schlüter, D.; Mänz, M. Ocular Toxoplasmosis: Recent Aspects of Pathophysiology and Clinical Implications. Ophthalmic Res. 2014, 52, 116–123.
- Ahn, S.J.; Woo, S.J.; Jin, Y.; Chang, Y.S.; Kim, T.W.; Ahn, J.; Heo, J.W.; Yu, H.G.; Chung, H.; Park, K.H.; et al. Clinical Features and Course of Ocular Toxocariasis in Adults. PLoS Negl. Trop. Dis. 2014, 8, e2938.
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50.
- Cuenca-Estrella, M.; Verweij, P.E.; Arendrup, M.C.; Arikan-Akdagli, S.; Bille, J.; Donnelly, J.P.; Jensen, H.E.; Lass-Flörl, C.; Richardson, M.D.; Akova, M.; et al. ESCMID* Guideline for the Diagnosis and Management of Candida Diseases 2012: Diagnostic Procedures. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 9–18.
- The Royal College of Ophthalmologists Ophthalmic Services Guidance; Eye Care in the Intensive Care Unit (ICU): London, UK, 2017.
- Radwan, A.; Metzinger, J.L.; Hinkle, D.M.; Foster, C.S. Cytomegalovirus Retinitis in Immunocompetent Patients: Case Reports and Literature Review. Ocul. Immunol. Inflamm. 2013, 21, 324–328.
- Kalogeropoulos, D.; Sakkas, H.; Mohammed, B.; Vartholomatos, G.; Malamos, K.; Sreekantam, S.; Kanavaros, P.; Kalogeropoulos, C. Ocular Toxoplasmosis: A Review of the Current Diagnostic and Therapeutic Approaches. Int. Ophthalmol. 2022, 42, 295.
- Carpani, G.; Foresti, S.; Dell’Oro, R.; Grassi, G.; Bombelli, M. Severe Systemic Cytomegalovirus Infection in an Immunocompetent Patient Outside the Intensive Care Unit: A Case Report. BMC Infect. Dis. 2019, 19, 34.
- Razonable, R.R. Oral Antiviral Drugs for Treatment of Cytomegalovirus in Transplant Recipients. Clin. Microbiol. Infect. 2023, 29, 1144–1149.




