Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonino Tuttolomondo | -- | 2507 | 2023-12-12 08:26:06 | | | |
| 2 | Catherine Yang | Meta information modification | 2507 | 2023-12-12 09:06:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ciaccio, A.M.; Tuttolomondo, A. Exosomal miRNAs as Biomarkers of Ischemic Stroke. Encyclopedia. Available online: https://encyclopedia.pub/entry/52605 (accessed on 07 February 2026).
Ciaccio AM, Tuttolomondo A. Exosomal miRNAs as Biomarkers of Ischemic Stroke. Encyclopedia. Available at: https://encyclopedia.pub/entry/52605. Accessed February 07, 2026.
Ciaccio, Anna Maria, Antonino Tuttolomondo. "Exosomal miRNAs as Biomarkers of Ischemic Stroke" Encyclopedia, https://encyclopedia.pub/entry/52605 (accessed February 07, 2026).
Ciaccio, A.M., & Tuttolomondo, A. (2023, December 12). Exosomal miRNAs as Biomarkers of Ischemic Stroke. In Encyclopedia. https://encyclopedia.pub/entry/52605
Ciaccio, Anna Maria and Antonino Tuttolomondo. "Exosomal miRNAs as Biomarkers of Ischemic Stroke." Encyclopedia. Web. 12 December, 2023.
Copy Citation
Exosomes are small lipid bilayer membrane particles released from all living cells into the extracellular environment. They carry several molecules and have a critical role in cell–cell communication under physiological and pathological conditions. Ischemic stroke due to cerebral vascular occlusion is the most common form, while hemorrhagic stroke due to cerebral bleeding accounts for about 12% of cases.
exosome
stroke
miRNA
1. Exosomes in Ischemic Stroke
During brain injury, exosomes can be released by different cell types within the CNS and could have a prominent role in brain remodeling post-stroke.
Astrocytes represent the most abundant glial cells within the CNS and play a critical role in cerebral homeostasis [1]. Under physiological and pathological conditions, astrocytes release sEVs. Astrocyte-derived sEVs contain different biological molecules, including DNA, miRNA, and proteins, but the composition varies according to the stimuli. Interestingly, under physiological conditions, astrocyte-derived sEVs are enriched with neuroprotective and neurotrophic elements as well as molecules to stimulate neurite outgrowth, synaptic transmission, and neuronal survival. Increasing evidence suggests that astrocytes are activated during cerebral ischemia and could secrete exosomes to protect the CNS. Pei et al. showed that astrocyte-derived exosomes inhibit autophagy and improve neuronal viability in ischemic stroke models [2]. Additionally, astrocyte-derived exosomes contain miRNAs, such as miR-34c and miR-361, that can, respectively, protect neurons and prevent nerve damage after cerebral ischemia [3][4]. Xin et al., in an in vitro model, showed that oxygen–glucose depletion (OGD) in astrocyte-derived exosomes enriched miR-133b-induced neuron outgrowth post-stroke [5]. Pei et al. revealed that miR-190b shuttled via exosomes is involved in preventing OGD-induced autophagy and inhibiting neuronal apoptosis [2]. Beyond miRNA, astrocytes also shuttle prion proteins, which could ameliorate neuronal survival. Finally, astrocyte-derived sEVs can also deliver apolipoprotein D, a classical neuroprotective protein, to neurons, improving neuronal survival [6].
The CNS is regarded as an immune-privileged organ in which adaptive immunity and inflammation are highly controlled to protect neural cells from possible immune response-mediated injury [7]. Microglia are the primary resident innate immune cells of the CNS, and upon activation, they participate in immunoregulation. They are highly dynamic cells that can vary in morphology, from ramified to amoeboid, and phenotype, from pro-inflammatory M1 to anti-inflammatory M2 types [8]. The M1 phenotype secretes cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNFα), nitric oxide (NO), and reactive oxygen species (ROS). In contrast, the M2 phenotype releases anti-inflammatory biomarkers, such as IL-10, transforming growth factor-β (TGF-β), and IL-4, and exerts neuroprotective effects, including cellular debris removal, angiogenesis promotion, and repair mechanisms. During ischemic stroke, microglia have a “double-edged sword” function, switching between pro- and anti-inflammatory phenotypes [9]. Specifically, it has been shown that the M2 phenotype is prevalent during the early stage of ischemic stroke and then progressively switches to the M1 phenotype. Several mechanisms regulate microglia polarization [10]. The content of sEVs derived from microglia varies based on the M1/M2 phenotypes. Song et al. showed that microglia-derived sEVs intravenously injected into mouse brains immediately after middle cerebral artery occlusion reduced ischemic brain injury and promoted neuronal survival via exosomal miR-124 and its downstream target USP14 [11]. Similarly, Zhang et al. found that miRNA-137 targeting the Notch-1 gene participated in the neuroprotective effect in OGD-treated neurons and transient middle cerebral artery occlusion (TMCAO)-treated mice [12]. On the other hand, some sEV-associated factors could worsen the ischemic injury. Xie et al. showed that exosomal shuttled miR-424-5p induces brain microvascular endothelial cell injury targeting the FGF2-mediated STAT3 signaling pathway [13].
A crosstalk between microglia and neurons has been described both under physiological and pathological conditions, including stroke. Emerging data indicate neurons can release sEV, guiding post-stroke recovery [14]. It has been suggested that neuron-derived sEVs could regulate microglial activation and function, promoting neuronal survival during ischemic stroke. Some authors showed that neuron-derived sEV could induce M2-type microglia polarization [15][16]. Neuron sEV-derived miR-98 emerged as an intercellular signal mediating neurons and microglia communication during brain remodeling after ischemic stroke. Yang et al. revealed that miR-98 regulates microglial phagocytosis by targeting platelet-activating factor receptor (PAFR), which is involved in neuronal pyroptosis during ischemia/reperfusion (I/R) and thus plays neuroprotection in stroke [17][18].
Neurons also communicate with astrocytes through sEV. Oligodendrocytes, neural tube-derived cells producing myelin, a lipid-rich membrane that wraps axons, providing insulation and metabolic support, have been proven to release sEV, which is captured by neurons in in vitro models [19][20]. Fröhlich et al., in an in vitro model of ischemia, showed that oligodendrocyte-derived sEV promoted neuronal survival [21].
Finally, brain microvascular endothelial cells secrete sEVs, which contain multiple factors with a critical role in protecting neurons under hypoxia through several mechanisms, including apoptosis inhibition, angiogenesis, and neurogenesis promotion.
Overall, cerebral exosomes protect the CNS after cerebral ischemia and contribute to post-ischemic recovery (Figure 1).
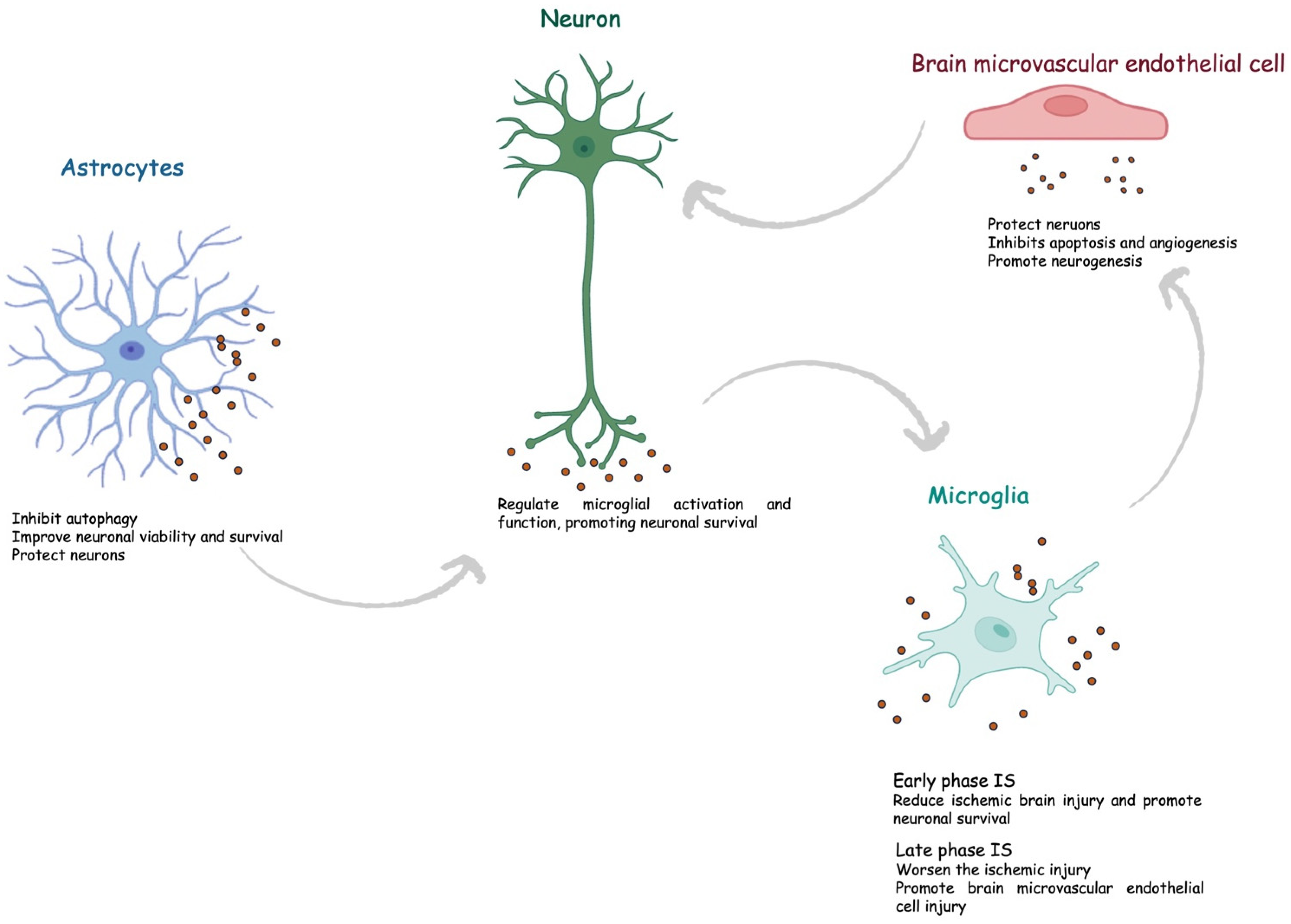
Figure 1. Role of exosomes in ischemic stroke. IS, ischemic stroke.
2. Exosomal miRNAs as Biomarkers of Ischemic Stroke
Biomarkers are defined as a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention, according to the U.S. National Institutes of Health working group [17][22]. There is intensive research to find reliable biomarkers to guide clinicians to early and differential diagnosis, prognosis, and monitoring. To date, a plethora of biomarkers of brain injury have been assessed, and the list continues to increase, but, so far, no stroke biomarker is available in clinical practice. The ideal biomarker should have high sensitivity and specificity, be cost-effective, and be easy to measure in emergencies. The biological matrix should be readily accessible and not invasive. The ideal biological fluid for assessing stroke biomarkers should be cerebrospinal fluid (CSF) because it is within the CNS and, thus, reflects the pathological alterations during an acute event. However, it has important limitations, including the invasiveness of the collection procedure and the correlated risks, making it unsuitable in clinical practice.
The detection of circulating stroke biomarkers is hampered by the BBB, a dynamic interface between the peripheral circulation and CNS, limiting the transition of biomarkers from CSF to blood. Exosomes have great potential because they can cross the BBB and, consequently, can be easily measured in blood. Recently, some authors assessed the possible role of exosomes as stroke biomarkers. Patients with ischemic stroke have cargo exosomes different from controls. miRNAs, which represent the most investigated content of exosomes, could provide precious information.
To date, most studies have been performed in animal models and in vitro. Noteworthy, despite stroke being a major risk factor for stroke, most studies have been performed on young animals, limiting the reliability of the findings of these studies [23]. Less evidence comes from human studies.
Zhou et al., in an observational study, found higher expression levels of miR-134 in stroke patients than controls [24]. The receiver operating characteristic (ROC) curve analysis showed good performance for diagnosing stroke with an area under the curve (AUC) of 0.834 (95% confidence interval, 0.88–0.97). Additionally, they found a significant correlation between the expression of exosomal miR-134, the National Institutes of Health Stroke Scale (NIHSS) score, and the infarct volume. Also, increased levels of miR-134 in stroke patients were associated with a poor prognosis. Thus, the authors conclude that miR-134 could represent a diagnostic and prognostic biomarker of ischemic stroke.
Similarly, Chen et al., in a retrospective case–control study, showed that increased levels of exosomal miR223 were associated with acute ischemic stroke [25]. The ROC curve analysis showed good diagnostic performance of miR-223 with an AUC of 0.859, a sensitivity of 84.0%, and a specificity of 78.8%. Also, exosomal miR-223 expression is positively correlated with the NIHSS score and poor outcomes in stroke patients. Thus, the authors concluded that exosomal miR-223 could be a reliable biomarker of ischemic stroke.
Jiang et al. performed a bioinformatic analysis using the GEO database to explore the association between exosomal miRNA expression and ischemic stroke [26]. They found that three miRNAs, namely hsa-miR-15b-5p, hsa-miR-184, and hsa-miR-16-5p, were highly expressed in the exosomes of patients with ischemic stroke. Thus, these miRNAs could serve as diagnostic biomarkers.
Ji et al. showed that the levels of exosomal miR-9 and miR-124 were significantly elevated in the serum of patients with ischemic stroke compared to controls and correlated with both NIHSS scores and the infarct volume [27]. They also showed that miR-9 and miR-124 could be sensitive biomarkers for diagnosing ischemic stroke with an AUC of 0.8026 (95% CI: 0.7235–0.8816) and 0.6976, respectively. Similarly, Qi et al. assessed the performance of serum exosomal miR-124-3p as a diagnostic biomarker of ischemic stroke [28]. They showed that the expression of miR-124-3p significantly increased in ischemic stroke and was negatively correlated with pro-inflammatory cytokines, including IL1β, IL6, and TNF-α, and the severity of disease. Serum exosomal miR-124-3p had high sensitivity and accuracy in diagnosing ischemic stroke. The authors also found that the overexpression of miR-124-3p mitigated inflammation in murine LPS-induced BV2 microglial cells via regulating p38 MAPK, Erk1/2, and PI3K/Akt signaling pathways. Overall, these findings support the use of miR-124-3p as a diagnostic and predictive marker for early-stage acute ischemic stroke.
Interestingly, Kalani et al. found that a subset of miRNA could differentiate between ischemic and hemorrhagic strokes [29]. Specifically, they found increased expression of miR-27b-3p and miR-146b-5p in ischemic strokes compared to hemorrhagic strokes.
On the contrary, levels of some exosomal miRNA could be decreased in patients with ischemic stroke. Song et al. found lower levels of miR-152-3p in the serum of stroke patients than controls, and the decrease was significantly related to the severity of endothelial injury [30]. Moreover, ROC curve analysis displayed that the AUC of the exosomal miR-152-3p level was 0.935, suggesting that it could be a reliable stroke biomarker.
Wang et al. revealed the role of serum exosomal miR-328-3p as a predictor of short-term prognosis in patients with stroke, with an odd ratio of 5.276 [31]. Similarly, He et al. demonstrated the prognostic role of exosomal miRNAs in acute ischemic stroke [32]. They measured the plasma levels of exosomal miR-125b-5p, miR-15a-3p, miR-15a-5p, and miR-206 24 h after thrombolysis with or without endovascular treatment in 94 patients. The authors found that miR-125b-5p and miR-206 levels were correlated with NIHSS scores and cerebral infarction volumes. Additionally, miR-125b-5p concentrations were significantly increased in patients with an unfavorable outcome. Thus, miR-125b-5p and miR-206 may be considered prognostic biomarkers of ischemic stroke.
Interestingly, some authors did not simply evaluate the different miRNA expressions between patients and controls but explored the possible differences among various stroke subtypes.
Ischemic stroke spans a temporal continuum from hyperacute (<6 h) to acute (1–3 and 4–7 days), subacute (8–14 days), and recovery (>14 days) phases [33]. Wang et al. explored the role of plasma exosomal microRNA-21-5p and microRNA-30a-5p in the different phases of ischemic stroke [34]. They found that miR-21-5p is higher in patients in the subacute and recovery phases than in controls. miR-30a-5p was increased in the hyperacute phase but decreased in the acute phase compared to controls. In the acute phase, both miRNAs were lower than in the hyperacute phase. The ROC curve analysis showed a good accuracy of miR-30a-5p for detecting the hyperacute phase, with an AUC of 0.826. Also, Li et al. evaluated the role of two plasma exosomal miRNAs according to the different phases of ischemic stroke [35]. The authors found that in the subacute phase, miR-422a and miR-125b-2-3p levels significantly decreased compared to both the controls and the acute phase. In the acute phase, miR-422a levels were increased as compared to controls. ROC analysis revealed good performance for miR-422a and miR-125b-2-3p in the subacute phase, with an AUC of 0.971 and 0.889, respectively, and miR-422a in the acute phase, with an AUC of 0.769. Taken together, these findings pave the way for new avenues for using miRNAs to classify stroke and, consequently, provide helpful information to guide appropriate treatment.
Beyond the timely classification, several classification systems have been proposed and are currently used in clinical practice [36]. Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification categorizes stroke patients, based on the etiology, into five subtypes, namely large artery atherosclerosis (LAA), cardioembolism (CE), small artery occlusion (SAO), stroke of other determined cause (SOC), and stroke of undetermined cause [37]. Among these, LAA is the most common subtype. Niu et al. explored the exosomal miRNA profile in patients with ischemic stroke grouped according to TOAST classification [38]. The authors showed that four miRNAs, miR-369-3p, miR-493-3p, miR-379-5p, and miR1296-5p, could reliably identify LAA. Moreover, the combination of three miRNAs improved the accuracy of the single for diagnosing LAA. Finally, the authors found that miR-493-3p and miR-1296-5p were negatively correlated with the NIHSS score. Interestingly, the authors also compared exosomal miRNAs with their counterparts in plasma. They did not find any correlation between exosomal and plasmatic miRNAs, supporting the protective effect of exosomes on miRNAs. Also, van Kralingen et al. assessed the miRNA profile expression in stroke subtypes according to TOAST classification [39]. First, they found that exosomal miRNA-17-5p, miRNA-20b-5p, miRNA-27b-3p, and miRNA-93-5p were significantly increased in patients with ischemic stroke compared to controls, with SAO patients showing the highest levels. Noteworthy, in this study, controls were not healthy subjects as in most studies, but patients with stroke mimic diseases. This represents a strength of the study, conferring greater clinical relevance to the investigated miRNAs as biomarkers of ischemic stroke.
Otero-Ortega et al. explored the role of exosomal miRNAs in ischemic stroke, classified according to their topography, subcortical and cortical–subcortical involvement [40]. Patients with cortical–subcortical ischemic stroke had decreased levels of miR-15a, miR-424, miR-100, and miR-339 compared with subcortical ischemic stroke, and miR-339, miR-100, miR-199a, miR-369a, miR-424, and miR-15a levels were lower than healthy controls. Thus, this study revealed significant differences in miRNA profiles according to stroke topography.
Table 1 summarizes the characteristics of the main human studies investigating the role of exosomal miRNA as a biomarker of ischemic stroke. As is evident from the literature, there is high heterogeneity among miRNAs investigated in the different studies, making it difficult to identify a unique candidate biomarker to introduce in clinical practice. Only miR124 has been evaluated by different authors achieving exciting findings. Thus, future research should test the potential to translate into clinical practice the use of miR124.
Table 1. Studies on exosome as diagnostic biomarker of ischemic stroke.
| Authors | Study Population | Time Sample Collection of Stroke Onset |
Exosomal miRNA | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Zhou et al. [23] | 50 patients and 50 controls |
Within 24 h | miR-134 | 0.834 (0.88–0.97) |
75.3% | 72.8% |
| Chen et al. [24] | 50 patients and 33 controls |
Within 72 h | miRNA-223 | 0.859 | 84% | 78.8% |
| Ji et al. [26] | 65 patients and 66 controls |
NA | miR-9 and miR-124 | miR-9: 0.8026 (0.7235–0.8816) miR-124: 0.6976 (0.6506–0.7895) |
NA | NA |
| Kalani et al. [28] | 21 patients with ischemic stroke and 36 patients with hemorrhagic stroke | Within 24 h | miR-27b-3p and miR-146b-5p |
NA | NA | NA |
| Qi et al. [27] | 10 patients and 10 controls |
At 2 h, 4 h, and 6 h | miR-124-3p | At 2 h: 0.81 At 4 h: 0.90 At 6 h: 0.94 |
NA | NA |
| Song et al. [29] | 93 patients and 70 controls |
NA | miR-152-3p | 0.935 (0.826–0.998) |
92.54% | 94.19% |
AUC, area under the curve; NA, information not available.
References
- Gharbi, T.; Zhang, Z.; Yang, G.Y. The Function of Astrocyte Mediated Extracellular Vesicles in Central Nervous System Diseases. Front. Cell Dev. Biol. 2020, 8, 568889.
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp. Cell Res. 2019, 382, 111474.
- Bu, X.; Li, D.; Wang, F.; Sun, Q.; Zhang, Z. Protective role of astrocyte-derived exosomal microRNA-361 in cerebral ischemic-reperfusion injury by regulating the AMPK/mTOR signaling pathway and targeting CTSB. Neuropsychiatr. Dis. Treat. 2020, 16, 1863–1877.
- Wu, W.; Liu, J.; Yang, C.; Xu, Z.; Huang, J.; Lin, J. Astrocyte-derived exosome-transported microRNA-34c is neuroprotective against cerebral ischemia/reperfusion injury via TLR7 and the NF-kappaB/MAPK pathways. Brain Res. Bull. 2020, 163, 84–94.
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosomeenriched extracellular particles. Stem Cells 2013, 31, 2737–2746.
- Pascua-Maestro, R.; Gonzalez, E.; Lillo, C.; Ganfornina, M.D.; Falcon-Perez, J.M.; Sanchez, D. Extracellular vesicles secreted by astroglial cells transport apolipoprotein D to neurons and mediate neuronal survival upon oxidative stress. Front. Cell Neurosci. 2018, 12, 526.
- Harris, M.G.; Hulseberg, P.; Ling, C.; Karman, J.; Clarkson, B.D.; Harding, J.S.; Zhang, M.; Sandor, A.; Christensen, K.; Nagy, A.; et al. Immune privilege of the CNS is not the consequence of limited antigen sampling. Sci. Rep. 2014, 4, 4422.
- Liu, G.; Li, T.; Yang, A.; Zhang, X.; Qi, S.; Feng, W. Knowledge domains and emerging trends of microglia research from 2002 to 2021: A bibliometric analysis and visualization study. Front Aging Neurosci. 2023, 14, 1057214.
- Dong, R.; Huang, R.; Wang, J.; Liu, H.; Xu, Z. Effects of Microglial Activation and Polarization on Brain Injury After Stroke. Front. Neurol. 2021, 12, 620948.
- Jiang, C.T.; Wu, W.F.; Deng, Y.H.; Ge, J.W. Modulators of Microglia Activation and Polarization in Ischemic Stroke. Mol. Med. Rep. 2020, 21, 2006–2018.
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019, 9, 2910–2923.
- Zhang, D.; Cai, G.; Liu, K.; Zhuang, Z.; Jia, K.; Pei, S.; Wang, X.; Wang, H.; Xu, S.; Cui, C.; et al. Microglia exosomal miRNA-137 attenuates ischemic brain injury through targeting Notch1. Aging 2021, 13, 4079–4095.
- Xie, L.; Zhao, H.; Wang, Y.; Chen, Z. Exosomal shuttled miR-424-5p from ischemic preconditioned microglia mediates cerebral endothelial cell injury through negatively regulation of FGF2/STAT3 pathway. Exp. Neurol. 2020, 333, 113411.
- Li, F.; Kang, X.; Xin, W.; Li, X. The Emerging Role of Extracellular Vesicle Derived from Neurons/Neurogliocytes in Central Nervous System Diseases: Novel Insights Into Ischemic Stroke. Front. Pharmacol. 2022, 13, 890698.
- Norris, G.T.; Smirnov, I.; Filiano, A.J.; Shadowen, H.M.; Cody, K.R.; Thompson, J.A.; Harris, T.H.; Gaultier, A.; Overall, C.C.; Kipnis, J. Neuronal integrity and complement control synaptic material clearance by microglia after CNS injury. J. Exp. Med. 2018, 215, 1789–1801.
- Pluvinage, J.V.; Haney, M.S.; Smith, B.A.H.; Sun, J.; Iram, T.; Bonanno, L.; Li, L.; Lee, D.P.; Morgens, D.W.; Yang, A.C.; et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 2019, 56, 187–192.
- Zhao, B.; Fei, Y.; Zhu, J.; Yin, Q.; Fang, W.; Li, Y. PAF Receptor Inhibition Attenuates Neuronal Pyroptosis in Cerebral Ischemia/Reperfusion Injury. Mol. Neurobiol. 2021, 58, 6520–6539.
- Yang, M.; Weng, T.; Zhang, W.; Zhang, M.; He, X.; Han, C.; Wang, X. The Roles of Non-coding RNA in the Development and Regeneration of Hair Follicles: Current Status and Further Perspectives. Front. Cell Dev. Biol. 2021, 9, 720879.
- Domingues, H.S.; Falcão, A.M.; Mendes-Pinto, I.; Salgado, A.J.; Teixeira, F.G. Exosome Circuitry During (De)(Re)Myelination of the Central Nervous System. Front. Cell Dev. Biol. 2020, 8, 483.
- Frühbeis, C.; Kuo-Elsner, W.P.; Müller, C.; Barth, K.; Peris, L.; Tenzer, S.; Möbius, W.; Werner, H.B.; Nave, K.A.; Fröhlich, D.; et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 2020, 18, e3000621.
- Fröhlich, D.; Kuo, W.P.; Frühbeis, C.; Sun, J.J.; Zehendner, C.M.; Luhmann, H.J.; Pinto, S.; Toedling, J.; Trotter, J.; Krämer-Albers, E.M. Multifaceted effects of oligodendroglial exosomes on neurons: Impact on neuronal firing rate, signal transduction and gene regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130510.
- Sun, J.; Yuan, Q.; Guo, L.; Xiao, G.; Zhang, T.; Liang, B.; Yao, R.; Zhu, Y.; Li, Y.; Hu, L. Brain Microvascular Endothelial Cell-Derived Exosomes Protect Neurons from Ischemia–Reperfusion Injury in Mice. Pharmaceuticals 2022, 15, 1287.
- Hermann, D.M.; Popa-Wagner, A.; Kleinschnitz, C.; Doeppner, T.R. Animal models of ischemic stroke and their impact on drug discovery. Expert. Opin. Drug Discov. 2019, 14, 315–326.
- Zhou, J.; Chen, L.; Chen, B.; Huang, S.; Zeng, C.; Wu, H.; Chen, C.; Long, F. Increased serum exosomal miR-134 expression in the acute ischemic stroke patients. BMC Neurol. 2018, 18, 198.
- Chen, Y.; Song, Y.; Huang, J.; Qu, M.; Zhang, Y.; Geng, J.; Zhang, Z.; Liu, J.; Yang, G.Y. Increased Circulating Exosomal miRNA-223 Is Associated with Acute Ischemic Stroke. Front. Neurol. 2017, 8, 57.
- Jiang, S.; Wu, J.; Geng, Y.; Zhang, Y.; Wang, Y.; Wu, J.; Lu, C.; Luo, G.; Zan, J.; Zhang, Y. Identification of Differentially Expressed microRNAs Associated with Ischemic Stroke by Integrated Bioinformatics Approaches. Int. J. Genom. 2022, 2022, 9264555.
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0163645.
- Qi, Z.; Zhao, Y.; Su, Y.; Cao, B.; Yang, J.J.; Xing, Q. Serum Extracellular Vesicle-Derived miR-124-3p as a Diagnostic and Predictive Marker for Early-Stage Acute Ischemic Stroke. Front. Mol. Biosci. 2021, 8, 685088.
- Kalani, M.Y.S.; Alsop, E.; Meechoovet, B.; Beecroft, T.; Agrawal, K.; Whitsett, T.G.; Huentelman, M.J.; Spetzler, R.F.; Nakaji, P.; Kim, S.; et al. Extracellular microRNAs in blood differentiate between ischaemic and haemorrhagic stroke subtypes. J. Extracell. Vesicles 2020, 9, 1713540.
- Song, P.; Sun, H.; Chen, H.; Wang, Y.; Zhang, Q. Decreased Serum Exosomal miR-152-3p Contributes to the Progression of Acute Ischemic Stroke. Clin. Lab. 2020, 66, 1615–1622.
- Wang, S.; Jun, J.; Cong, L.; Du, L.; Wang, C. miR-328-3p, a Predictor of Stroke, Aggravates the Cerebral Ischemia-Reperfusion Injury. Int. J. Gen. Med. 2021, 14, 2367–2376.
- He, X.W.; Shi, Y.H.; Zhao, R.; Liu, Y.S.; Li, G.F.; Hu, Y.; Chen, W.; Cui, G.H.; Su, J.J.; Liu, J.R. Plasma Levels of miR-125b-5p and miR-206 in Acute Ischemic Stroke Patients After Recanalization Treatment: A Prospective Observational Study. J. Stroke Cerebrovasc. Dis. 2019, 28, 1654–1661.
- Allen, L.M.; Hasso, A.N.; Handwerker, J.; Farid, H. Sequence-specific MR imaging findings that are useful in dating ischemic stroke. Radiographics 2012, 32, 1285–1299.
- Wang, W.; Li, D.B.; Li, R.Y.; Zhou, X.; Yu, D.J.; Lan, X.Y.; Li, J.P.; Liu, J.L. Diagnosis of Hyperacute and Acute Ischaemic Stroke: The Potential Utility of Exosomal MicroRNA-21-5p and MicroRNA-30a-5p. Cerebrovasc. Dis. 2018, 45, 204–212.
- Li, D.B.; Liu, J.L.; Wang, W.; Li, R.Y.; Yu, D.J.; Lan, X.Y.; Li, J.P. Plasma Exosomal miR-422a and miR-125b-2-3p Serve as Biomarkers for Ischemic Stroke. Curr. Neurovasc. Res. 2017, 14, 330–337.
- Chen, P.H.; Gao, S.; Wang, Y.J.; Xu, A.D.; Li, Y.S.; Wang, D. Classifying Ischemic Stroke, from TOAST to CISS. CNS Neurosci. Ther. 2012, 18, 452–456.
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41.
- Niu, M.; Li, H.; Li, X.; Yan, X.; Ma, A.; Pan, X.; Zhu, X. Circulating Exosomal miRNAs as Novel Biomarkers Perform Superior Diagnostic Efficiency Compared with Plasma miRNAs for Large-Artery Atherosclerosis Stroke. Front. Pharmacol. 2021, 12, 791644.
- Van Kralingen, J.C.; McFall, A.; Ord, E.N.J.; Coyle, T.F.; Bissett, M.; McClure, J.D.; McCabe, C.; Macrae, I.M.; Dawson, J.; Work, L.M. Altered Extracellular Vesicle MicroRNA Expression in Ischemic Stroke and Small Vessel Disease. Transl. Stroke Res. 2019, 10, 495–508.
- Otero-Ortega, L.; Alonso-López, E.; Pérez-Mato, M.; Laso-García, F.; Gómez-de Frutos, M.C.; Diekhorst, L.; García-Bermejo, M.L.; Conde-Moreno, E.; Fuentes, B.; de Leciñana, M.A.; et al. Circulating Extracellular Vesicle Proteins and MicroRNA Profiles in Subcortical and Cortical-Subcortical Ischaemic Stroke. Biomedicines 2021, 9, 786.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
721
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
2 times
(View History)
Update Date:
12 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




