Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
The imine bond, also referred to as the Schiff base, is one of the reversible covalent bonds that can participate in both associative and dissociative reactions. This opens up possibilities for mechanical and chemical recycling as well as self-healing.
- epoxy thermoset
- bio-based
- imine bond
- recyclable
1. Introduction
The rapid expansion of plastic pollution and environmental concern motivate scientific research to use renewable resources and recyclability of the utilised polymeric materials that can be effective for packaging, construction, furniture and automotive industries [1,2,3]. There are two types of conventional synthetic polymer materials: thermoplastic and thermosetting polymers. While a certain temperature is applied, the thermoplastic polymer is broken its intermolecular connections, such as hydrogen bonds, Van der Waals forces and molecular chain entanglement, resulting in fluidity, and it can be easily reprocessed [4]. Among thermoset polymers, epoxy thermosets are considered the most adaptable and commonly used material due to their resilience to chemicals and heat, high mechanical strength and magnificent insulation [5,6]. Based on its chemical structure, the molecule has at least two epoxy groups and can be found in solid, viscous and liquid forms [7]. Therefore, it is often used in construction, electronic packaging, adhesives, coatings and composites [8]. However, diglycidyl ether of bisphenol A (DGEBA), which is not renewable, makes up about 90% of the epoxy thermosets used today [9]. However, after the completion of their service life, this thermoset decreases the composite material’s feasibility and creates massive environmental and waste resource difficulties. Concerning environmental and human health issues, the utilization of bisphenol A BPA in materials that come into touch with food is also prohibited by several nations, and they are concentrating on BPA replacement in research [10,11,12]. Keep focusing on petroleum availability and price uncertainty; the biobased materials and chemicals have to be synthesised from renewable resources. Therefore, epoxy made by BPA can be replaced by using renewable chemicals and derivatives [10]. Biomass renewable resources are cellulose [13,14,15], lignin [16,17,18], vanillin [19,20,21], vegetable oil [22,23,24], furan [25,26,27], cardanol [28,29,30], rosin [31,32,33], itaconic acid [34,35,36], quercetin [37,38] and so on.
Moreover, conventional and biobased thermosetting polymeric materials cannot be recycled or mended due to irreversible crosslinking networks, so they are treated as less environmentally friendly than thermoplastics [39,40,41]. The problems of the recycling and degradation, reprocessability, and self-healing of epoxy thermosets can be solved by incorporating reversible dynamic covalent bonds in the polymer network. Wuld and coworkers first proposed the concept of adaptive covalent chemistry (ACC) in 2002 [42], while Bowman and coworkers initially used the term covalent adaptive networks, also known as CANs, regarding crosslinked polymer networks with reversible bonds [43]. If certain stimuli and equilibrium controls allow for the reversible formation and breaking of covalent bonds, these bonds can be categorised as dynamic. In addition, these covalent bonds will be constructed as sustainable thermosets, eliminating the use of petroleum-based components and the depletion of fossil resources [44,45,46,47]. Several numbers of reversible covalent bonds are disulfide linkages [48,49,50], boronic ester bonds [31,51], ester bonds [37,52,53], acetal linkages [54,55,56], Schiff bases [2,57], carbamate linkages [58,59], Diels–Alder additions [60,61] and silicon oxygen bonds [62,63]. The dynamic covalent bond containing crosslinked network demonstrates repairability, malleability, degradability, reprocessability and self-healing properties under several extrinsic stimuli, such as solvent, pressure, light and heat [1,64,65,66]. CANs may be divided into two general categories according to how their dynamic structures are created: either bond exchange done kinetically (associative), expressed in Figure 1, or through equilibrium changes that result in reversible depolymerization (dissociative), expressed in Figure 2 [67,68]. In the case of associative dynamic bonds, a new bond is formed as the previous one breaks at the same time, keeping a constant crosslink density (Figure 1) [68,69]. In the case of dissociative dynamic bonds, the covalent networks are initially disrupted and then rebuilt at a different location within the network (Figure 2). During crosslinked network breakdown, the molecular density declines, resulting in network connectivity to be lost or completely degraded. This breakdown of the network might result in either the recovery of molecules or the formation of free chains, according to the composition of the dissociative network. Photo-triggerable reactions and Diels–Alder adducts are two of the most well-known examples of dissociative reversible networks [70,71]. In both situations, the polymeric thermosets are malleable and reshapable, and the crosslinks can be reformed to restore their original mechanical characteristics.
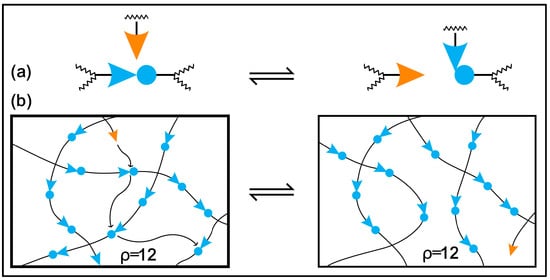
Figure 1. The mechanism of bond exchange in the polymer networks: (a) an exchange reaction that an active species goes through results in a bond exchange and the creation of a new active species, which then goes through more exchange reactions; (b) effective dispersion of reaction events rearranges the overall network connectivity while maintaining the total bond and crosslink density when the exchange reaction takes place in the backbone of a polymer network.
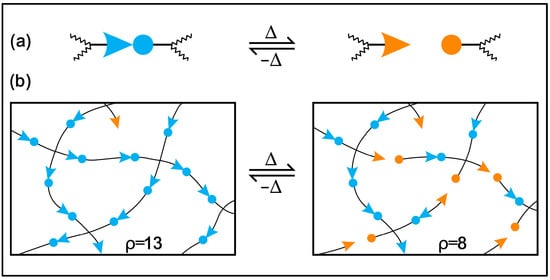
Figure 2. Reversible polymerization mechanism in a polymer network: (a) depolymerization event enabled by reversible crosslinking and (b) covalently modification of a crosslinked network.
Recently, it has been established that various chemical linkages have adaptive abilities, making the resulting polymer network characteristics typically of CANs [72]. Moreover, the imine bond is a viable choice for CANs and epoxy thermosets among the many dynamic bonds. An imine (C=N) bond is formed in the reaction between the activated carbonyl and primary nucleophilic amine groups [2]. These linkages give polymers exceptional characteristics, including stimuli adaptability, reprocessability, degradability and self-healability.
2. Imine-Based Adaptive Covalent Chemistry
A well-known process that produces imines is the condensation reaction of an aldehyde or ketone with a primary amine. Water and acidic environments, in particular, can cause the imine to hydrolyse, which results in the reformation of the original functions (Figure 3a). In addition to this dissociative process, two exchange reaction-based associative pathways can occur without water: transamination (Figure 3b) and imine metathesis (Figure 3c) [73,74]. Transamination is defined as the process of changing an imine into an additional imine and another primary amine. On the other hand, the metathesis route involves an interaction between two imines that produces two new imines. According to some reports, the transamination route does not need a catalyst and typically takes place when so many amines exist, resulting in the generation of aminal precursors [75]. L-proline is an example of an organic base accelerator that might enhance imine metathesis [76]. As a result, the imine linkages can induce the materials to have qualities such as self-healing, degradation and recyclability.
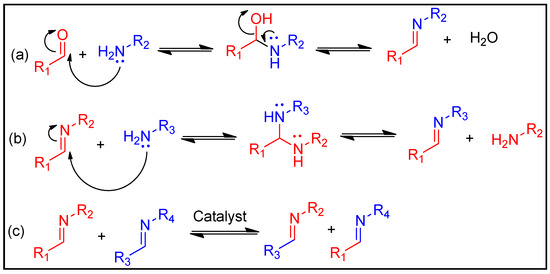
Figure 3. Basic exchange reaction of imine linkages: (a) imine hydrolysis, (b) transamination and (c) imine metathesis.
The water-based solubility of imine linkages is a common problem of imine chemistry, although the possibility for imine linkages to undergo heat-induced associative techniques has been utilised to generate thermally reprocessable thermosets [77,78,79]. However, this characteristic has also produced chemically recyclable polymers [80]. For example, Taynton and coworkers developed a reworkable and reproducible polyimine compound using the associative and dissociative imine systems [81]. The polyimine structures were developed using readily existing aldehyde, diamine and triamine combinations, demonstrating 100% recycling efficiency, stress-relaxation and Arrhenius-like malleability performance. Moreover, these thermosets also showed water-induced relaxation and were chemically recoverable at an ambient temperature. The imine metathesis and transimination were used to explain the malleability and rapid stress–relaxation of polyimine thermosets. In contrast, the combined reaction of imine dissociation and transimination caused by accessible amine functions was used to describe the quick water-induced stress relaxation. Besides water-induced methods, organic chemicals are additionally examined to promote the reversible reaction of imine linkages. In this context, Zhang and coworkers produced an imine-crosslinked self-healing organogel and exhibited that the synergistic impact of extra primary amine and organic solvent could accelerate the transimination process [78]. Schoustra and coworkers emphasised the effect of polar elements in molecular networks on the exchange of imine linkages to gain a deeper understanding of the associative connections of imine linkages and the impact of network and molecular components on the dynamics of the associative networks [82]. According to the study, adding higher polar elements, such as ethylene oxide, could increase the imine exchange reaction rate five times more than adding less polar components, such as aliphatic carbon chains. Based on the finding, cured polymer products containing polar functionalities in their network exhibited much higher temperatures for the glass-to-rubber and rubber-to-liquid phase transitions than polar components. The examination of stress–relaxation activity of polymer chains revealed a progressive three-phase relaxing approach that largely involved chain rearrangement inside the polymeric structure, imine interchange on a regional scale and imine interchange following diffusion across the chain.
This entry is adapted from the peer-reviewed paper 10.3390/reactions4040043
This entry is offline, you can click here to edit this entry!
