Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Abdur Rashid | -- | 1356 | 2023-12-11 13:09:54 | | | |
| 2 | Camila Xu | Meta information modification | 1356 | 2023-12-12 03:14:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Emon, J.H.; Rashid, M.A.; Islam, M.A.; Hasan, M.N.; Patoary, M.K. Imine-Based Adaptive Covalent Chemistry. Encyclopedia. Available online: https://encyclopedia.pub/entry/52582 (accessed on 07 February 2026).
Emon JH, Rashid MA, Islam MA, Hasan MN, Patoary MK. Imine-Based Adaptive Covalent Chemistry. Encyclopedia. Available at: https://encyclopedia.pub/entry/52582. Accessed February 07, 2026.
Emon, Jabed Hossen, Muhammad Abdur Rashid, Md. Ariful Islam, Md. Nabiul Hasan, Mohammed Kayes Patoary. "Imine-Based Adaptive Covalent Chemistry" Encyclopedia, https://encyclopedia.pub/entry/52582 (accessed February 07, 2026).
Emon, J.H., Rashid, M.A., Islam, M.A., Hasan, M.N., & Patoary, M.K. (2023, December 11). Imine-Based Adaptive Covalent Chemistry. In Encyclopedia. https://encyclopedia.pub/entry/52582
Emon, Jabed Hossen, et al. "Imine-Based Adaptive Covalent Chemistry." Encyclopedia. Web. 11 December, 2023.
Copy Citation
The imine bond, also referred to as the Schiff base, is one of the reversible covalent bonds that can participate in both associative and dissociative reactions. This opens up possibilities for mechanical and chemical recycling as well as self-healing.
epoxy thermoset
bio-based
imine bond
recyclable
1. Introduction
The rapid expansion of plastic pollution and environmental concern motivate scientific research to use renewable resources and recyclability of the utilised polymeric materials that can be effective for packaging, construction, furniture and automotive industries [1][2][3]. There are two types of conventional synthetic polymer materials: thermoplastic and thermosetting polymers. While a certain temperature is applied, the thermoplastic polymer is broken its intermolecular connections, such as hydrogen bonds, Van der Waals forces and molecular chain entanglement, resulting in fluidity, and it can be easily reprocessed [4]. Among thermoset polymers, epoxy thermosets are considered the most adaptable and commonly used material due to their resilience to chemicals and heat, high mechanical strength and magnificent insulation [5][6]. Based on its chemical structure, the molecule has at least two epoxy groups and can be found in solid, viscous and liquid forms [7]. Therefore, it is often used in construction, electronic packaging, adhesives, coatings and composites [8]. However, diglycidyl ether of bisphenol A (DGEBA), which is not renewable, makes up about 90% of the epoxy thermosets used today [9]. However, after the completion of their service life, this thermoset decreases the composite material’s feasibility and creates massive environmental and waste resource difficulties. Concerning environmental and human health issues, the utilization of bisphenol A BPA in materials that come into touch with food is also prohibited by several nations, and they are concentrating on BPA replacement in research [10][11][12]. Keep focusing on petroleum availability and price uncertainty; the biobased materials and chemicals have to be synthesised from renewable resources. Therefore, epoxy made by BPA can be replaced by using renewable chemicals and derivatives [10]. Biomass renewable resources are cellulose [13][14][15], lignin [16][17][18], vanillin [19][20][21], vegetable oil [22][23][24], furan [25][26][27], cardanol [28][29][30], rosin [31][32][33], itaconic acid [34][35][36], quercetin [37][38] and so on.
Moreover, conventional and biobased thermosetting polymeric materials cannot be recycled or mended due to irreversible crosslinking networks, so they are treated as less environmentally friendly than thermoplastics [39][40][41]. The problems of the recycling and degradation, reprocessability, and self-healing of epoxy thermosets can be solved by incorporating reversible dynamic covalent bonds in the polymer network. Wuld and coworkers first proposed the concept of adaptive covalent chemistry (ACC) in 2002 [42], while Bowman and coworkers initially used the term covalent adaptive networks, also known as CANs, regarding crosslinked polymer networks with reversible bonds [43]. If certain stimuli and equilibrium controls allow for the reversible formation and breaking of covalent bonds, these bonds can be categorised as dynamic. In addition, these covalent bonds will be constructed as sustainable thermosets, eliminating the use of petroleum-based components and the depletion of fossil resources [44][45][46][47]. Several numbers of reversible covalent bonds are disulfide linkages [48][49][50], boronic ester bonds [31][51], ester bonds [37][52][53], acetal linkages [54][55][56], Schiff bases [2][57], carbamate linkages [58][59], Diels–Alder additions [60][61] and silicon oxygen bonds [62][63]. The dynamic covalent bond containing crosslinked network demonstrates repairability, malleability, degradability, reprocessability and self-healing properties under several extrinsic stimuli, such as solvent, pressure, light and heat [1][64][65][66]. CANs may be divided into two general categories according to how their dynamic structures are created: either bond exchange done kinetically (associative), expressed in Figure 1, or through equilibrium changes that result in reversible depolymerization (dissociative), expressed in Figure 2 [67][68]. In the case of associative dynamic bonds, a new bond is formed as the previous one breaks at the same time, keeping a constant crosslink density (Figure 1) [68][69]. In the case of dissociative dynamic bonds, the covalent networks are initially disrupted and then rebuilt at a different location within the network (Figure 2). During crosslinked network breakdown, the molecular density declines, resulting in network connectivity to be lost or completely degraded. This breakdown of the network might result in either the recovery of molecules or the formation of free chains, according to the composition of the dissociative network. Photo-triggerable reactions and Diels–Alder adducts are two of the most well-known examples of dissociative reversible networks [70][71]. In both situations, the polymeric thermosets are malleable and reshapable, and the crosslinks can be reformed to restore their original mechanical characteristics.
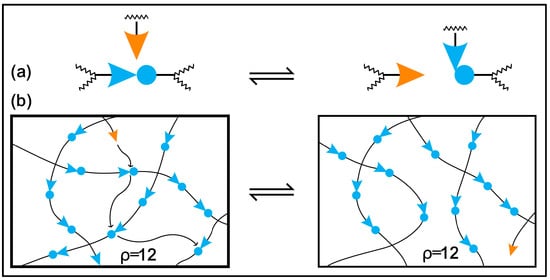
Figure 1. The mechanism of bond exchange in the polymer networks: (a) an exchange reaction that an active species goes through results in a bond exchange and the creation of a new active species, which then goes through more exchange reactions; (b) effective dispersion of reaction events rearranges the overall network connectivity while maintaining the total bond and crosslink density when the exchange reaction takes place in the backbone of a polymer network.
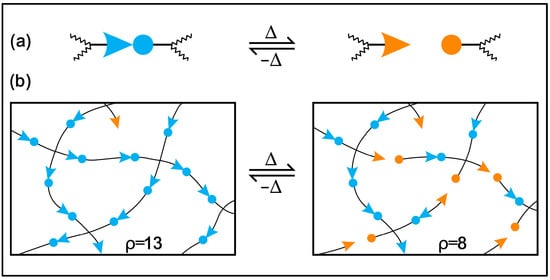
Figure 2. Reversible polymerization mechanism in a polymer network: (a) depolymerization event enabled by reversible crosslinking and (b) covalently modification of a crosslinked network.
Recently, it has been established that various chemical linkages have adaptive abilities, making the resulting polymer network characteristics typically of CANs [72]. Moreover, the imine bond is a viable choice for CANs and epoxy thermosets among the many dynamic bonds. An imine (C=N) bond is formed in the reaction between the activated carbonyl and primary nucleophilic amine groups [2]. These linkages give polymers exceptional characteristics, including stimuli adaptability, reprocessability, degradability and self-healability.
2. Imine-Based Adaptive Covalent Chemistry
A well-known process that produces imines is the condensation reaction of an aldehyde or ketone with a primary amine. Water and acidic environments, in particular, can cause the imine to hydrolyse, which results in the reformation of the original functions (Figure 3a). In addition to this dissociative process, two exchange reaction-based associative pathways can occur without water: transamination (Figure 3b) and imine metathesis (Figure 3c) [73][74]. Transamination is defined as the process of changing an imine into an additional imine and another primary amine. On the other hand, the metathesis route involves an interaction between two imines that produces two new imines. According to some reports, the transamination route does not need a catalyst and typically takes place when so many amines exist, resulting in the generation of aminal precursors [75]. L-proline is an example of an organic base accelerator that might enhance imine metathesis [76]. As a result, the imine linkages can induce the materials to have qualities such as self-healing, degradation and recyclability.
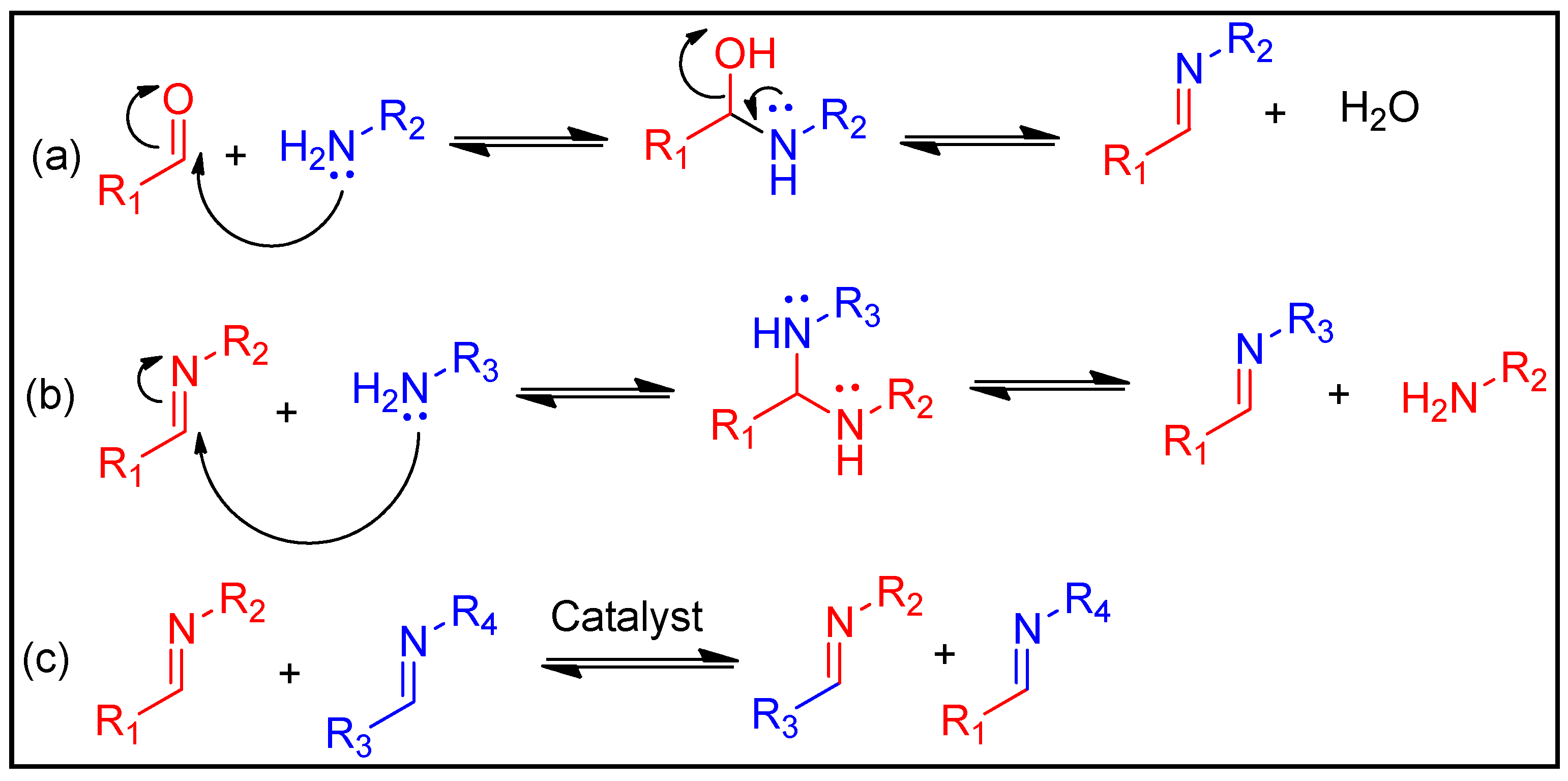
Figure 3. Basic exchange reaction of imine linkages: (a) imine hydrolysis, (b) transamination and (c) imine metathesis.
The water-based solubility of imine linkages is a common problem of imine chemistry, although the possibility for imine linkages to undergo heat-induced associative techniques has been utilised to generate thermally reprocessable thermosets [77][78][79]. However, this characteristic has also produced chemically recyclable polymers [80]. For example, Taynton and coworkers developed a reworkable and reproducible polyimine compound using the associative and dissociative imine systems [81]. The polyimine structures were developed using readily existing aldehyde, diamine and triamine combinations, demonstrating 100% recycling efficiency, stress-relaxation and Arrhenius-like malleability performance. Moreover, these thermosets also showed water-induced relaxation and were chemically recoverable at an ambient temperature. The imine metathesis and transimination were used to explain the malleability and rapid stress–relaxation of polyimine thermosets. In contrast, the combined reaction of imine dissociation and transimination caused by accessible amine functions was used to describe the quick water-induced stress relaxation. Besides water-induced methods, organic chemicals are additionally examined to promote the reversible reaction of imine linkages. In this context, Zhang and coworkers produced an imine-crosslinked self-healing organogel and exhibited that the synergistic impact of extra primary amine and organic solvent could accelerate the transimination process [78]. Schoustra and coworkers emphasised the effect of polar elements in molecular networks on the exchange of imine linkages to gain a deeper understanding of the associative connections of imine linkages and the impact of network and molecular components on the dynamics of the associative networks [82]. According to the study, adding higher polar elements, such as ethylene oxide, could increase the imine exchange reaction rate five times more than adding less polar components, such as aliphatic carbon chains. Based on the finding, cured polymer products containing polar functionalities in their network exhibited much higher temperatures for the glass-to-rubber and rubber-to-liquid phase transitions than polar components. The examination of stress–relaxation activity of polymer chains revealed a progressive three-phase relaxing approach that largely involved chain rearrangement inside the polymeric structure, imine interchange on a regional scale and imine interchange following diffusion across the chain.
References
- Xu, Y.; Odelius, K.; Hakkarainen, M. Photocurable, Thermally Reprocessable, and Chemically Recyclable Vanillin-Based Imine Thermosets. ACS Sustain. Chem. Eng. 2020, 8, 17272–17279.
- Liguori, A.; Hakkarainen, M. Designed from Biobased Materials for Recycling: Imine-Based Covalent Adaptable Networks. Macromol. Rapid Commun. 2022, 43, e2100816.
- Rashid, M.A.; Liu, W.; Wei, Y.; Jiang, Q. Review of intrinsically recyclable biobased epoxy thermosets enabled by dynamic chemical bonds. Polym.-Plast. Technol. Mater. 2022, 61, 1740–1782.
- Aiswarya, S.; Awasthi, P.; Banerjee, S.S. Self-healing thermoplastic elastomeric materials: Challenges, opportunities and new approaches. Eur. Polym. J. 2022, 181, 111658.
- Zhao, X.-L.; Li, Y.-D.; Zeng, J.-B. Progress in the design and synthesis of biobased epoxy covalent adaptable networks. Polym. Chem. 2022, 13, 6573–6588.
- Ma, S.; Webster, D.C. Degradable thermosets based on labile bonds or linkages: A review. Prog. Polym. Sci. 2018, 76, 65–110.
- Memon, H.; Wei, Y.; Zhu, C. Recyclable and reformable epoxy resins based on dynamic covalent bonds–Present, past, and future. Polym. Test. 2022, 105, 107420.
- Ding, C.; Matharu, A.S. Recent Developments on Biobased Curing Agents: A Review of Their Preparation and Use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236.
- Rashid, M.A.; Liu, W.; Wei, Y.; Jiang, Q. Review of reversible dynamic bonds containing intrinsically flame retardant biomass thermosets. Eur. Polym. J. 2022, 173, 111263.
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased thermosetting epoxy: Present and future. Chem. Rev. 2014, 114, 1082–1115.
- Jian, X.-Y.; He, Y.; Li, Y.-D.; Wang, M.; Zeng, J.-B. Curing of epoxidized soybean oil with crystalline oligomeric poly(butylene succinate) towards high performance and sustainable epoxy resins. Chem. Eng. J. 2017, 326, 875–885.
- Zhao, S.; Huang, X.; Whelton, A.J.; Abu-omar, M.M. Renewable Epoxy Thermosets from Fully Lignin-Derived Triphenols. ACS Sustain. Chem. Eng. 2018, 6, 7600–7608.
- Huang, B.; He, H.; Dufresne, A.; He, X.; Wang, S. Enhancing toughness, healing and reprocessability of sustainable epoxy vitrimer composites by PEG-assisted regenerated cellulose. Ind. Crops Prod. 2021, 170, 113804.
- Zhao, W.; Feng, Z.; Liang, Z.; Lv, Y.; Xiang, F.; Xiong, C.; Duan, C.; Dai, L.; Ni, Y. Vitrimer-Cellulose Paper Composites: A New Class of Strong, Smart, Green, and Sustainable Materials. ACS Appl. Mater. Interfaces 2019, 11, 36090–36099.
- Qin, X.; Ge, W.; Mei, H.; Li, L.; Zheng, S. Toughness improvement of epoxy thermosets with cellulose nanocrystals. Polym. Int. 2021, 70, 1640–1648.
- Xue, B.; Tang, R.; Xue, D.; Guan, Y.; Sun, Y.; Zhao, W.; Tan, J.; Li, X. Sustainable alternative for bisphenol A epoxy resin high-performance and recyclable lignin-based epoxy vitrimers. Ind. Crops Prod. 2021, 168, 113583.
- Tang, R.; Xue, B.; Tan, J.; Guan, Y.; Wen, J.; Li, X.; Zhao, W. Regulating lignin-based epoxy vitrimer performance by fine-tuning the lignin structure. ACS Appl. Polym. Mater. 2022, 4, 1117–1125.
- Moreno, A.; Morsali, M.; Sipponen, M.H. Catalyst-free synthesis of lignin vitrimers with tunable mechanical properties: Circular polymers and recoverable adhesives. ACS Appl. Mater. Interfaces 2021, 13, 57952–57961.
- Rashid, M.A.; Hasan, M.N.; Dayan, M.A.R.; Jamal, M.S.I.; Patoary, M.K. A Critical Review of Sustainable Vanillin-modified Vitrimers: Synthesis, Challenge and Prospects. Reactions 2023, 4, 66–91.
- Rashid, M.A.; Mian, M.M.; Wei, Y.; Liu, W. A vanillin-derived hardener for recyclable, degradable and self-healable high-performance epoxy vitrimers based on transimination. Mater. Today Commun. 2023, 35, 106178.
- Su, X.; Zhou, Z.; Liu, J.; Luo, J.; Liu, R. A recyclable vanillin-based epoxy resin with high-performance that can compete with DGEBA. Eur. Polym. J. 2020, 140, 110053.
- Fei, M.; Liu, T.; Zhao, B.; Otero, A.; Chang, Y.-C.; Zhang, J. From Glassy Plastic to Ductile Elastomer: Vegetable Oil-Based UV-Curable Vitrimers and Their Potential Use in 3D Printing. ACS Appl. Polym. Mater. 2021, 3, 2470–2479.
- Liu, Y.-Y.; He, J.; Li, Y.-D.; Zhao, X.-L.; Zheng, J.-B. Biobased, reprocessable and weldable epoxy vitrimers from epoxidized soybean oil. Ind. Crops Prod. 2020, 153, 112576.
- Zhao, X.-L.; Liu, Y.-Y.; Weng, Y.; Li, Y.-D.; Zheng, J.-B. Sustainable Epoxy Vitrimers from Epoxidized Soybean Oil and Vanillin. ACS Sustain. Chem. Eng. 2020, 8, 15020–15029.
- Dhers, S.; Vantomme, G.; Avérous, L. A fully bio-based polyimine vitrimer derived from fructose. Green. Chem. 2019, 21, 1596–1601.
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. A furan-derived epoxy thermoset with inherent anti-flammability, degradability, and raw material recycling. Mater. Today Chem. 2023, 27, 101315.
- Jiang, Y.; Yun, J.; Pan, X. Renewable Furan-Based Epoxy Resins Derived from 5-Hydroxymethylfurfural and Furfural. ACS Sustain. Chem. Eng. 2022, 10, 16555–16562.
- Trejo-Machin, A.; Puchot, L.; Verge, P. A cardanol-based polybenzoxazine vitrimer: Recycling, reshaping and reversible adhesion. Polym. Chem. 2020, 11, 7026–7034.
- Liu, G.; Jin, C.; Huo, S.; Kong, Z.; Chu, F. Preparation and properties of novel bio-based epoxy resin thermosets from lignin oligomers and cardanol. Int. J. Biol. Macromol. 2021, 193, 1400–1408.
- Hu, Y.; Tong, S.; Sha, Y.; Yu, J.; Hu, L.; Huang, Q.; Jia, P.; Zhou, Y. Cardanol-based epoxy vitrimer/carbon fiber composites with integrated mechanical, self-healing, reprocessable, and welding properties and degradability. Chem. Eng. J. 2023, 471, 144633.
- Zeng, Y.; Li, J.; Liu, S.; Yang, B. Rosin-Based Epoxy Vitrimers with Dynamic Boronic Ester Bonds. Polymers 2021, 13, 3386.
- Zhang, H.; Li, W.; Xu, J.; Shang, S.; Song, Z. Synthesis and characterization of bio-based epoxy thermosets using rosin-based epoxy monomer. Iran. Polym. J. 2021, 30, 643–654.
- Zeng, Y.; Yang, B.; Luo, Z.; Pan, X.; Ning, Z. Fully rosin-based epoxy vitrimers with high mechanical and thermostability properties, thermo-healing and closed-loop recycling. Eur. Polym. J. 2022, 181, 111643.
- Huang, J.; Zhang, J.; Zhu, G.; Yu, X.; Hu, Y.; Shang, Q.; Chen, J.; Hu, L.; Zhou, Y. Self-healing, high-performance, and high-biobased-content UV-curable coatings derived from rubber seed oil and itaconic acid. Prog. Org. Coat. 2021, 159, 106391.
- Liu, Y.; Wang, B.; Ma, S.; Xu, X.; Qiu, J.; Li, Q.; Wang, S.; Lu, N.; Ye, J.; Zhu, J. Phosphate-based covalent adaptable networks with recyclability and flame retardancy from bioresources. Eur. Polym. J. 2021, 144, 110236.
- Zhang, J.; Gong, Z.; Wu, C.; Li, T.; Tang, Y.; Wu, J.; Jiang, C.; Miao, M.; Zhang, D. Itaconic acid-based hyperbranched polymer toughened epoxy resins with rapid stress relaxation, superb solvent resistance and closed-loop recyclability. Green. Chem. 2022, 24, 6900–6911.
- Rashid, M.A.; Zhu, S.; Zhang, L.; Wei, Y.; Liu, W. A Quercetin-Derived Polybasic Acid Hardener for Reprocessable and Degradable Epoxy Resins Based on Transesterification. ACS Appl. Polym. Mater. 2022, 4, 5708–5716.
- Kristufek, S.L.; Yang, S.; Link, L.A.; Rohde, B.J.; Robertson, M.L.; Wooley, K.L. Synthesis, Characterization, and Cross-Linking Strategy of a Quercetin-Based Epoxidized Monomer as a Naturally-Derived Replacement for BPA in Epoxy Resins. ChemSusChem 2016, 9, 2135–2142.
- Baroncini, E.A.; Yadav, S.K.; Palmese, G.R.; Stanzione, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 44103.
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Development of Biobased Epoxy Resins: A Review. Polym.-Plast. Technol. Eng. 2018, 57, 133–155.
- Rashid, M.A.; Liu, W.; Wei, Y.; Jiang, Q. Review on intrinsically recyclable flame retardant thermosets enabled through covalent bonds. J. Appl. Polym. Sci. 2022, 139, e52493.
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A Thermally Re-mendable Cross-Linked Polymeric Material. Science 2002, 295, 1698–1702.
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent Adaptable Networks (CANs): A Unique Paradigm in Cross-Linked Polymers. Macromolecules 2010, 43, 2643–2653.
- Wang, B.; Ma, M.; Yan, S.; Zhu, J. Readily recyclable carbon fiber reinforced composites based on degradable thermosets: A review. Green. Chem. 2019, 21, 5781–5796.
- Wang, S.; Ma, S.; Li, Q.; Xu, X.; Wang, B.; Yuan, W.; Zhou, S.; You, S.; Zhu, J. Facile in situ preparation of high-performance epoxy vitrimer from renewable resources and its application in nondestructive recyclable carbon fiber composite. Green. Chem. 2019, 21, 1484–1497.
- Yu, K.; Shi, Q.; Dunn, M.L.; Wang, T.; Qi, H.J. Carbon Fiber Reinforced Thermoset Composite with Near 100% Recyclability. Adv. Funct. Mater. 2016, 26, 6098–6106.
- Yuan, Y.; Sun, Y.; Yan, S.; Zhao, J.; Liu, S.; Zhang, M.; Zheng, X.; Jia, L. Multiply fully recyclable carbon fibre reinforced heat-resistant covalent thermosetting advanced composites. Nat. Commun. 2017, 8, 14657.
- Tratnik, N.; Tanguy, N.R.; Yan, N. Recyclable, self-strengthening starch-based epoxy vitrimer facilitated by exchangeable disulfide bonds. Chem. Eng. J. 2023, 451, 138610.
- Zhou, F.; Guo, Z.; Wang, W.; Lei, X.; Zhang, B.; Zhang, H.; Zhang, Q. Preparation of self-healing, recyclable epoxy resins and low-electrical resistance composites based on double-disulfide bond exchange. Compos. Sci. Technol. 2018, 167, 79–85.
- Li, X.; Zhang, J.; Zhang, L.; Luzuriaga, A.R.D.; Rekondo, A.; Wang, D.Y. Recyclable flame-retardant epoxy composites based on disulfide bonds: Flammability and recyclability. Compos. Commun. 2021, 25, 100754.
- Zeng, Y.; Liu, S.; Xu, X.; Chen, Y.; Zhang, F. Fabrication and curing properties of o-cresol formaldehyde epoxy resin with reversible cross-links by dynamic boronic ester bonds. Polymer 2020, 211, 123116.
- Zhao, W.; An, L.; Wang, S. Recyclable High-Performance Epoxy-Anhydride Resins with DMP-30 as the Catalyst of Transesterification Reactions. Polymers 2021, 13, 296.
- Chen, J.-H.; Lu, J.H.; Pu, X.L.; Chen, L.; Wang, Y.Z. Recyclable, malleable and intrinsically flame-retardant epoxy resin with catalytic transesterification. Chemosphere 2022, 294, 133778.
- Toendepi, I.; Zhu, S.; Liu, Y.; Zhang, L.; Wei, Y.; Liu, W. Synthesis and structure-property relationship of epoxy vitrimers containing different acetal structures. Polymer 2023, 272, 125862.
- Chen, J.; Zhang, K.; Zhang, K.; Jiang, B.; Huang, Y. Facile preparation of reprocessable and degradable phenolic resin based on dynamic acetal motifs. Polym. Degrad. Stab. 2022, 196, 109818.
- Kuroyanagi, M.; Yamaguchi, A.; Hashimoto, T.; Urushisaki, M.; Sakaguchi, T.; Kawabe, K. Novel degradable acetal-linkage-containing epoxy resins with high thermal stability: Synthesis and application in carbon fiber-reinforced plastics. Polym. J. 2022, 54, 313–322.
- Jiang, Y.; Wang, S.; Dong, W.; Kaneko, T.; Chen, M.; Shi, D. High-Strength, Degradable and Recyclable Epoxy Resin Based on Imine Bonds for Its Carbon-Fiber-Reinforced Composites. Materials 2023, 16, 1604.
- Qin, J.; Liu, X.; Chen, B.; Liu, J.; Wu, M.; Tan, L.; Yang, C.; Liang, L. Thermo-healing and recyclable epoxy thermosets based on dynamic phenol-carbamate bonds. React. Funct. Polym. 2022, 180, 105411.
- Fortman, D.J.; Sheppard, D.T.; Dichtel, W.R. Reprocessing Cross-Linked Polyurethanes by Catalyzing Carbamate Exchange. Macromolecules 2019, 52, 6330–6335.
- Lorero, I.; Rodriguez, A.; Campo, M.; Prolongo, S.G. Thermally remendable, weldable, and recyclable epoxy network crosslinked with reversible Diels-alder bonds. Polymer 2022, 259, 125334.
- Puyadena, M.; Calafel, I.; Roman, E.G.D.S.; Martin, L.; Gonzalez, A.; Irusta, L. Recyclable Epoxy Resin via Simultaneous Dual Permanent/Reversible Crosslinking Based on Diels–Alder Chemistry. Macromol. Chem. Phys. 2021, 222, 2100146.
- Nishimura, Y.; Chung, J.; Muradyan, H.; Guan, Z. Silyl Ether as a Robust and Thermally Stable Dynamic Covalent Motif for Malleable Polymer Design. J. Am. Chem. Soc. 2017, 139, 14881–14884.
- Liu, Q.; Jiang, L.; Zhao, Y.; Wang, Y.; Lei, J. Reprocessable and Shape Memory Thermosetting Epoxy Resins Based on Silyl Ether Equilibration. Macromol. Chem. Phys. 2019, 220, 1900149.
- Shi, Q.; Yu, K.; Dunn, M.L.; Wang, T.; Qi, H.J. Solvent Assisted Pressure-Free Surface Welding and Reprocessing of Malleable Epoxy Polymers. Macromolecules 2016, 49, 5527–5537.
- Xu, W.M.; Rong, M.Z.; Zhang, M.Q. Sunlight driven self-healing, reshaping and recycling of a robust, transparent and yellowing-resistant polymer. J. Mater. Chem. A 2016, 4, 10683–10690.
- Mai, V.D.; Shin, S.R.; Lee, D.S.; Kang, I. Thermal Healing, Reshaping and Ecofriendly Recycling of Epoxy Resin Crosslinked with Schiff Base of Vanillin and Hexane-1,6-Diamine. Polymers 2019, 11, 293.
- Bowman, C.N.; Kloxin, C.J. Covalent adaptable networks: Reversible bond structures incorporated in polymer networks. Angew. Chem. Int. Ed. Engl. 2012, 51, 4272–4274.
- Denissen, W.; Winne, J.M.; Prez, F.E.D. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38.
- Van Zee, N.J.; Nicolaÿ, R. Vitrimers: Permanently crosslinked polymers with dynamic network topology. Prog. Polym. Sci. 2020, 104, 101233.
- Gandini, A. The furan/maleimide Diels–Alder reaction: A versatile click–unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29.
- Kaur, G.; Johnston, P.; Saito, K. Photo-reversible dimerisation reactions and their applications in polymeric systems. Polym. Chem. 2014, 5, 2171–2186.
- Huang, S.; Kong, X.; Xiong, Y.; Zhang, X.; Chen, H.; Jiang, W.; Niu, Y.; Xu, W.; Ren, C. An overview of dynamic covalent bonds in polymer material and their applications. Eur. Polym. J. 2020, 141, 110094.
- Ciaccia, M.; Di Stefano, S. Mechanisms of imine exchange reactions in organic solvents. Org. Biomol. Chem. 2015, 13, 646–654.
- Ciaccia, M.; Cacciapaglia, R.; Mencarelli, P.; Mandolini, L.; Stefano, S.D. Fast transimination in organic solvents in the absence of proton and metal catalysts. A key to imine metathesis catalyzed by primary amines under mild conditions. Chem. Sci. 2013, 4, 2253–2261.
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. A high performance fully bio-based epoxy thermoset from a syringaldehyde-derived epoxy monomer cured by furan-derived amine. Green. Chem. 2021, 23, 501–510.
- Wilhelms, N.; Kulchat, S.; Lehn, J.M. Organocatalysis of CN/CN and CC/CN Exchange in Dynamic Covalent Chemistry. Helv. Chim. Acta 2012, 95, 2635–2651.
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024.
- Chao, A.; Negulescu, I.; Zhang, D. Dynamic Covalent Polymer Networks Based on Degenerative Imine Bond Exchange: Tuning the Malleability and Self-Healing Properties by Solvent. Macromolecules 2016, 49, 6277–6284.
- Godoy-Alcántar, C.; Yatsimirsky, A.K.; Lehn, J.M. Structure-stability correlations for imine formation in aqueous solution. J. Phys. Org. Chem. 2005, 18, 979–985.
- Liu, Y.; Yu, Z.; Wang, B.; Li, P.; Zhu, J.; Ma, S. Closed-loop chemical recycling of thermosetting polymers and their applications: A review. Green. Chem. 2022, 24, 5691–5708.
- Taynton, P.; Yu, K.; Shoemaker, R.K.; Jin, Y.; Qi, H.J.; Zhang, W. Heat- or Water-Driven Malleability in a Highly Recyclable Covalent Network Polymer. Adv. Mater. 2014, 26, 3938–3942.
- Schoustra, S.K.; Groeneveld, T.; Smulders, M.M.J. The effect of polarity on the molecular exchange dynamics in imine-based covalent adaptable networks. Polym. Chem. 2021, 12, 1635–1642.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
12 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




