
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zheng Chen | -- | 3842 | 2023-12-15 06:02:37 | | | |
| 2 | Lindsay Dong | -3 word(s) | 3839 | 2023-12-15 09:08:54 | | |
Video Upload Options
Lithium-ion batteries (LIBs) have become popular for energy storage due to their high energy density, storage capacity, and long-term cycle life. Although binders make up only a small proportion of LIBs, they have become the key to promoting the transformation of the battery preparation process. Along with the development of binders, the battery manufacturing process has evolved from the conventional slurry-casting (SC) process to a more attractive solvent-free (SF) method. Compared with traditional LIBs manufacturing method, the SF method could dramatically reduce and increase the energy density due to the reduced preparation steps and enhanced electrode loading. Polytetrafluoroethylene (PTFE), as a typical binder, has played an important role in fabricating high-performance LIBs, particularly in regards to the SF technique.
1. Introduction
2. Developments in SF Processes and Binders
2.1. SF Processes
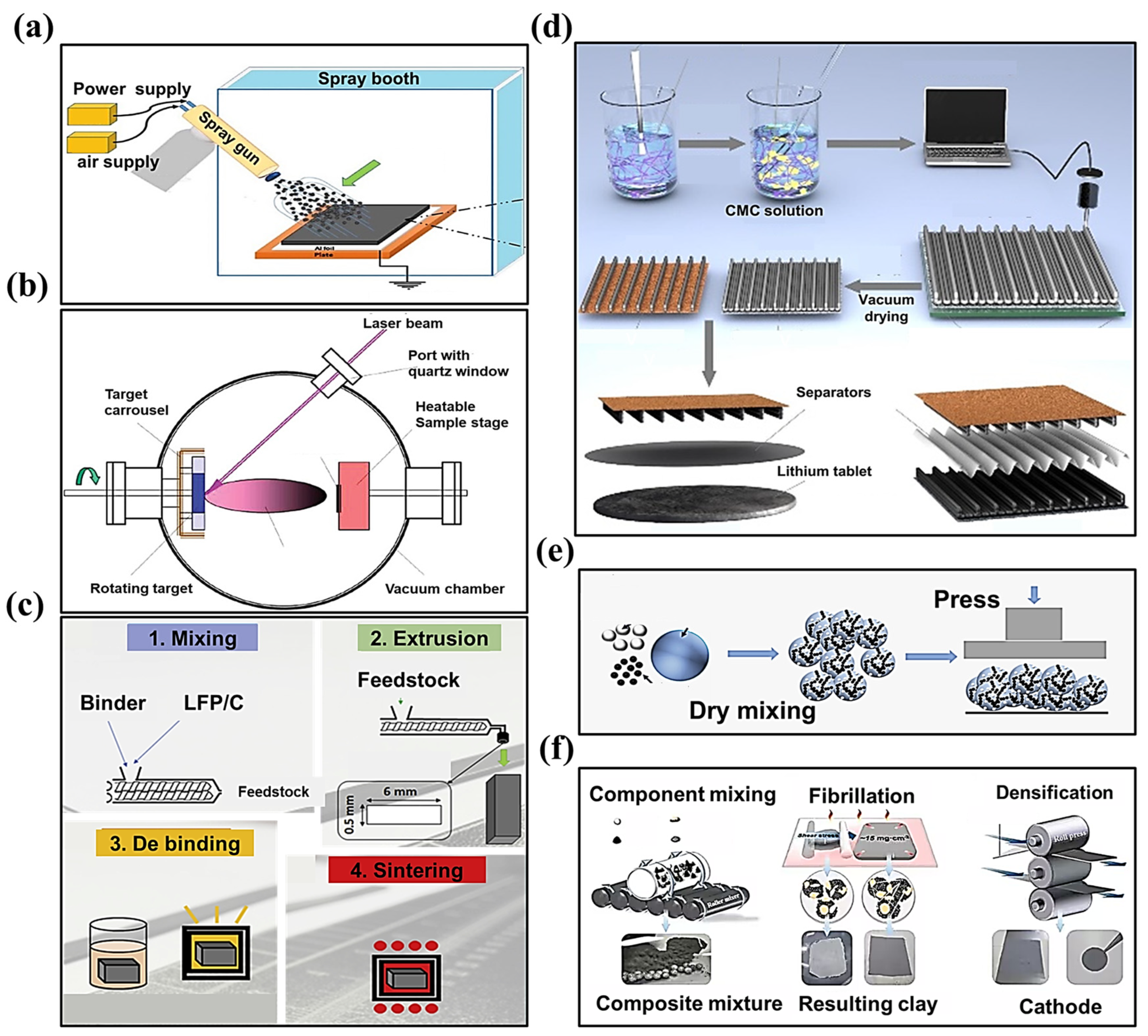
- I.
-
Dry spray deposition
- II.
-
Vapor deposition
- III.
-
Melting and extrusion
- IV.
-
3D printing
- V.
-
Direct pressing
- VI.
-
Polymer fibrillation
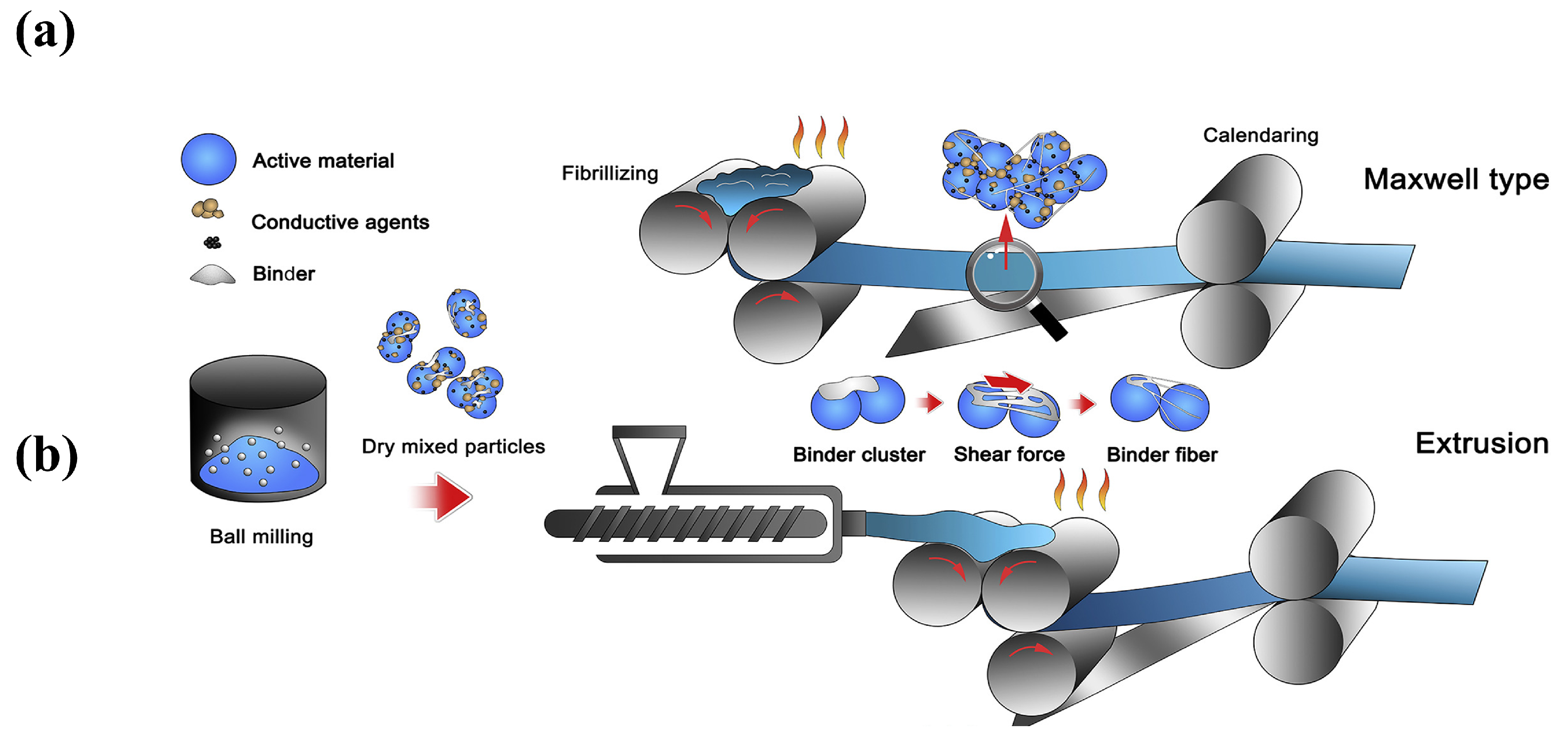
2.2. Binders
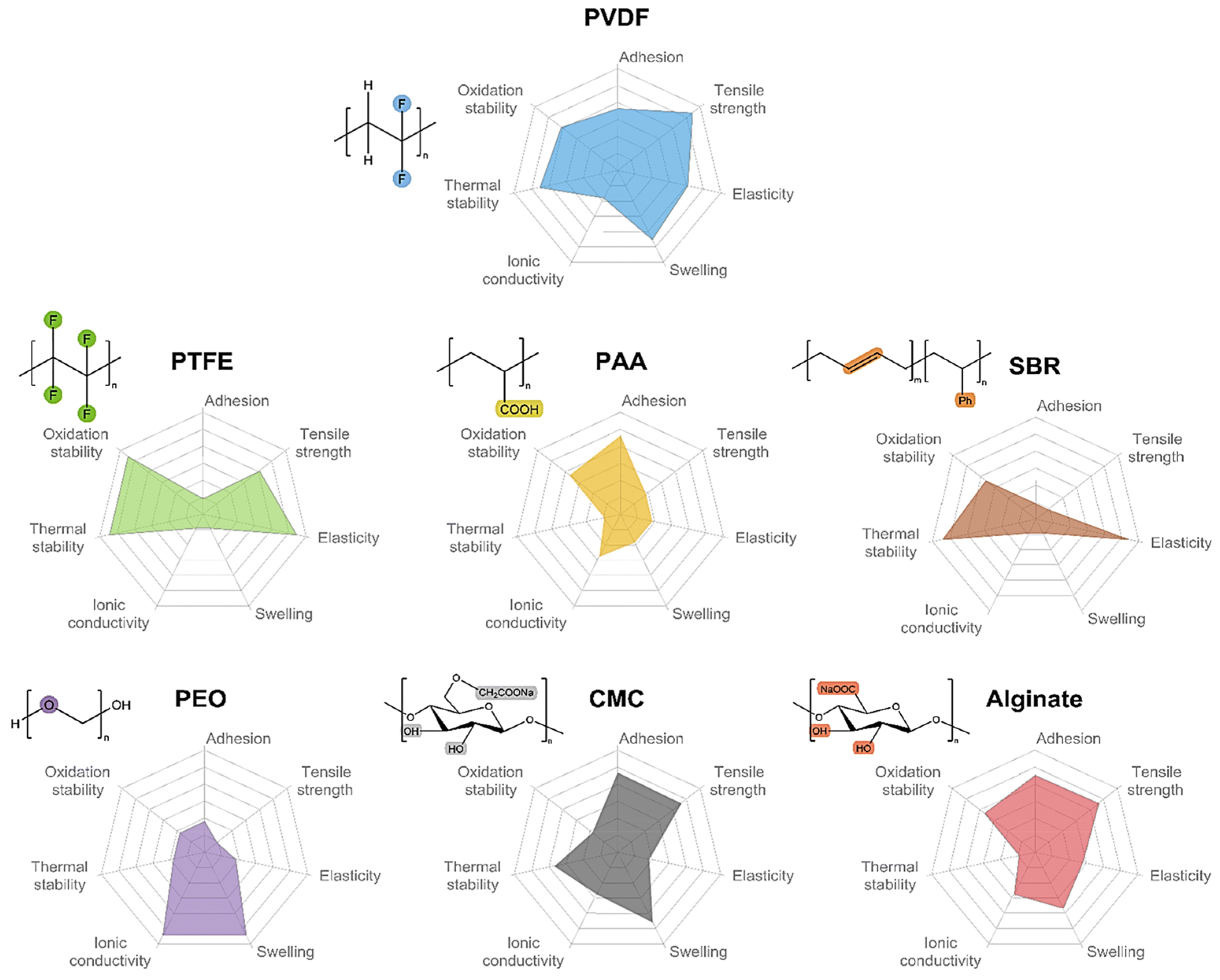
3. Binders of PTFE
3.1. Aqueous Binders of PTFE
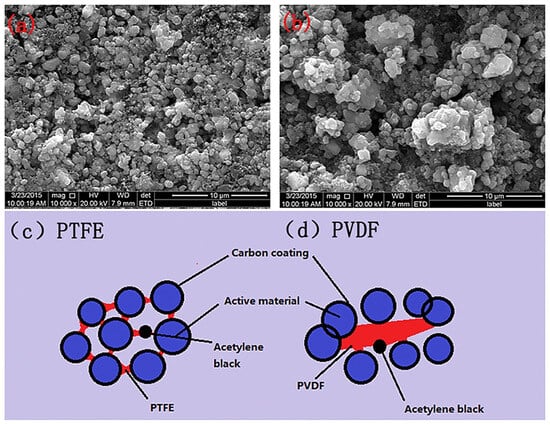
3.2. SF Binders of PTFE
3.2.1. Molecular Structure
3.2.2. The Principle of Polymer Fibrillation
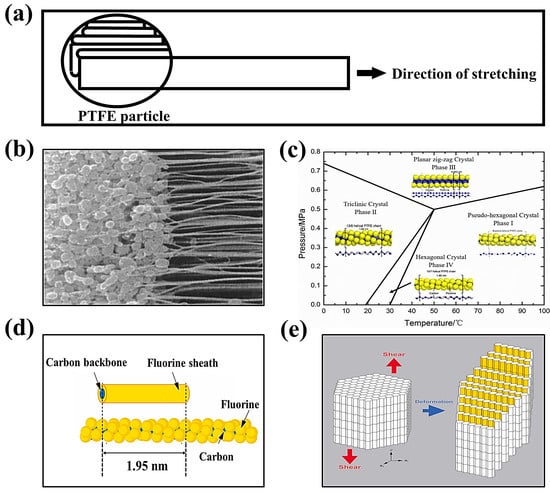
3.2.3. Factors Affecting PTFE Fibrillation
4. SF Process with PTFE Binder
4.1. Positive Characteristics
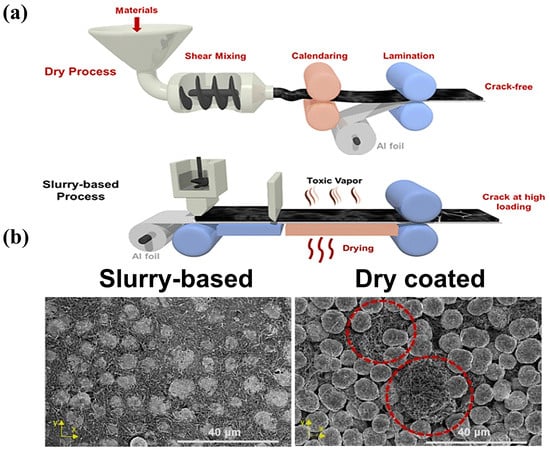
- I.
-
It is environmentally friendly and suitable for large-scale production.
- II.
-
It exhibits a flatter electrode shape than that from the wet process.
- III.
-
It offers greater compaction density.
- IV.
-
It improves the performance of the battery
- V.
-
It allows for the possibility of prepare solid-state batteries.
4.2. Development Status of SF Process with PTFE Binder
4.2.1. Effect of PTFE on SF Batteries
- I.
-
Side reactions of PTFE binders
- II.
-
Crystallinity
- III.
-
Modified materials

4.2.2. Influence of Components Other Than Binders
- I.
-
Conductive additives
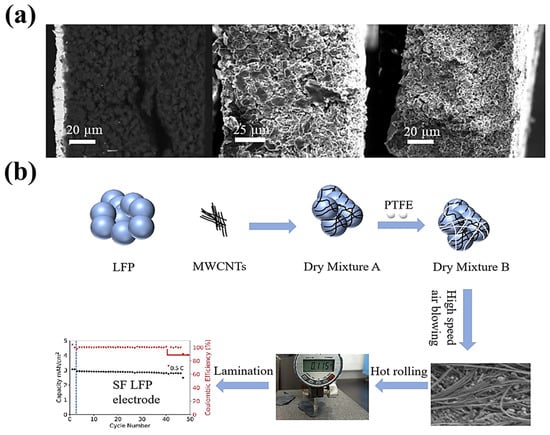
- II.
-
Electrode materials
- III.
-
Collectors
4.2.3. Innovative Technology and System
- I.
-
High-speed airflow technology
- II.
-
Lithium-sulfur (Li-S) batteries
- III.
-
High-voltage batteries
- IV.
-
High-load batteries
- V.
-
Solid-state batteries
References
- Dang, C.C.; Mu, Q.; Xie, X.B.; Sun, X.Q.; Yang, X.Y.; Zhang, Y.P.; Maganti, S.; Huang, M.N.; Jiang, Q.L.; Seok, I.; et al. Recent Progress in Cathode Catalyst for Nonaqueous Lithium Oxygen Batteries: A review. Adv. Compos. Hybrid Mater. 2022, 5, 606–626.
- Eng, A.Y.S.; Soni, C.B.; Lum, Y.; Khoo, E.; Yao, Z.; Vineeth, S.K.; Kumar, V.; Lu, J.; Johnson, C.S.; Wolverton, C.; et al. Theory-Guided Experimental Design in Battery Materials Research. Sci. Adv. 2022, 8, eabm2422.
- Jiang, M.; Danilov, D.L.; Eichel, R.A.; Notten, P.H.L. A Review of Degradation Mechanisms and Recent Achievements for Ni-Rich Cathode-Based Li-Ion Batteries. Adv. Energy Mater. 2021, 11, 2103005.
- Kalnaus, S.; Dudney, N.J.; Westover, A.S.; Herbert, E.; Hackney, S. Solid-State Batteries: The Critical Role of Mechanics. Science 2023, 381, eabg5998.
- Li, J.L.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956.
- Liu, W.; Placke, T.; Chau, K.T. Overview of Batteries and Battery Management for Electric Vehicles. Energy Rep. 2022, 8, 4058–4084.
- Wang, W.; Yuan, B.Q.; Sun, Q.; Wennersten, R. Application of Energy Storage in Integrated Energy Systems-A Solution to Fluctuation and Uncertainty of Renewable Energy. J. Energy Storage 2022, 52, 104812.
- Viswanathan, V.; Epstein, A.H.; Chiang, Y.M.; Takeuchi, E.; Bradley, M.; Langford, J.; Winter, M. The Challenges and Opportunities of Battery-Powered Flight. Nature 2022, 601, 519–525.
- Xing, C.W.; Li, M.C.; Liu, L.Y.; Lu, R.; Liu, N.; Wu, W.J.; Yuan, D.D. A Comprehensive Review on the Blending Condition Between Virgin and RAP Asphalt Binders in Hot Recycled Asphalt Mixtures: Mechanisms, Evaluation Methods, and Influencing Factors. J. Clean. Prod. 2023, 398, 136515.
- Zhu, C.; Usiskin, R.E.; Yu, Y.; Maier, J. The Nanoscale Circuitry of Battery Electrodes. Science 2017, 358, eaao2808.
- Ludwig, A.; Wu, M.; Kharicha, A. On The Importance of Modeling 3D Shrinkage Cavities for the Prediction of Macrosegregation in Steel Ingots. CFD Model. Simu. Mat. Pro. 2016, 2016, 1–10.
- Pillai, A.M.; Salini, P.S.; John, B.; Devassy, M.T. Aqueous Binders for Cathodes: A Lodestar for Greener Lithium Ion Cells. Energy Fuels 2022, 36, 5063–5087.
- Guo, R.N.; Han, W.Q. Effects of Structure and Properties of Polar Polymeric Binders on Lithium-ion Batteries. Inorg. Mater. 2019, 34, 1021–1029.
- Wang, Y.B.; Yang, Q.; Guo, X.; Yang, S.; Chen, A.; Liang, G.J.; Zhi, C.Y. Strategies of Binder Design for High-Performance Lithium-Ion Batteries: A Mini Review. Rare Metals 2022, 41, 745–761.
- Pettinger, K.-H.; Dong, W. When Does the Operation of a Battery Become Environmentally Positive? J. Electrochem. Soc. 2017, 164, A6274.
- Al-Shroofy, M.; Zhang, Q.; Xu, J.; Chen, T.; Kaur, A.P.; Cheng, Y.-T. Solvent-Free Dry Powder Coating Process for Low-Cost Manufacturing of LiNi1/3Mn1/3Co1/3O2 Cathodes in Lithium-Ion Batteries. J. Power Sources 2017, 352, 187–193.
- Ludwig, B.; Liu, J.; Chen, I.M.; Liu, Y.; Shou, W.; Wang, Y.; Pan, H. Understanding Interfacial-Energy-Driven Dry Powder Mixing for Solvent-Free Additive Manufacturing of Li-Ion Battery Electrodes. Ad. Mater. Interfaces 2017, 4, 1700570.
- Shiraki, S.; Oki, H.; Takagi, Y.; Suzuki, T.; Kumatani, A.; Shimizu, R.; Haruta, M.; Ohsawa, T.; Sato, Y.; Ikuhara, Y.; et al. Fabrication of All-Solid-State Battery Using Epitaxial LiCoO2 Thin Films. J. Power Sources 2014, 267, 881–887.
- Subramanyam, G.; Cole, M.W.; Sun, N.X.; Kalkur, T.S.; Sbrockey, N.M.; Tompa, G.S.; Guo, X.; Chen, C.; Alpay, S.P.; Rossetti, G.A.; et al. Challenges and Opportunities for Multi-Functional Oxide Thin Films for Voltage Tunable Radio Frequency/Microwave Components. J. Appl. Phys. 2013, 114, 191301.
- Sotomayor, M.E.; Torre-Gamarra, C.d.l.; Levenfeld, B.; Sanchez, J.-Y.; Varez, A.; Kim, G.-T.; Varzi, A.; Passerini, S. Ultra-Thick Battery Electrodes for High Gravimetric and Volumetric Energy Density Li-Ion Batteries. J. Power Sources 2019, 437, 226923.
- Trembacki, B.; Duoss, E.; Oxberry, G.; Stadermann, M.; Murthy, J. Mesoscale Electrochemical Performance Simulation of 3D Interpenetrating Lithium-Ion Battery Electrodes. J. Electrochem. Soc. 2019, 166, A923.
- Carneiro, O.S.; Silva, A.F.; Gomes, R. Fused Deposition Modeling with Polypropylene. Mater. Des. 2015, 83, 768–776.
- Kirsch, D.J.; Lacey, S.D.; Kuang, Y.; Pastel, G.; Xie, H.; Connell, J.W.; Lin, Y.; Hu, L. Scalable Dry Processing of Binder-Free Lithium-Ion Battery Electrodes Enabled by Holey Graphene. ACS Appl. Energy Mater. 2019, 2, 2990–2997.
- Han, X.; Funk, M.R.; Shen, F.; Chen, Y.-C.; Li, Y.; Campbell, C.J.; Dai, J.; Yang, X.; Kim, J.W.; Liao, Y.; et al. Scalable Holey Graphene Synthesis and Dense Electrode Fabrication toward High-Performance Ultracapacitors. ACS Nano 2014, 8, 8255–8265.
- Lee, D.J.; Jang, J.; Lee, J.-P.; Wu, J.; Chen, Y.-T.; Holoubek, J.; Yu, K.; Ham, S.-Y.; Jeon, Y.; Kim, T.-H.; et al. Physio-Electrochemically Durable Dry-Processed Solid-State Electrolyte Films for All-Solid-State Batteries. Adv. Funct. Mater. 2023, 33, 2301341.
- Wood, D.L.; Wood, M.; Li, J.; Du, Z.; Ruther, R.E.; Hays, K.A.; Muralidharan, N.; Geng, L.; Mao, C.; Belharouak, I. Perspectives on The Relationship Between Materials Chemistry and Roll-to-Roll Electrode Manufacturing for High-Energy Lithium-Ion Batteries. Energy Stor. Mater. 2020, 29, 254–265.
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-ion Batteries. Sci. Rep. 2016, 6, 23150.
- Li, Y.; Wu, Y.; Wang, Z.; Xu, J.; Ma, T.; Chen, L.; Li, H.; Wu, F. Progress in Solvent-Free Dry-Film Technology for Batteries and Supercapacitors. Mater. Today 2022, 55, 92–109.
- Lu, Y.; Zhao, C.-Z.; Yuan, H.; Hu, J.-K.; Huang, J.-Q.; Zhang, Q. Dry Electrode Technology, The Rising Star in Solid-state Battery Industrialization. Matter 2022, 5, 876–898.
- Zou, F.; Manthiram, A. A Review of the Design of Advanced Binders for High-Performance Batteries. Adv. Energy Mater. 2020, 10, 2002508.
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and Prospects of PVDF Based Polymer Electrolytes. J. Energy Chem. 2022, 64, 62–84.
- Zhu, T.; Sternlicht, H.; Ha, Y.; Fang, C.; Liu, D.; Savitzky, B.H.; Zhao, X.; Lu, Y.; Fu, Y.; Ophus, C.; et al. Formation of Hierarchically Ordered Structures in Conductive Polymers to Enhance the Performances of Lithium-Ion Batteries. Nat. Energy 2023, 8, 129–137.
- Oh, J.; Choi, S.H.; Chang, B.; Lee, J.; Lee, T.; Lee, N.; Kim, H.; Kim, Y.; Im, G.; Lee, S.; et al. Elastic Binder for High-Performance Sulfide-Based All-Solid-State Batteries. ACS Energy Lett. 2022, 7, 1374–1382.
- Mu, P.; Zhang, H.; Jiang, H.; Dong, T.; Zhang, S.; Wang, C.; Li, J.; Ma, Y.; Dong, S.; Cui, G. Bioinspired Antiaging Binder Additive Addressing the Challenge of Chemical Degradation of Electrolyte at Cathode/Electrolyte Interphase. J. Am. Chem. Soc. 2021, 143, 18041–18051.
- Maleki, H.; Deng, G.; Kerzhner-Haller, I.; Anani, A.; Howard, J.N. Thermal Stability Studies of Binder Materials in Anodes for Lithium-Ion Batteries. J. Electrochem. Soc 2000, 147, 4470.
- Yonaga, A.; Kawauchi, S.; Mori, Y.; Xuanchen, L.; Ishikawa, S.; Nunoshita, K.; Inoue, G.; Matsunaga, T. Effects of Dry Powder Mixing on Electrochemical Performance of Lithium-ion Battery Electrode Using Solvent-Free Dry Forming Process. J. Power Sources 2023, 581, 233466.
- Zhang, Z.; Han, D.; Xiao, M.; Wang, S.; Feng, Y.; Huang, S.; Meng, Y. New Potential Substitute of PVDF Binder: Poly(propylene carbonate) for Solvent-Free Manufacturing High-Loading Cathodes of LiFePO4|Li Batteries. Ionics 2023, 29, 3895–3906.
- Abdel-Hakim, A.; El-Basheer, T.M.; Abdelkhalik, A. Mechanical, Acoustical and Flammability Properties of SBR and SBR-PU Foam Layered Structure. Poly. Test. 2020, 88, 106536.
- Li, Y.; Wu, Y.; Ma, T.; Wang, Z.; Gao, Q.; Xu, J.; Chen, L.; Li, H.; Wu, F. Long-Life Sulfide All-Solid-State Battery Enabled by Substrate-Modulated Dry-Process Binder. Adv. Energy Mater. 2022, 12, 2201732.
- Park, J.; Willenbacher, N.; Ahn, K.H. How the Interaction Between Styrene-Butadiene-Rubber (SBR) Binder and a Secondary Fluid Affects the Rheology, Microstructure and Adhesive Properties of Capillary-Suspension-Type Graphite Slurries Used for Li-ion Battery Anodes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123692.
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H.; Kita, R. Properties Enhancement of Carboxymethyl Cellulose with Thermo-Responsive Polymer as Solid Polymer Electrolyte for Zinc Ion Battery. Sci. Rep. 2020, 10, 12587.
- Kim, J.; Choi, J.; Park, K.; Kim, S.; Nam, K.W.; Char, K.; Choi, J.W. Host–Guest Interlocked Complex Binder for Silicon–Graphite Composite Electrodes in Lithium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103718.
- Ibrahim, S.M.; El Salmawi, K.M. Preparation and Properties of Carboxymethyl Cellulose (CMC)/Sodium alginate (SA) Blends Induced by Gamma Irradiation. J. Polym. Environ. 2013, 21, 520–527.
- Xu, Z.; Yang, J.; Zhang, T.; Nuli, Y.; Wang, J.; Hirano, S.-i. Silicon Microparticle Anodes with Self-Healing Multiple Network Binder. Joule 2018, 2, 950–961.
- Hu, Y.; Shao, D.; Chen, Y.; Peng, J.; Dai, S.; Huang, M.; Guo, Z.-H.; Luo, X.; Yue, K. A Physically Cross-Linked Hydrogen-Bonded Polymeric Composite Binder for High-Performance Silicon Anodes. ACS Appl. Energy Mater. 2021, 4, 10886–10895.
- Senthil, C.; Kim, S.-S.; Jung, H.Y. Flame Retardant High-Power Li-S Flexible Batteries Enabled by Bio-macromolecular Binder Integrating Conformal Fractions. Nat. Commun. 2022, 13, 145.
- Mackanic, D.G.; Yan, X.; Zhang, Q.; Matsuhisa, N.; Yu, Z.; Jiang, Y.; Manika, T.; Lopez, J.; Yan, H.; Liu, K.; et al. Decoupling of Mechanical Properties and Ionic Conductivity in Supramolecular Lithium Ion Conductors. Nat. Commun. 2019, 10, 5384.
- Dong, T.; Zhang, H.; Hu, R.; Mu, P.; Liu, Z.; Du, X.; Lu, C.; Lu, G.; Liu, W.; Cui, G. A Rigid-Flexible Coupling Poly (vinylene carbonate) Based Cross-Linked Network: A versatile Polymer Platform for Solid-state Polymer Lithium Batteries. Energy Stor. Mater. 2022, 50, 525–532.
- Xia, J.; Wang, Z.; Rodrig, N.D.; Nan, B.; Zhang, J.; Zhang, W.; Lucht, B.L.; Yang, C.; Wang, C. Super-Reversible CuF2 Cathodes Enabled by Cu2+-Coordinated Alginate. Adv. Mater. 2022, 34, 2205229.
- Jeong, Y.K.; Kwon, T.-w.; Lee, I.; Kim, T.-S.; Coskun, A.; Choi, J.W. Millipede-inspired structural design principle for high performance polysaccharide binders in silicon anodes. Energy Environ. Sci. 2015, 8, 1224–1230.
- Strand, A.; Kouko, J.; Oksanen, A.; Salminen, K.; Ketola, A.; Retulainen, E.; Sundberg, A. Enhanced Strength, Stiffness and Elongation Potential of Paper by Spray Addition of Polysaccharides. Cellulose 2019, 26, 3473–3487.
- Qin, T.; Yang, H.; Li, Q.; Yu, X.; Li, H. Design of Functional Binders for High-Specific-Energy Lithium-Ion Batteries: From Molecular Structure to Electrode Properties. Ind. Eng. Chem. Res. 2023.
- Wang, X.; Liu, S.; Zhang, Y.; Wang, H.; Aboalhassan, A.A.; Li, G.; Xu, G.; Xue, C.; Yu, J.; Yan, J.; et al. Highly Elastic Block Copolymer Binders for Silicon Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 38132–38139.
- Ignatieva, L.N.; Mashchenko, V.A.; Zverev, G.A.; Ustinov, A.Y.; Slobodyuk, A.B.; Bouznik, V.M. Study of The Manufactured Copolymers of Ethylene with Tetrafluoroethylene. J. Fluor. Chem. 2020, 231, 109460.
- Zhang, Y.; Lu, S.; Wang, Z.; Volkov, V.; Lou, F.; Yu, Z. Recent Technology Development in Solvent-Free Electrode Fabrication for Lithium-Ion Batteries. Renew. Sust. Energ. Rev. 2023, 183, 113515.
- Huang, S.; Huang, X.T.; Huang, Y.Y.; He, X.Q.; Zhuo, H.T.; Chen, S.J. Rational Design of Effective Binders for LiFePO4 Cathodes. Polymers 2021, 13, 3146.
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and Future Lithium-Ion Battery Manufacturing. iScience 2021, 24, 102332.
- Gao, S.; Su, Y.; Bao, L.; Li, N.; Chen, L.; Zheng, Y.; Tian, J.; Li, J.; Chen, S.; Wu, F. High-Performance LiFePO4/C Electrode with Polytetrafluoroethylene as An Aqueous-Based Binder. J. Power Sources 2015, 298, 292–298.
- Li, W.; Mays, S.; Lam, D. Material and Finite Element Analysis of Poly(tetrafluoroethylene) otary Seals. Plast. Rubber Compos. 2002, 31, 359–363.
- Brown, E.N.; Rae, P.J.; Bruce Orler, E.; Gray, G.T.; Dattelbaum, D.M. The Effect of Crystallinity on the Fracture of Polytetrafluoroethylene (PTFE). Mater. Sci. Eng. C 2006, 26, 1338–1343.
- Brown, E.N.; Dattelbaum, D.M. The Role of Crystalline Phase on Fracture and Microstructure Evolution of Polytetrafluoroethylene (PTFE). Polymer 2005, 46, 3056–3068.
- Joyce, J.A. Fracture Toughness Evaluation of Polytetrafluoroethylene. Polym. Eng. Sci. 2003, 43, 1702–1714.
- Rae, P.J.; Brown, E.N. The Properties of Poly(tetrafluoroethylene) (PTFE) in Tension. Polymer 2005, 46, 8128–8140.
- Pruitt, L.A. Deformation, Yielding, Fracture and Fatigue Behavior of Conventional and Highly Cross-Linked Ultra High Molecular Weight Polyethylene. Biomaterials 2005, 26, 905–915.
- Brown, E.N.; Trujillo, C.P.; Gray, G.T.; Rae, P.J.; Bourne, N.K. Soft Recovery of Polytetrafluoroethylene Shocked Through The Crystalline Phase II-III Transition. J. Appl. Phys. 2007, 101, 024916.
- Millett, J.C.F.; Brown, E.N.; Gray, G.T.; Bourne, N.K.; Wood, D.C.; Appleby-Thomas, G. The Effects of Changing Chemistry on the Shock Response of Basic Polymers. J. Dyn. Behav. Mater. 2016, 2, 326–336.
- Lee, D.; Manthiram, A. Stable Cycling with Intimate Contacts Enabled by Crystallinity-Controlled PTFE-Based Solvent-Free Cathodes in All-Solid-State Batteries. Small Methods 2023, 7, 2201680.
- Kitamura, T.; Okabe, S.; Tanigaki, M.; Kurumada, K.; Ohshima, M.; Kanazawa, S. Morphology Change in Polytetrafluoroethylene (PTFE) Porous Membrane Caused by Heat Treatment. Polym. Eng. Sci. 2000, 40, 809–817.
- Wecker, S.M.; Davidson, T.; Baker, D.W. Preferred Orientation of Crystallites in Uniaxially Deformed Polytetrafluoroethylene. J. Appl. Phys. 1972, 43, 4344–4348.
- Puts, G.J.; Crouse, P.; Ameduri, B.M. Polytetrafluoroethylene: Synthesis and Characterization of the Original Extreme Polymer. Chem. Rev. 2019, 119, 1763–1805.
- Wu, J.; Wang, H.; Feng, B.; Li, Y.; Wu, S.; Yin, Q.; Yu, Z.; Huang, J. The Effect of Temperature-Induced Phase Transition of PTFE on The Dynamic Mechanical Behavior and Impact-Induced Initiation Characteristics of Al/PTFE. Polym. Test. 2020, 91, 106835.
- Sato, K.; Tominaga, Y.; Imai, Y.; Yoshiyama, T.; Aburatani, Y. Deformation Capability of Poly(tetrafluoroethylene) Materials: Estimation with X-ray Diffraction Measurements. Polym. Test 2022, 113, 107690.
- Hieu, D.; Joon, S.; Yudi, Y. Dry Electrode Coating Technology; Maxwell Technologies: San Diego, CA, USA, 2018; Available online: https://api.semanticscholar.org/CorpusID:201928996 (accessed on 19 October 2023).
- Zhang, A.; Chai, J.; Yang, C.; Zhao, J.; Zhao, G.; Wang, G. Fibrosis Mechanism, Crystallization Behavior and Mechanical Properties of In-Situ Fibrillary PTFE Reinforced PP Composites. Mater. Des. 2021, 211, 110157.
- Yao, W.; Chouchane, M.; Li, W.; Bai, S.; Liu, Z.; Li, L.; Chen, A.X.; Sayahpour, B.; Shimizu, R.; Raghavendran, G.; et al. A 5 V-Class Cobalt-Free Battery Cathode with High Loading Enabled by Dry Coating. Energy Environ. Sci. 2023, 16, 1620–1630.
- Wu, Q.; Zheng, J.P.; Hendrickson, M.; Plichta, E.J. Dry Process for Fabricating Low Cost and High Performance Electrode for Energy Storage Devices. MRS Adv. 2019, 4, 857–863.
- Li, G. The Influence of Polytetrafluorethylene Reduction on the Capacity Loss of the Carbon Anode for Lithium Ion Batteries. Solid State Ion. 1996, 90, 221–225.
- Tao, R.; Tan, S.; Meyer Iii, H.M.; Sun, X.-G.; Steinhoff, B.; Sardo, K.; Bishtawi, A.; Gibbs, T.; Li, J. Insights into the Chemistry of the Cathodic Electrolyte Interphase for PTFE-Based Dry-Processed Cathodes. ACS Appl. Mater. Interfaces 2023, 15, 40488–40495.
- Hong, S.B.; Lee, Y.J.; Kim, U.H.; Bak, C.; Lee, Y.M.; Cho, W.; Hah, H.J.; Sun, Y.K.; Kim, D.W. All-Solid-State Lithium Batteries: Li+-Conducting Ionomer Binder for Dry-Processed Composite Cathodes. ACS Energy Letter. 2022, 7, 1092–1100.
- Zhang, Y.; Huld, F.; Lu, S.; Jektvik, C.; Lou, F.; Yu, Z. Revisiting Polytetrafluorethylene Binder for Solvent-Free Lithium-Ion Battery Anode Fabrication. Batteries 2022, 8, 57.
- Zhang, Y.; Lu, S.; Lou, F.; Yu, Z. Solvent-free Lithium Iron Phosphate Cathode Fabrication with Fibrillation of Polytetrafluoroethylene. Electrochim. Acta 2023, 456, 142469.
- Zhou, H.; Liu, M.; Gao, H.; Hou, D.; Yu, C.; Liu, C.; Zhang, D.; Wu, J.-C.; Yang, J.; Chen, D. Dense Integration of Solvent-Free Electrodes for Li-Ion Supercabattery with Boosted Low Temperature Performance. J. Power Sources 2020, 473, 228553.
- Fiedler, M.; Cangaz, S.; Hippauf, F.; Dörfler, S.; Abendroth, T.; Althues, H.; Kaskel, S. Mechanistic Insights into the Cycling Behavior of Sulfur Dry-Film Cathodes. Adv. Sustain. Syst. 2023, 7, 2200439.
- Tao, R.; Steinhoff, B.; Sun, X.-G.; Sardo, K.; Skelly, B.; Meyer, H.M.; Sawicki, C.; Polizos, G.; Lyu, X.; Du, Z.; et al. High-Throughput and High-performance Lithium-ion Batteries via Dry Processing. Chem. Eng. J. 2023, 471, 144300.
- Shin, D.; Nam, J.S.; Linh Nguyen, C.T.; Jo, Y.; Lee, K.; Hwang, S.M.; Kim, Y.J. Design of Densified Nickel-Rich Layered Composite Cathode via The Dry-Film Process for Sulfide-Based Solid-State Batteries. J. Mater. Chem. A 2022, 10, 23222–23231.




