Examining the ocular fundus demonstrates significant clinical relevance in systemic infections among inpatients. The necessity of such evaluations is not universal for all infectious cases but critical when systemic infections, notably those demonstrating a potential for ocular involvement. The utilization of a collaborative decision tree is proposed to guide clinicians in identifying patients who may derive substantial benefit from fundus examinations, thereby enhancing diagnostic accuracy and tailoring therapeutic interventions. A well-structured, interdisciplinary approach, combining systemic and ocular assessments, is crucial to establish diagnostic clarity and refine therapeutic approaches, especially in the complex clinical scenarios often presented by inpatients with systemic infections. Ultimately, adopting this strategic framework aims to promote better patient outcomes through informed and timely intervention strategies.
- systemic infections
- bedside diagnosis
- ocular fundus examination
- ophalmoscopy
1. Introduction
3. Ocular Fundus Examination
Ocular fundus examination allows for the inspection of the posterior segment of the eye, including the vitreous, retina, optic nerve, macula, and retinal vessels [14][13]. Ocular fundus can be explored by direct or indirect ophthalmoscopy [15][14]. Direct ophthalmoscopy procures an upright, unreversed image with a magnification of around 15 times, whereas indirect ophthalmoscopy delivers a reversed, inverted image, magnified between two to five times. The latter, which has a longer learning curve, can be subdivided into monocular indirect ophthalmoscopy (MIO) and binocular indirect ophthalmoscopy (BIO). Direct ophthalmoscopy is suitable for swift assessments of the optic nerve head or evaluating the red reflex, and is frequently employed by non-ophthalmologist clinicians, such as neurologists and pediatricians [16,17][15][16]. Conversely, indirect ophthalmoscopy, particularly the BIO, provides a stereoscopic, extensive view of the retina, encompassing around a 40–45-degree field when utilizing a 20D lens, enabling a more detailed examination and evaluation of peripheral retinal structures, and allowing dynamic observation through lens movement and scleral depression [18][17]. Furthermore, during ophthalmoscopy, the anterior segment of the eye can also be evaluated at the bedside. Notably, signs of anterior segment involvement, such as corneal ulcers or abscesses, or the presence of synechiae can be also detected, even if in a ward context [7]. In Figure 1, two examples from real-world fundus examinations conducted in infectious disease wards using a 20D lens are shown.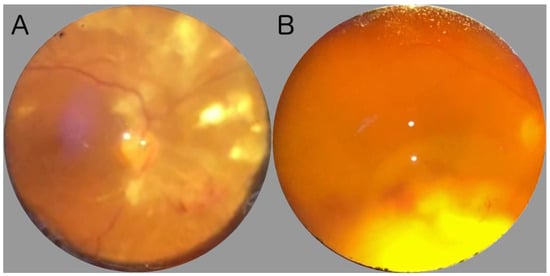
4. Principal Systemic Infections at Bedside
.1. Systemic Bacterial Infections
4.2. Systemic Viral Infections
Ocular fundus examination could uncover underlying systemic viral infections and potentially severe health conditions, providing critical insights particularly relevant in immunocompromised populations [41,42][28][29]. In naïve HIV patients, retinal microangiopathy is commonly observed. It frequently presents as cotton wool patches, which are pale, fluffy lesions on the retina, caused by microinfarctions of the retinal nerve fiber layer due to obstructed retinal capillaries [43,44][30][31]. This manifestation is especially prevalent with decreased CD4 lymphocyte counts, serving as a potential marker for monitoring disease progression and severity. Additionally, viral infections such as CMV retinitis, present with distinctive retinal findings, including vascular-distributed, hemorrhagic, or granular retinitis, providing a diagnostic marker, especially crucial in contexts of immunosuppression, whether due to HIV or other etiologies like organ transplantation [45,46][32][33]. Herpes viruses can cause serious necrotizing conditions that affect the retina. In this context, necrotizing herpetic retinopathy (NHR) is a collective term for a group of diseases that cause acute retinal necrosis due to herpes viruses, encompassing conditions such as CMV retinitis, acute retinal necrosis (ARN), and progressive outer retinal necrosis (PORN) [55][34]. CMV retinitis typically occurs in immunocompromised individuals, such as those with AIDS or undergoing immunosuppressive therapy, and presents with a distinctive appearance often described as resembling “cottage cheese with ketchup”, primarily affecting the posterior pole. Vitritis is generally absent, but signs of periphlebitis may be present [56,57][35][36]. The primary treatment for CMV retinitis is antiviral therapy, commonly with valganciclovir. In contrast, ARN can affect both immunocompetent and immunocompromised individuals and usually begins in the peripheral retina. It can present with mild hemorrhages and is often accompanied by severe vitritis. ARN typically requires systemic antiviral treatment, such as aciclovir. It is often diagnosed in an outpatient setting, as inpatients may not exhibit systemic symptoms indicative of this condition. PORN, primarily seen in severely immunocompromised patients, particularly those with advanced AIDS, is characterized by rapid progression and extensive necrosis of the outer retina, often starting at the posterior pole. Unlike CMV retinitis, PORN does not typically present with significant intraocular inflammation, which is reflected in the minimal anterior chamber reaction and vitreous cell presence. The most common causative agent is the Varicella Zoster Virus (VZV), followed by HSV. Treatment for PORN includes aggressive antiviral therapy administered both intravitreally and intravenously, along with the management of disease sequelae, such as retinal detachment. Despite therapy, the visual prognosis for PORN remains poor [41,51,55][28][34][37]. A particular discourse warrants allocation to emerging viral diseases such as SARS-CoV-2, given the proliferation of case reports delineating varied retinal findings in the context of COVID-19 [58][38]. Observations have highlighted various retinal changes, including cotton wool spots, inner retinal optical coherence tomography (OCT) hyperreflective spots, and retinal microhemorrhages, suggesting the systemic impact of SARS-CoV-2 beyond respiratory complications. The occurrence of vascular occlusions such as Central Retinal Vein Occlusion (CRVO) and Central Retinal Artery Occlusion (CRAO), and conditions like Acute Macular Neuroretinopathy (AMN) and Paracentral Acute Middle Maculopathy (PAMM) further emphasize the potential ocular involvement in infected patients. That being said, there is still no clear evidence regarding specific findings in COVID-19 infection.4.3. Systemic Fungal Infections
In the context of systemic fungal infections, which predominantly affect immunocompromised individuals, conducting an ocular fundus examination is generally recommended, particularly in high-risk patients such as those who are immunocompromised, receiving intensive care, or with long-term catheterization [61,62][39][40]. Ocular manifestations often start with retinitis, then extend to the vitreous and ultimately evolve into endophthalmitis. Hence, early detection of initial ocular involvement can deter the progression to a more severe condition. When a systemic fungal infection is identified, clinicians may initiate an empirical fungal treatment (e.g., Caspofungin) that strategically considers drug toxicity and pharmacokinetic properties [66,67][41][42]. However, ensuring optimal therapeutic outcomes has frequently involved a comprehensive assessment of the ocular fundus, especially since identifying ocular candidiasis dictates a pivot towards utilizing agents, such as Fluconazole, that traverse the BRB effectively [68,69][43][44].4.4. Systemic Parasitic Infections
Parasitic incursions into the ocular milieu may emanate from a diverse array of organisms, including protozoa, nematodes, and cestodes, each engendering distinct pathological sequela within the ocular fundus [71][45]. Such pathologies, whether directly attributed to parasitic activity or indirectly mediated through host immune responses, pervade both the anterior and posterior ocular segments. The latter, which encompasses choroiditis, retinichoroiditis, retinal vasculitis, and additional deleterious conditions, warrants rigorous investigation to safeguard against irreversible retinal damage and concomitant visual impairment [72][46]. Ocular toxoplasmosis, commonly resulting from T. gondii, typically presents notable retinal findings such as a distinctive white focal retinitis with concurrent vitreous inflammation, often described as a “headlight in the fog” [73,74][47][48]. Alternatively, ocular toxocariasis, often stemming from Toxocara infestation, might display as granulomatous posterior uveitis, peripheral inflammatory masses, or even, in severe cases, retinal detachment [75][49].5. When Assessing Ocular Fundus in Systemic Infections
5.1. The Evidence about Ophthalmoscopy in Systemic Fungal Infections
Generally, ophthalmologists have been routinely consulted in hospitals to screen for intraocular infections in patients with Candida bloodstream infections. This approach originated before the advent of systemic antifungal medications and before the establishment of clear definitions of ocular disease associated with candidemia. The Infectious Diseases Society of America (IDSA) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) have provided insights into the role of fundus examinations in the context of candidemia [84,85][50][51]. The IDSA, in 2016, specifically recommends fundoscopy screening within the first week for all patients who test positive for fungal blood cultures, highlighting the potential ocular complications that can arise from candidemia. This proactive stance is driven by the fact that many patients with candidemia can be asymptomatic or may be too systemically unwell to report visual disturbances [84,85][50][51]. In stark contrast, ESCMID’s guidelines on the Diagnosis and Management of Candida Diseases make no explicit mention of ocular involvement, indicating a more conservative stance. The Royal College of Ophthalmologists (RCOphth) has also entered the discussion, collaborating with the Intensive Care Society to recommend fundoscopy screenings for Intensive Care Unit (ICU) patients with positive fungal cultures, emphasizing that such patients are more likely to be non-verbal and, therefore, less likely to communicate visual symptoms [86][52]. More recent recommendations by the AAO aimed at eliminating low-value care practices, which not only prove inefficient but may also pose risks to patient safety. The Academy’s position on routine screening for intraocular infections resulting from Candida bloodstream infections seeks to minimize unnecessary examinations and aligns with the evidence presented in various studies on endogenous Candida endophthalmitis.[1]5.2. A Decision Tree for Clinicians
While the significance of routine ophthalmoscopy remains a topic of discussion, various guidelines emphasize the necessity of a personalized approach. This approach should account for the distinct clinical conditions of each patient, while also staying aligned with the latest scientific consensus. Consequently, we have formulated a comprehensive set of criteria to delineate the specific scenarios where ocular fundus examination could impact patient outcomes. To begin, even if ocular symptoms are not readily apparent, practitioners should maintain a heightened level of vigilance for patients with systemic infections known for ocular involvement or possessing a notable propensity for dissemination, such as toxoplasmosis or CMV [52,77][53][54]. Firstly, even in the absence of ocular symptoms, a high index of suspicion should be reserved for patients where the systemic infection is notorious for ocular involvement or has a propensity to disseminate, e.g., toxoplasmosis or CMV [91][55]. It is advisable to categorize patients into risk strata, considering factors such as immunosuppression, prolonged hospitalization, or the presence of a central venous catheter which are commonly associated with systemic fungal infections. Secondly, the temporality and nature of ocular symptoms, within the framework of systemic infection, must be judiciously assessed. A decision tree for assessing the ocular fundus in inpatients with systemic infections is shown in Figure 2. Figure 2. The flowchart outlines the clinical process for evaluating and treating patients with suspected systemic infections. It includes guidelines on when to conduct an ocular fundus examination.
To date, comprehensive data on the implications of ocular fundus examinations for inpatients with systemic infections remain sparse, especially in terms of understanding its diagnostic efficacy, impact on treatment modifications, and ultimate contribution to patient outcomes in a hospital setting. Further retrospective studies examining historical patient data could shed light on the clinical trajectories of inpatients undergoing fundus examination versus those who were not during systemic infections.
Figure 2. The flowchart outlines the clinical process for evaluating and treating patients with suspected systemic infections. It includes guidelines on when to conduct an ocular fundus examination.
To date, comprehensive data on the implications of ocular fundus examinations for inpatients with systemic infections remain sparse, especially in terms of understanding its diagnostic efficacy, impact on treatment modifications, and ultimate contribution to patient outcomes in a hospital setting. Further retrospective studies examining historical patient data could shed light on the clinical trajectories of inpatients undergoing fundus examination versus those who were not during systemic infections.
6. Patient Management after Ocular Fundus Examination
Depending on the findings of the examination, clinical strategies will differ depending on conclusive and inconclusive findings. In scenarios with inconclusive evidence, monitoring ocular symptoms could be useful to understand whether ocular fundus could be re-evaluated based on the patient’s progress and the efficacy of systemic interventions. On the other hand, in cases with conclusive evidence pointing towards specific pathologies, the therapeutic strategy might necessitate modifications. This could involve enhancing the systemic antimicrobial regimen or incorporating specific antiviral/fungal treatments. Moreover, active collaboration with ophthalmology specialists becomes paramount to crafting a comprehensive care pathway. The utility of ocular fundus examination in guiding patient management is a nuanced topic. In the context of bacterial infections, detecting ocular involvement might suggest a systemic dissemination of the infection. Yet, conventional treatments for bacterial infections typically involve broad-spectrum antibiotics. These medications would inherently address bacterial pathogens impacting the eye. Therefore, the presence of ocular involvement might not substantially alter the foundational approach to treating bacterial infections. However, if the systemic infection is controlled, prompt treatment of the eye is crucial. Early intervention can help prevent potential vision loss, underscoring the significance of considering targeted eye therapies in such cases, such as performing aqueous/vitreous taps for antibiograms or contemplating intravitreal antibiotic injections [9]. Concerning some viral infections such as CMV, ocular changes can be instrumental in dictating treatment modalities. Detecting CMV retinitis, particularly in immunocompromised individuals, could necessitate the introduction or adjustment of specific antiviral agents. This finding may also underscore the importance of evaluating and addressing the patient’s overall immunological status [47][56]. A flowchart about patient management after ocular fundus examination is shown in Figure 3, and it emphasizes an interdisciplinary methodology that integrates diagnostic insights with the broader clinical picture to optimize patient outcomes.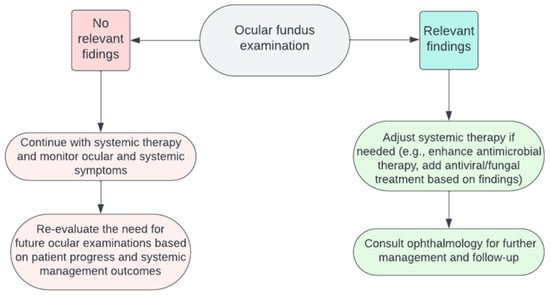
References
- Mark P. Breazzano; John B. Bond; Srilaxmi Bearelly; Donna H. Kim; Sean P. Donahue; Flora Lum; Timothy W. Olsen; American Academy of Ophthalmology Recommendations on Screening for Endogenous Candida Endophthalmitis. Ophthalmol.. 2021, 129, 73-76.Pirani, V.; Pelliccioni, P.; De Turris, S.; Rosati, A.; Franceschi, A.; Cesari, C.; Nicolai, M.; Mariotti, C. The Eye as a Window to Systemic Infectious Diseases: Old Enemies, New Imaging. J. Clin. Med. 2019, 8, 1392.
- Yannuzzi, L.A.; Ober, M.D.; Slakter, J.S.; Spaide, R.F.; Fisher, Y.L.; Flower, R.W.; Rosen, R. Ophthalmic Fundus Imaging: Today and Beyond. Am. J. Ophthalmol. 2004, 137, 511–524.
- Salvetat, M.L.; Musa, M.; Pellegrini, F.; Salati, C.; Spadea, L.; Zeppieri, M. Considerations of COVID-19 in Ophthalmology. Microorganisms 2023, 11, 2220.
- Ong, J.; Zarnegar, A.; Corradetti, G.; Singh, S.R.; Chhablani, J. Advances in Optical Coherence Tomography Imaging Technology and Techniques for Choroidal and Retinal Disorders. J. Clin. Med. 2022, 11, 5139.
- Bypareddy, R.; Sujatha Rathod, B.L.; Shilpa, Y.D.; Hithashree, H.R.; Nagaraj, K.B.; Hemalatha, B.C.; Basumatary, J.; Bekal, D.; Niranjan, R.; Anusha, P.G. Fundus Evaluation in COVID-19 Positives with Non-Severe Disease. Indian. J. Ophthalmol. 2021, 69, 1271.
- Narayana, S.; McGee, S. Bedside Diagnosis of the ‘Red Eye’: A Systematic Review. Am. J. Med. 2015, 128, 1220–1224.e1.
- Pirraglia, M.P.; Ceccarelli, G.; Cerini, A.; Visioli, G.; d’Ettorre, G.; Mastroianni, C.M.; Pugliese, F.; Lambiase, A.; Gharbiya, M. Retinal Involvement and Ocular Findings in COVID-19 Pneumonia Patients. Sci. Rep. 2020, 10, 17419.
- Mark, H.H. On the Evolution of Binocular Ophthalmoscopy. Arch. Ophthalmol. 2007, 125, 830–833.
- Lynn, W.A.; Lightman, P.S. The Eye in Systemic Infection. Lancet 2004, 364, 1439–1450.
- Uchio, E.; Ohno, S. Ocular Manifestations of Systemic Infections. Curr. Opin. Ophthalmol. 1999, 10, 452–457.
- Madu, A.A.; Mayers, M. Ocular Manifestation of Systemic Infections. Curr. Opin. Ophthalmol. 1996, 7, 85–90.
- 20 Surprising Health Problems an Eye Exam Can Catch—American Academy of Ophthalmology. Available online: https://www.aao.org/eye-health/tips-prevention/surprising-health-conditions-eye-exam-detects (accessed on 12 October 2023).
- Keeler, C.R. The Ophthalmoscope in the Lifetime of Hermann von Helmholtz. Arch. Ophthalmol. 2002, 120, 194–201.
- Benbassat, J.; Polak, B.C.P.; Javitt, J.C. Objectives of Teaching Direct Ophthalmoscopy to Medical Students. Acta Ophthalmol. 2012, 90, 503–507.
- Rodenbeck, S.J.; MacKay, D.D. Examining the Ocular Fundus in Neurology. Curr. Opin. Neurol. 2019, 32, 105–110.
- Sun, M.; Ma, A.; Li, F.; Cheng, K.; Zhang, M.; Yang, H.; Nie, W.; Zhao, B. Sensitivity and Specificity of Red Reflex Test in Newborn Eye Screening. J. Pediatr. 2016, 179, 192–196.e4.
- Cordero, I. Understanding and Caring for an Indirect Ophthalmoscope. Community Eye Health 2016, 29, 57.
- Coburn, P.S.; Wiskur, B.J.; Astley, R.A.; Callegan, M.C. Blood–Retinal Barrier Compromise and Endogenous Staphylococcus aureus Endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7303–7311.
- Yang, X.; Yu, X.W.; Zhang, D.D.; Fan, Z.G.; Wei, P.F. Blood-Retinal Barrier as a Converging Pivot in Understanding the Initiation and Development of Retinal Diseases. Chin. Med. J. 2020, 133, 2586.
- Coburn, P.S.; Wiskur, B.J.; Miller, F.C.; Lagrow, A.L.; Astley, R.A.; Elliott, M.H.; Callegan, M.C. Bloodstream-To-Eye Infections Are Facilitated by Outer Blood-Retinal Barrier Dysfunction. PLoS ONE 2016, 11, e0154560.
- Jackson, T.L.; Paraskevopoulos, T.; Georgalas, I. Systematic Review of 342 Cases of Endogenous Bacterial Endophthalmitis. Surv. Ophthalmol. 2014, 59, 627–635.
- Ho, V.; Ho, L.Y.; Ranchod, T.M.; Drenser, K.A.; Williams, G.A.; Garretson, B.R. Endogenous Methicillin-Resistant Staphylococcus aureus Endophthalmitis. Retina 2011, 31, 596–601.
- Levison, M.E.; Levison, J.H. Pharmacokinetics and Pharmacodynamics of Antibacterial Agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815.
- Sallam, A.B.; Kirkland, K.A.; Barry, R.; Soliman, M.K.; Ali, T.K.; Lightman, S. A Review of Antimicrobial Therapy for Infectious Uveitis of the Posterior Segment. Med. Hypothesis Discov. Innov. Ophthalmol. 2018, 7, 140.
- Restivo, L.; Abbouda, A.; Nardella, C.; Bruscolini, A.; Pirraglia, M.P.; Pezzi, P.P. Uveitis Heralding Previously Unknown Luetic and HIV Infection. Syphilitic Uveitis in an Italian Referral Center. Ann. Ist. Super. Sanita 2013, 49, 133–137.
- Wathek, C.; Rannen, R.; Wathek, C.; Rannen, R. Ocular Manifestations of Endocarditis. In Contemporary Challenges in Endocarditis; IntechOpen: London, UK, 2016.
- Arora, N.; Dhibar, D.P.; Bashyal, B.; Agarwal, A. Roth’s Spots, a Clinical Diagnostic Clue for Infective Endocarditis. Perm. J. 2020, 24, 20.038.
- Ganatra, J.B.; Chandler, D.; Santos, C.; Kuppermann, B.; Margolis, T.P. Viral Causes of the Acute Retinal Necrosis Syndrome. Am. J. Ophthalmol. 2000, 129, 166–172.
- Wu, X.N.; Lightman, S.; Tomkins-Netzer, O. Viral Retinitis: Diagnosis and Management in the Era of Biologic Immunosuppression: A Review. Clin. Exp. Ophthalmol. 2019, 47, 381–395.
- Pathai, S.; Deshpande, A.; Gilbert, C.; Lawn, S.D. Prevalence of HIV-Associated Ophthalmic Disease among Patients Enrolling for Antiretroviral Treatment in India: A Cross-Sectional Study. BMC Infect. Dis. 2009, 9, 158.
- Sudharshan, S.; Nair, N.; Curi, A.; Banker, A.; Kempen, J. Human Immunodeficiency Virus and Intraocular Inflammation in the Era of Highly Active Anti Retroviral Therapy—An Update. Indian. J. Ophthalmol. 2020, 68, 1787.
- Carmichael, A. Cytomegalovirus and the Eye. Eye 2012, 26, 237.
- Accorinti, M.; Abbouda, A.; Gilardi, M.; Zito, R.; Iannetti, L. Cytomegalovirus-Related Scleritis. Ocul. Immunol. Inflamm. 2013, 21, 413–415.
- Loubsens, E.; Adam, R.; Debard, A.; Barioulet, L.; Varenne, F.; Fournié, P.; Sales de Gauzy, T.; Ollé, P.; Martin-Blondel, G.; Soler, V. First-Line Management of Necrotizing Herpetic Retinitis by Prioritizing the Investigation of Immune Status and Prognostic Factors for Poor Visual Outcomes. Int. Ophthalmol. 2023, 43, 2545.
- Jabs, D.A.; Belfort, R.; Bodaghi, B.; Graham, E.; Holland, G.N.; Lightman, S.L.; Oden, N.; Palestine, A.G.; Smith, J.R.; Thorne, J.E.; et al. Classification Criteria for Cytomegalovirus Retinitis. Am. J. Ophthalmol. 2021, 228, 245.
- Ho, M.; Invernizzi, A.; Zagora, S.; Tsui, J.; Oldani, M.; Lui, G.; McCluskey, P.; Young, A.L. Presenting Features, Treatment and Clinical Outcomes of Cytomegalovirus Retinitis: Non-HIV Patients Vs HIV Patients. Ocul. Immunol. Inflamm. 2020, 28, 651–658.
- Vasudevan, A.; Rojas-Moreno, C.; Tarun, T. Acute Retinal Necrosis Secondary to Varicella Zoster Virus. IDCases 2019, 18, e00585.
- Venkatesh, A.; Patel, R.; Goyal, S.; Rajaratnam, T.; Sharma, A.; Hossain, P. Ocular Manifestations of Emerging Viral Diseases. Eye 2021, 35, 1117–1139.
- El-Abiary, M.; Jones, B.; Williams, G.; Lockington, D. Fundoscopy Screening for Intraocular Candida in Patients with Positive Blood Cultures—Is It Justified? Eye 2018, 32, 1697.
- Vinikoor, M.J.; Zoghby, J.; Cohen, K.L.; Tucker, J.D. Do All Candidemic Patients Need an Ophthalmic Examination? Int. J. Infect. Dis. 2013, 17, e146–e148.
- Klastersky, J. Empirical Antifungal Therapy. Int. J. Antimicrob. Agents 2004, 23, 105–112.
- Gauthier, G.M.; Nork, T.M.; Prince, R.; Andes, D. Subtherapeutic Ocular Penetration of Caspofungin and Associated Treatment Failure in Candida Albicans Endophthalmitis. Clin. Infect. Dis. 2005, 41, e27–e28.
- Zhang, M.K.; Rao, Z.G.; Ma, T.; Tang, M.; Xu, T.Q.; He, X.X.; Li, Z.P.; Liu, Y.; Xu, Q.J.; Yang, K.Y.; et al. Appropriate Empirical Antifungal Therapy Is Associated with a Reduced Mortality Rate in Intensive Care Unit Patients with Invasive Fungal Infection: A Real-World Retrospective Study Based on the MIMIC-IV Database. Front. Med. 2022, 9, 952611.
- Danielescu, C.; Stanca, H.T.; Iorga, R.E.; Darabus, D.M.; Potop, V. The Diagnosis and Treatment of Fungal Endophthalmitis: An Update. Diagnostics 2022, 12, 679.
- Das, D.; Ramachandra, V.; Islam, S.; Bhattacharjee, H.; Biswas, J.; Koul, A.; Deka, P.; Deka, A. Update on Pathology of Ocular Parasitic Disease. Indian. J. Ophthalmol. 2016, 64, 794.
- El-Sayed, N.M.; Safar, E.H. Characterization of the Parasite-Induced Lesions in the Posterior Segment of the Eye. Indian. J. Ophthalmol. 2015, 63, 881.
- Song, H.B.; Jun, H.O.; Kim, J.H.; Lee, Y.H.; Choi, M.H.; Kim, J.H. Disruption of Outer Blood-Retinal Barrier by Toxoplasma gondii-Infected Monocytes Is Mediated by Paracrinely Activated FAK Signaling. PLoS ONE 2017, 12, e0175159.
- Pleyer, U.; Schlüter, D.; Mänz, M. Ocular Toxoplasmosis: Recent Aspects of Pathophysiology and Clinical Implications. Ophthalmic Res. 2014, 52, 116–123.
- Ahn, S.J.; Woo, S.J.; Jin, Y.; Chang, Y.S.; Kim, T.W.; Ahn, J.; Heo, J.W.; Yu, H.G.; Chung, H.; Park, K.H.; et al. Clinical Features and Course of Ocular Toxocariasis in Adults. PLoS Negl. Trop. Dis. 2014, 8, e2938.
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50.
- Cuenca-Estrella, M.; Verweij, P.E.; Arendrup, M.C.; Arikan-Akdagli, S.; Bille, J.; Donnelly, J.P.; Jensen, H.E.; Lass-Flörl, C.; Richardson, M.D.; Akova, M.; et al. ESCMID* Guideline for the Diagnosis and Management of Candida Diseases 2012: Diagnostic Procedures. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 9–18.
- The Royal College of Ophthalmologists Ophthalmic Services Guidance; Eye Care in the Intensive Care Unit (ICU): London, UK, 2017.
- Radwan, A.; Metzinger, J.L.; Hinkle, D.M.; Foster, C.S. Cytomegalovirus Retinitis in Immunocompetent Patients: Case Reports and Literature Review. Ocul. Immunol. Inflamm. 2013, 21, 324–328.
- Kalogeropoulos, D.; Sakkas, H.; Mohammed, B.; Vartholomatos, G.; Malamos, K.; Sreekantam, S.; Kanavaros, P.; Kalogeropoulos, C. Ocular Toxoplasmosis: A Review of the Current Diagnostic and Therapeutic Approaches. Int. Ophthalmol. 2022, 42, 295.
- Carpani, G.; Foresti, S.; Dell’Oro, R.; Grassi, G.; Bombelli, M. Severe Systemic Cytomegalovirus Infection in an Immunocompetent Patient Outside the Intensive Care Unit: A Case Report. BMC Infect. Dis. 2019, 19, 34.
- Razonable, R.R. Oral Antiviral Drugs for Treatment of Cytomegalovirus in Transplant Recipients. Clin. Microbiol. Infect. 2023, 29, 1144–1149.
