
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Krzysztof Gomułka | -- | 3459 | 2023-12-13 11:54:40 | | | |
| 2 | Lindsay Dong | -3 word(s) | 3456 | 2023-12-19 04:08:52 | | | | |
| 3 | Sarmoko Sarmoko | -6 word(s) | 3409 | 2024-08-05 12:42:03 | | |
Video Upload Options
Diabetes mellitus (DM) is a growing problem nowadays, and diabetic retinopathy (DR) is its predominant complication.
1. Introduction
2. Risk Factors
2.1 Duration of Diabetes and Hyperglycemia
2.2 Nephropathy and High BMI
2.3 Smoking
2.4 Pregnancy
3. Pathophysiology
4. Molecular Biomarkers
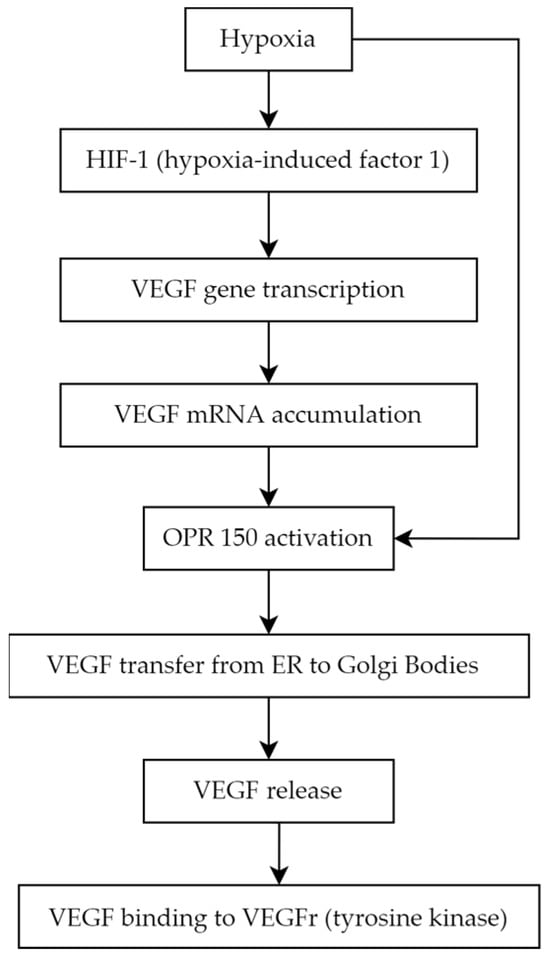
4.1. Vascular Endothelial Growth Factor
- VEGF-A (also called VEGF or vascular permeability factor, the first discovered molecule of the whole family in 1983)
- VEGF-B
- VEGF-C (essential for the formation of lymphatic vessels) [27]
- VEGF-D (known as c-Fos-induced growth factor, FIGF)
- VEGF-E (connected with parapoxvirus Orf, which causes pustular dermatitis) [28]
- placenta growth factor (PGF) [29][30].
4.2. Asymmetric Dimethylarginine
4.3. MicroRNAs
4.4. Endothelin-1
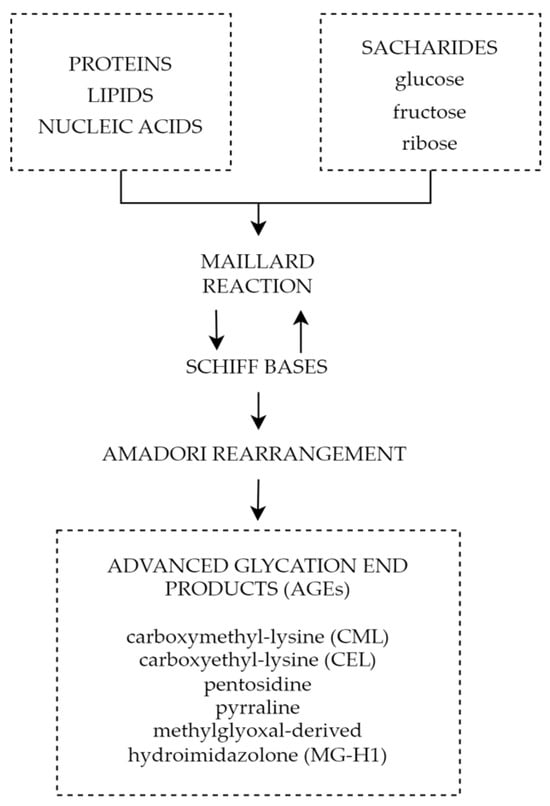
4.5. Advanced Glycation End Products
5. Summary
ADMA inhibits the activity of NOS, which results in decreased levels of NO and leads to vasoconstriction and endothelial dysfunction. Increased ADMA levels may be considered an early prognostic factor of diabetes complications such as PDR. The use of ADMA as a biomarker may help in early diagnosis, monitoring, and effective therapeutic management of the disease. Reducing ADMA levels in patients with diabetes may be a new therapeutic target to prevent the development of diabetic retinopathy. Endothelin-1 is another factor with an undoubted relationship to diabetic retinopathy. Increased serum and aqueous humor levels are observed in patients with ET-1 elevation dependent on the severity of the progression of the disease. This, juxtaposed with promising results of ET-1 receptor antagonist animal studies, showcases the potential of ET-1 as a possible target for future therapy. It is important to note that miRNAs are not only supposed to be an innovative predictive biomarker and progression indicator in DR but also a potential therapeutic target. Different miRNAs can be found in T1DM and T2DM as well depending on sample type, moreover, some of them differ depending on DR type. The variety of miRNAs and frequently high amounts of particles involved in several pathogenesis pathways can be at the same time the advantage and disadvantage of that prospective novel biomarkers group; hence, miRNAs panels are more adequate than a single biomarker rating. Finally, advanced glycation end products play a significant role in the pathophysiology of diabetic retinopathy causing impairment of the neurovascular units through reactive oxygen species, inflammatory reactions, and cell death pathways. All the above mechanisms play a significant role not only in diabetic retinal disorders, but also other chronic oxidative-based diseases; therefore, a thorough understanding of their properties and mechanisms will allow advances in the diagnosis and treatment of chronic diseases and most importantly diabetic retinopathy. The above factors and signaling pathways can help to create multimodal and highly specified therapies for patients suffering from DR. It is crucial to investigate molecular agents participating in DR pathogenesis. Hopefully, it will provide the ability to inhibit this progressive disease at its early stage.
References
- Tan, T.E.; Wong, T.Y. Diabetic retinopathy: Looking forward to 2030. Front. Endocrinol. 2023, 13, 1077669.
- International Diabetes Federation. International Diabetes Federation—Facts & Figures. Idf.org. Published 12 September 2021. Available online: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 15 May 2023).
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136.
- Modjtahedi, B.S.; Wu, J.; Luong, T.Q.; Gandhi, N.K.; Fong, D.S.; Chen, W. Severity of Diabetic Retinopathy and the Risk of Future Cerebrovascular Disease, Cardiovascular Disease, and All-Cause Mortality. Ophthalmology 2021, 128, 1169–1179.
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591.
- Lin, K.Y.; Hsih, W.H.; Lin, Y.B.; Wen, C.Y.; Chang, T.J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J. Diabetes Investig. 2021, 12, 1322–1325.
- Chew, E.Y.; Davis, M.D.; Danis, R.P.; Locato, J.F. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014, 121, 2443–2451.
- Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group; The Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Study Group. Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) follow-on study. Diabetes Care 2016, 39, 1089–1100.
- Kaštelan, S.; Tomić, M.; Gverović Antunica, A.; Ljubić, S.; Salopek Rabatić, J.; Karabatić, M. Body mass index: A risk factor for retinopathy in type 2 diabetic patients. Mediat. Inflamm. 2013, 2013, 436329.
- Chang, Y.C.; Wu, W.C. Dyslipidemia and diabetic retinopathy. Rev. Diabet. Stud. 2013, 10, 121–132.
- Kohner, E.M.; Aldington, S.J.; Stratton, I.M.; Manley, S.E.; Holman, R.R.; Matthews, D.R.; Turner, R.C. United Kingdom Prospective Diabetes Study 30. Diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch. Ophthalmol. 1998, 116, 297–303.
- Klein, R.; Sharrett, A.R.; Klein, B.E.; Moss, S.E.; Folsom, A.R.; Wong, T.Y.; Brancati, F.L.; Hubbard, L.D.; Couper, D.; ARIC Group. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: The Atherosclerosis Risk in Communities study. Ophthalmology 2002, 109, 1225–1234.
- Klein, R.; Marino, E.K.; Kuller, L.H.; Polak, J.F.; Tracy, R.P.; Gottdiener, J.S.; Burke, G.L.; Hubbard, L.D.; Boineau, R. The relation of atherosclerotic cardiovascular disease to retinopathy in people with diabetes in the Cardiovascular Health Study. Br. J. Ophthalmol. 2002, 86, 84–90.
- van Leiden, H.A.; Dekker, J.M.; Moll, A.C.; Nijpels, G.; Heine, R.J.; Bouter, L.M.; Stehouwer, C.D.; Polak, B.C. Blood pressure, lipids, and obesity are associated with retinopathy: The Hoorn study. Diabetes Care 2002, 25, 1320–1325.
- Vujosevic, S.; Aldington, S.J.; Silva, P.; Peto, P. Screening for diabetic retinopathy: New perspectives and challenges. Lancet Diabetes Endocrinol. 2020, 8, 337–347.
- Cai, X.; Chen, Y.; Yang, W.; Gao, X.; Han, X.; Ji, L. The association of smoking and risk of diabetic retinopathy in patients with type 1 and type 2 diabetes: A meta-analysis. Endocrine 2018, 62, 299–306.
- Rasmussen, K.L.; Laugesen, C.S.; Ringholm, L.; Vestgaard, M.; Damm, P.; Mathiesen, E.R. Progression of diabetic retinopathy during pregnancy in women with type 2 diabetes. Diabetologia 2010, 53, 1076–1083.
- Rubsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942.
- Simo, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912.
- Ansari, P.; Tabasumma, N.; Snigdha, N.N.; Siam, N.H.; Panduru, R.V.N.R.S.; Azam, S.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy. Diabetology 2022, 3, 159–175.
- Kowluru, R.A.; Santos, J.M.; Mishra, M. Epigenetic modifications and diabetic retinopathy. Biomed. Res. Int. 2013, 2013, 635284.
- Kollias, A.N.; Ulbig, M.W. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch. Arztebl. Int. 2010, 107, 75–83.
- Williams, B.; Baker, A.Q.; Gallacher, B.; Lodwick, D. Angiotensin II increases vascular permeability factor gene expression by human vascular smooth muscle cells. Hypertension 1995, 25, 913–917.
- Viswanath, K.; McGavin, D.D. Diabetic retinopathy: Clinical findings and management. Community Eye Health 2003, 16, 21–24.
- Roy, S.; Kim, D. Retinal capillary basement membrane thickening: Role in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2021, 82, 100903.
- Nawaz, I.M.; Rezzola, S.; Cancarini, A.; Russo, A.; Costagliola, C.; Semeraro, F.; Presta, M. Human vitreous in proliferative diabetic retinopathy: Characterization and translational implications. Prog. Retin. Eye Res. 2019, 72, 100756.
- Homsi, J.; Daud, A.I. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control 2007, 14, 285–294.
- Shibuya, M. Vascular endothelial growth factor receptor-2: Its unique signaling and specific ligand, VEGF-E. Cancer Sci. 2005, 94, 751–756.
- Arrigo, A.; Aragona, E.; Bandello, F. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann. Med. 2022, 54, 1089–1111.
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209.
- Gupta, N.; Mansoor, S.; Sharma, A.; Sapkal, A.; Sheth, J.; Falatoonzadeh, P.; Kuppermann, B.; Kenney, M. Diabetic retinopathy and VEGF. Open Ophthalmol. J. 2013, 7, 4–10.
- Ferrara, N. Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocr. Rev. 2004, 25, 581–611.
- Stuttfeld, E.; Ballmer-Hofer, K. Structure and function of VEGF receptors. IUBMB Life 2009, 61, 915–922.
- Clauss, M. Molecular Biology of the VEGF and the VEGF Receptor Family. Semin. Thromb. Hemost. 2000, 26, 561–570.
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281.
- Claesson-Welsh, L. VEGF receptor signal transduction—A brief update. Vasc. Pharmacol. 2016, 86, 14–17.
- Carmeliet, P.; Ferreira, V.; Breier, G.; Harpal, K. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439.
- Bucolo, C.; Barbieri, A.; Vigano, I.; Band, F. Short-and Long-Term Expression of Vegf: A Temporal Regulation of a Key Factor in Diabetic Retinopathy. Front. Pharmacol. 2021, 12, 707909.
- Grillo, M.A.; Colombatto, S. Arginine revisited: Minireview article. Amino Acids 2004, 26, 345–351.
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992.
- Yonem, A.; Duran, C.; Unal, M.; Ipcioglu, O.M.; Ozcan, O. Plasma apelin and asymmetric dimethylarginine levels in type 2 diabetic patients with diabetic retinopathy. Diabetes Res. Clin. Pract. 2009, 84, 219–223.
- Narayanan, P.S.; Rojas, M.; Suwanpradid, J.; Toque, H.A.; Caldwell, W.R.; Caldwell, R.B. Arginase in retinopathy. Prog. Retin. Eye Res. 2013, 36, 260–280.
- Sena, C.M.; Pereira, A.M.; Seica, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231.
- Forstermann, U.; Closs, E.I.; Pollock, J.S.; Nakane, M.; Schwarz, P.; Gath, I.; Kleinert, H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 1994, 23, 1121–1131.
- Toutouzas, K.; Riga, M.; Stefanadi, E.; Stefanadis, C. Asymmetric dimethylarginine (ADMA) and other endogenous nitric oxide synthase (NOS) inhibitors as an important cause of vascular insulin resistance. Horm. Metab. Res. 2008, 40, 655–659.
- Bode-Boger, S.M.; Scalera, F.; Martens-Lobenhoffer, J. Asymmetric dimethylarginine (ADMA) accelerates cell senescence. Vasc. Med. 2005, 10, 65–71.
- Sirman, Y.V.; Savytskyi, I.V. Study of endothelial dysfunction and asymmetric dimethylarginine levels. J. Educ. Health Sport 2019, 9, 395–412.
- Leiper, J.M.; Vallance, P. The synthesis and metabolism of asymmetric dimethylarginine (ADMA). Eur. J. Clin. Pharmacol. 2006, 62, 33–38.
- Morris, S.M. Arginine metabolism revisited. J. Nutr. 2016, 146, 2579–2586.
- Trocha, M.; Merwid-Lad, A.; Szuba, A.; Sozanski, T.; Magdalan, J.; Szelag, A. Asymmetric dimethylarginine synthesis and degradation under physiological and pathological conditions. Adv. Clin. Exp. Med. 2010, 19, 233–243.
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575.
- Sydow, K.; Munzel, T. ADMA and oxidative stress. Atheroscler. Suppl. 2003, 4, 41–51.
- Cardounel, A.J.; Cui, H.; Samouilov, A.; Johnson, W.; Kearns, P.; Tsai, A.-L.; Berka, V.; Zweier, J.L. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J. Biol. Chem. 2007, 282, 879–887.
- Jian, Q.; Wu, Y.; Zhang, F. Metabolomics in diabetic retinopathy: From potential biomarkers to molecular basis of oxidative stress. Cells 2022, 11, 3005.
- Peters, K.S.; Rivera, E.; Warden, C.; Harlow, P.A.; Mitchell, S.L.; Calcutt, M.W.; Samuels, D.C.; Brantley, M.A. Plasma arginine and citrulline are elevated in diabetic retinopathy. Am. J. Ophthalmol. 2022, 235, 154–162.
- Sumarriva, K.; Uppal, K.; Ma, C.; Herren, D.J.; Wang, Y.; Chocron, I.M.; Warden, C.; Mitchell, S.L.; Burgess, G.L.; Goodale, M.P.; et al. Arginine and carnitine metabolites are altered in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3119–3126.
- Dag, U.; Caglayan, M.; Alakus, M.F.; Oncul, H. The relationship between reduced choroidal thickness due to high plasma asymmetrical dimethylarginine level and increased severity of diabetic retinopathy. Arq. Bras. Oftalmol. 2023, 86, 27–32.
- Lamprou, S.; Koletsos, N.; Mintziori, G.; Anyfanti, P.; Trakatelli, C.; Kotsis, V.; Gkaliagkousi, E.; Triantafyllou, A. Microvascular and endothelial dysfunction in prediabetes. Life 2023, 13, 644.
- Krasnicki, P.; Proniewska-Skretek, E.; Dmuchowska, D.A.; Dobrzycki, S.; Mariak, Z. Asymmetric dimethylarginine (ADMA) as a marker of blood flow disturbances in ocular circulation in patients with type 2 diabetes and coronary artery disease. Mag. Lek. Okulisty 2009, 3, 325–331.
- Tousoulis, D.; Kampoli, A.-M.; Stefanadis, C. Diabetes mellitus and vascular endothelial dysfunction: Current perspectives. Curr. Vasc. Pharmacol. 2012, 10, 19–32.
- Stepien, E.; Szuscik, I.; Tokarz, A.; Enguita, F.J.; Solnica, B.; Zurakowski, A.; Malecki, M. The role of microparticles in pathomechanisms of diabetic retinopathy—Analysis of intercellular communication mechanisms in endothelial aging. Case control study in patients with metabolic syndrome, diabetes type 1 and type 2. J. Med. Sci. 2014, 83, 322–327.
- Huang, C.-Y.; Zhou, T.; Li, G.; Li, M.-Y.; Xiong, X.-M.; Wu, M.-T.; Jiang, J.-L. Asymmetric dimethylarginine aggravates blood-retinal barrier breakdown of diabetic retinopathy via inhibition of intercellular communication in retinal pericytes. Amino Acids 2019, 51, 1515–1526.
- Liu, J.; Li, C.; Chen, W.; He, K.; Ma, H.; Ma, B.; Zhao, P.; Tian, L. Relationship between serum asymmetric dimethylarginine level and microvascular. Bio. Med. Res. Int. 2019, 2019, 2941861.
- Alpay, A.; Ozcan, O.; Ugurbas, S.C.; Ugurbas, S.H. Investigated of vitreous and serum asymmetric dimethylarginine levels in diabetic. Res. Sq. 2019, 2019.
- Sugai, M.; Ohta, A.; Ogata, Y.; Nakanishi, M.; Ueno, S.; Kawata, T.; Saito, N.; Tanaka, Y. Asymmetric dimethylarginine (ADMA) in the aqueous humor of diabetic patients. Endocr. J. 2007, 54, 303–309.
- Abhary, S.; Kasmeridis, N.; Burdon, K.P.; Kuot, A.; Whiting, M.J.; Yew, W.P.; Petrovsky, N.; Craig, J.E. Diabetic retinopathy is associated with elevated serum asymmetric and symmetric dimethylarginines. Diabetes Care 2009, 32, 2084–2086.
- Eliana, F.; Suwondo, P.; Makmun, L.H.; Harbuwono, D.S. ADMA as a marker of endothelial dysfunction in prediabetic women. Acta Medica Indones. 2011, 43, 92–98.
- Du, M.-R.; Yan, L.; Li, N.-S.; Wang, Y.-J.; Zhou, T.; Jiang, J.-L. Asymmetric dimethylarginine contributes to retinal neovascularization of diabetic retinopathy through EphrinB2 pathway. Vasc. Pharmacol. 2018, 108, 46–56.
- Yun, J.H.; Kim, J.-M.; Jeon, H.J.; Oh, T.; Choi, H.J.; Kim, B.-J. Metabolomics profiles associated with diabetic retinopathy in type 2 diabetes patients. PLoS ONE 2020, 15, e241365.
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402.
- Ha, M.; Kim, V. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524.
- Karbasforooshan, H.; Karimi, G. The role of SIRT1 in diabetic retinopathy. Biomed. Pharmacother. 2018, 97, 190–194.
- Chang, X.; Zhu, G.; Cai, Z.; Wang, Y.; Lian, R.; Tang, X.; Ma, C.; Fu, S. miRNA, lncRNA and circRNA: Targeted Molecules Full of Therapeutic Prospects in the Development of Diabetic Retinopathy. Front. Endocrinol. 2021, 12, 771552.
- Ji, Q.; Han, J.; Wang, L.; Liu, J.; Dong, Y.; Zhu, K.; Shi, L. MicroRNA-34a promotes apoptosis of retinal vascular endothelial cells by targeting SIRT1 in rats with diabetic retinopathy. Cell Cycle 2020, 19, 2886–2896.
- Pan, Q.; Gao, Z.; Zhu, C.; Peng, Z.; Song, M.; Li, L. Overexpression of histone deacetylase SIRT1 exerts an antiangiogenic role in diabetic retinopathy via miR-20a elevation and YAP/HIF1α/VEGFA depletion. Am. J. Physiol. Endocrinol. Metab. 2020, 319, 932–943.
- Qin, B.; Liu, J.; Liu, S.; Li, B.; Ren, J. MiR-20b targets AKT3 and modulates vascular endothelial growth factor-mediated changes in diabetic retinopathy. Acta Biochim. Biophys. Sin. 2016, 48, 732–740.
- Maisto, R.; Trotta, M.C.; Petrillo, F.; Izzo, S.; Cuomo, G.; Alfano, R.; Hermenean, A.; Barcia, J.M.; Galdiero, M.; Platania, C.B.M.; et al. Resolvin D1 Modulates the Intracellular VEGF-Related miRNAs of Retinal Photoreceptors Challenged With High Glucose. Front. Pharmacol. 2020, 11, 235.
- Liu, H.N.; Cao, N.J.; Li, X.; Qian, W.; Chen, X.L. Serum microRNA-211 as a biomarker for diabetic retinopathy via modulating Sirtuin 1. Biochem. Biophys. Res. Commun. 2018, 505, 1236–1243.
- Miao, C.; Chang, J.; Zhang, G.; Fang, Y. MicroRNAs in type 1 diabetes: New research progress and potential directions. Biochem. Cell Biol. 2018, 96, 498–506.
- Liang, Z.; Gao, K.P.; Wang, Y.X.; Liu, Z.C.; Tian, L.; Yang, X.Z.; Ding, J.Y.; Wu, W.T.; Yang, W.H.; Li, Y.L.; et al. RNA sequencing identified specific circulating miRNA biomarkers for early detection of diabetes retinopathy. Am. J. Physiol. Endocrinol. Metab. 2018, 315, 374–385.
- Santovito, D.; Toto, L.; De Nardis, V.; Ces, D. Plasma microRNA signature associated with retinopathy in patients with type 2 diabetes. Sci Rep. 2021, 11, 4136.
- Guo, J.; Zhou, P.; Liu, Z.; Dai, F.; Pan, M.; An, G.; Han, J.; Du, L.; Jin, X. The Aflibercept-Induced MicroRNA Profile in the Vitreous of Proliferative Diabetic Retinopathy Patients Detected by Next-Generation Sequencing. Front. Pharmacol. 2021, 12, 781276.
- Saleh, A.A.; El-Hefnawy, S.M.; Kasemy, Z.A.; Alhagaa, A.A.; Nooh, M.Z.; Arafat, E.S. Mi-RNA-93 and Mi-RNA-152 in the Diagnosis of Type 2 Diabetes and Diabetic Retinopathy. Br. J. Biomed. Sci. 2022, 79, 10192.
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817.
- Ko, G.Y.; Yu, F.; Bayless, K.J.; Ko, M.L. MicroRNA-150 (miR-150) and Diabetic Retinopathy: Is miR-150 Only a Biomarker or Does It Contribute to Disease Progression? Int. J. Mol. Sci. 2022, 23, 99.
- Jenkins, H.N.; Rivera-Gonzalez, O.; Gibert, Y.; Speed, J.S. Endothelin-1 in the pathophysiology of obesity and insulin resistance. Obes. Rev. 2020, 21, e13086.
- Ergul, A. Endothelin-1 and diabetic complications: Focus on the vasculature. Pharmacol. Res. 2011, 63, 477–482.
- Stow, L.R.; Jacobs, M.E.; Wingo, C.S.; Cain, B.D. Endothelin-1 gene regulation. FASEB J. 2010, 25, 16–28.
- Kostov, K. The causal relationship between endothelin-1 and hypertension: Focusing on endothelial dysfunction, arterial stiffness, vascular remodeling, and Blood Pressure Regulation. Life 2021, 11, 986.
- Abman, S.H. Role of endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Annu. Rev. Med. 2009, 60, 13–23.
- Cheung, S.S.; Leung, J.W.; Lam, A.K.; Acy, L. Selective over-expression of endothelin-1 in endothelial cells exacerbates inner retinal edema and neuronal death in ischemic retina. PLoS ONE 2011, 6, e26184.
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules 2020, 25, 5591.
- Ruiz, H.H.; Ramasamy, R.; Schmidt, A.M. Advanced Glycation End Products: Building on the Concept of the “Common Soil” in Metabolic Disease. Endocrinology 2020, 161, bqz006.
- Reddy, V.P.; Aryal, P.; Darkwah, E.K. Advanced Glycation End Products in Health and Disease. Microorganisms 2022, 10, 1848.
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542.
- Mao, L.; Yin, R.; Yang, L.; Zhao, D. Role of advanced glycation end products on vascular smooth muscle cells under diabetic atherosclerosis. Front. Endocrinol. 2022, 13, 983723.
- Vlassara, H.; Bucala, R.; Striker, L. Pathogenic effects of advanced glycosylation: Biochemical, biologic, and clinical implications for diabetes and aging. Lab. Investig. 1988, 58, 317–326.




