
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Riccardo Saro | -- | 1838 | 2023-12-20 19:28:31 | | | |
| 2 | Mona Zou | Meta information modification | 1838 | 2023-12-21 04:10:04 | | |
Video Upload Options
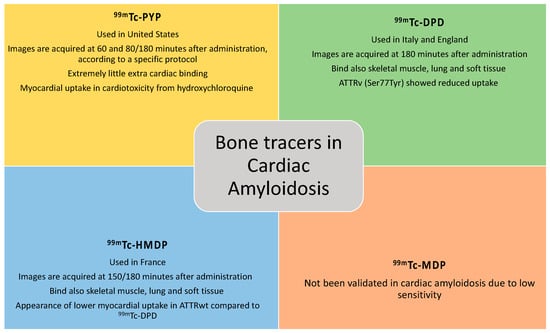
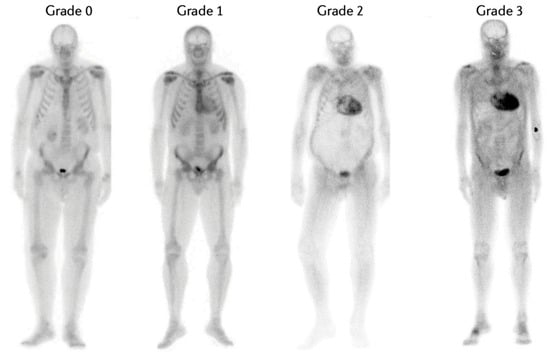
Radionuclide bone scintigraphy is the cornerstone of an imaging-based algorithm for accurate non-invasive diagnosis of transthyretin cardiac amyloidosis (ATTR-CA). In patients with heart failure and suggestive echocardiographic and/or cardiac magnetic resonance imaging findings, the positive predictive value of Perugini grade 2 or 3 myocardial uptake on a radionuclide bone scan approaches 100% for the diagnosis of ATTR-CA as long as there is no biochemical evidence of a clonal dyscrasia. The technetium-labelled tracers that are currently validated for non-invasive diagnosis of ATTR-CA include pyrophosphate (99mTc-PYP); hydroxymethylene diphosphonate (99mTc-HMDP); and 3,3-diphosphono-1,2-propanodicarboxylate (99mTc-DPD). Although nuclear scintigraphy has transformed the contemporary diagnostic approach to ATTR-CA, a number of grey areas remains, including the mechanism for binding tracers to the infiltrated heart, differences in the kinetics and distribution of these radiotracers, differences in protocols of image acquisition worldwide, the clinical significance of extra-cardiac uptake, and the use of this technique for prognostic stratification, monitoring disease progression and assessing the response to disease-modifying treatments.
1. Bone Tracers and Cardiac Amyloidosis
1.1. Pathophysiological Mechanisms of Amyloidogenic Cascade
1.2. Cardiac Scintigraphy
1.3. Bone Tracers
1.4. Cardiac Evaluation Methods
2. Clinical Application of Cardiac Scintigraphy with Bone Tracers
References
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. Systemic amyloidosis. Lancet 2016, 387, 2641–2654.
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68.
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016, 133, 2404–2412.
- Martinez-Naharro, A.; Baksi, A.J.; Hawkins, P.N.; Fontana, M. Diagnostic imaging of cardiac amyloidosis. Nat. Rev. Cardiol. 2020, 17, 413–426.
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084.
- Castano, A.; Haq, M.; Narotsky, D.L.; Goldsmith, J.; Weinberg, R.L.; Morgenstern, R.; Pozniakoff, T.; Ruberg, F.L.; Miller, E.J.; Berk, J.L.; et al. Multicenter Study of Planar Technetium 99m Pyrophosphate Cardiac Imaging: Predicting Survival for Patients With ATTR Cardiac Amyloidosis. JAMA Cardiol. 2016, 1, 880–889.
- Hutt, D.F.; Quigley, A.M.; Page, J.; Hall, M.L.; Burniston, M.; Gopaul, D.; Lane, T.; Whelan, C.J.; Lachmann, H.J.; Gillmore, J.D.; et al. Utility and limitations of 3,3-diphosphono-1, 2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1289–1298.
- Glaudemans, A.W.; van Rheenen, R.W.; van den Berg, M.P.; Noordzij, W.; Koole, M.; Blokzijl, H.; Dierckx, R.A.; Slart, R.H.; Hazenberg, B.P. Bone scintigraphy with 99mtechnetiumhydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis. Amyloid 2014, 21, 35–44.
- Rapezzi, C.; Gagliardi, C.; Milandri, A. Analogies and disparities among scintigraphic bone tracers in the diagnosis of cardiac and non-cardiac ATTR amyloidosis. J. Nucl. Cardiol. 2019, 26, 1638–1641.
- Thelander, U.; Westermark, G.T.; Antoni, G.; Estrada, S.; Zancanaro, A.; Ihse, E.; Westermark, P. Cardiac microcalcifications in transthyretin (ATTR) amyloidosis. Int. J. Cardiol. 2022, 352, 84–91.
- Stats, M.A.; Stone, J.R. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: Implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc. Pathol. 2016, 25, 413–417.
- Pilebro, B.; Suhr, O.B.; Näslund, U.; Westermark, P.; Lindqvist, P.; Sundström, T. 99mTc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis. Upsala J. Med. Sci. 2016, 121, 17–24.
- Sperry, B.W.; Gonzalez, M.H.; Brunken, R.; Cerqueira, M.D.; Hanna, M.; Jaber, W.A. Non-cardiac uptake of technetium-99m pyrophosphate in transthyretin cardiac amyloidosis. J. Nucl. Cardiol. 2019, 26, 1630–1637.
- Hutt, D.F.; Gilbertson, J.; Quigley, A.M.; Wechalekar, A.D. 99mTc-DPD scintigraphy as a novel imaging modality for identification of skeletal muscle amyloid deposition in light-chain amyloidosis. Amyloid 2016, 23, 134–135.
- Porcari, A.; Hutt, D.F.; Grigore, S.F.; Quigley, A.M.; Rowczenio, D.; Gilbertson, J.; Patel, R.; Razvi, Y.; Ioannou, A.; Rauf, M.U. Comparison of different technetium-99m labelled bone tracers for imaging cardiac amyloidosis. Eur. J. Prev. Cardiol. 2023, 30, E4–E6.
- Porcari, A.; Fontana, M.; Gillmore, J.D. Transthyretin cardiac amyloidosis. Cardiovasc. Res. 2022, 118, 3517–3535.
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.J.M.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 2 of 2—Diagnostic criteria and appropriate utilization. J. Nucl. Cardiol. 2019, 27, 659–673.
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.J.M.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2—Evidence base and standardized methods of imaging. J. Nucl. Cardiol. 2019, 26, 2065–2123.
- Sinagra, G.; Porcari, A.; Fabris, E.; Merlo, M. Standardizing the role of endomyocardial biopsy in current clinical practice worldwide. Eur. J. Heart Fail. 2021, 23, 1995–1998.
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568.
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626.
- Asif, T.; Gomez, J.; Singh, V.; Doukky, R.; Nedeltcheva, A.; Malhotra, S. Comparison of planar with tomographic pyrophosphate scintigraphy for transthyretin cardiac amyloidosis: Perils and pitfalls. J. Nucl. Cardiol. 2021, 28, 104–111.
- Mattana, F.; Muraglia, L.; Girardi, F.; Cerio, I.; Porcari, A.; Dore, F.; Bonfiglioli, R.; Fanti, S. Clinical application of cardiac scintigraphy with bone tracers: Controversies and pitfalls in cardiac amyloidosis. Vessel Plus 2022, 6, 13.
- Porcari, A.; Rossi, M.; Dore, F.; Imazio, M.; Fontana, M.; Merlo, M.; Sinagra, G. Ten questions for the cardiologist about cardiac scintigraphy with bone tracers, amyloidosis and the heart. G. Ital. Di Cardiol. 2022, 23, 424–432.
- Hanna, M.; Ruberg, F.L.; Maurer, M.S.; Dispenzieri, A.; Dorbala, S.; Falk, R.H.; Hoffman, J.; Jaber, W.; Soman, P.; Witteles, R.M.; et al. Cardiac Scintigraphy With Technetium-99m-Labeled Bone-Seeking Tracers for Suspected Amyloidosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 2851–2862.
- Porcari, A.; Fontana, M.; Gillmore, J.D. Letter by Porcari et al Regarding Article, Association Between Atrial Uptake on Cardiac Scintigraphy With Technetium-99m-Pyrophosphate Labeled Bone-Seeking Tracers and Atrial Fibrillation. Circ. Cardiovasc. Imaging 2022, 15, E014692.




