
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dillon P. Kiernan | -- | 3509 | 2023-12-08 15:04:46 | | | |
| 2 | Catherine Yang | Meta information modification | 3509 | 2023-12-11 02:22:08 | | |
Video Upload Options
The rise in antimicrobial-resistant pathogens has necessitated a decrease in the use of antibiotics and antimicrobials in commercial pig farming. Consequently, there has been a surge in research to pinpoint alternative dietary interventions that can contribute to the health and performance of pigs. The pivotal role of the gastrointestinal (GIT) microbiota in animal health and performance is gaining greater recognition. Therefore, enhancing the GIT microbiota, particularly the pioneer microbiota in young pigs, has become a central objective. The supplementation of probiotics emerges as a practical strategy to introduce beneficial bacteria into the pig's GIT, promoting improved animal health and performance.
1. Introduction
The gastrointestinal tract (GIT) microbiota plays a pivotal role in influencing critical factors such as feed efficiency [1] , growth performance [2] and susceptibility to diseases [3] in pigs. Interventions targeting improvements in the GIT microbiota through dietary adjustments have proven to be notably effective across all developmental stages of pigs, resulting in enhancements in average daily gain, food conversion ratio (FCR), and overall animal health [4][5][6]. A probiotic, defined as a "live microorganism which, when administered in adequate amounts, confers a health benefit on the host," offers a promising avenue for modulating the GIT microbiota. These health benefits can manifest through various mechanisms, encompassing both direct actions of the probiotic itself and indirect effects mediated by the production of beneficial metabolites and secretions.
2. Mechanisms of Action of Probiotics
There are numerous mechanisms through which probiotics can confer health benefits to the host, and these can be both direct, via the probiotic itself, and/or indirect, via the production of beneficial metabolites and secretions (Figure 1). The following is a description of the beneficial modes of action depicted in Figure 1.
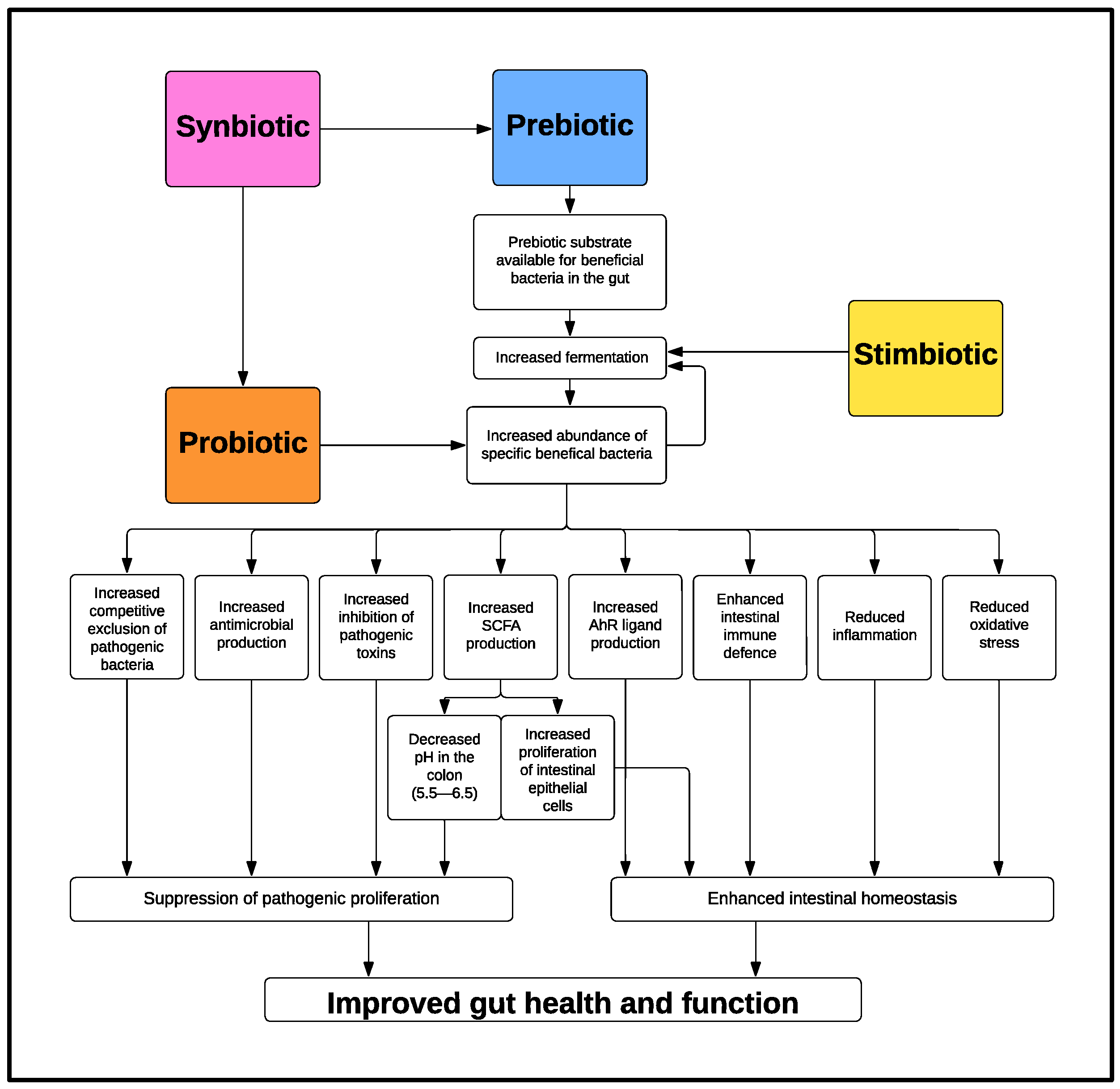
Figure 1. Mechanism of Action of Probiotics, Prebiotics, Synbiotics and Stimbiotics.
2.1. Competitive Exclusion of Pathogenic Bacteria
2.2. Antimicrobial Production
2.3. Inhibiting Pathogenic Toxins
2.4. Short-Chain Fatty Acid Production
2.5. Microbiota Derived Aryl Hydrocarbon Receptor Ligands
2.6. Enhancing Intestinal Immune Defence
- (1)
-
Epithelial barrier integrity
- (2)
-
Host defence peptides and secretory immunoglobulin A
- (3)
-
Mucus layer
2.7 Reducing Inflammation
2.8 Reducing Oxidative Stress
2.9 Other Potential Beneficial Effects: Vitamins and Enzymes
3. Conclusion
In conclusion, probiotics showcase a wide array of mechanisms, including competitive exclusion, antimicrobial production, and toxin inhibition, underscoring their efficacy against pathogenic bacteria. Additionally, the beneficial effects of microbiota-derived metabolites such as short-chain fatty acid and the AhR ligands can fortify immune defences, mitigate inflammation, and reduce oxidative stress. In essence, the diverse and intricate modes of action outlined underscore the significance of probiotics as agents capable of positively influencing various aspects of gastrointestinal health. Further research and exploration into specific strains and their applications are warranted to discover the full potential of probiotics in promoting health and performance.
References
- Déru, V.; Bouquet, A.; Zemb, O.; Blanchet, B.; De Almeida, M.L.; Cauquil, L.; Carillier-Jacquin, C.; Gilbert, H. Genetic relationships between efficiency traits and gut microbiota traits in growing pigs being fed with a conventional or a high-fiber diet. J. Anim. Sci. 2022, 100, skac183.
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109.
- Yang, Q.; Huang, X.; Zhao, S.; Sun, W.; Yan, Z.; Wang, P.; Li, S.; Huang, W.; Zhang, S.; Liu, L. Structure and function of the fecal microbiota in diarrheic neonatal piglets. Front. Microbiol. 2017, 8, 502.
- Lu, X.; Zhang, M.; Zhao, L.; Ge, K.; Wang, Z.; Jun, L.; Ren, F. Growth performance and post-weaning diarrhea in piglets fed a diet supplemented with probiotic complexes. J. Microbiol. Biotechnol. 2018, 28, 1791–1799.
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Patel, B.H.M.; Singh, P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest. Sci. 2017, 195, 74–79.
- Cao, M.; Li, Y.; Wu, Q.J.; Zhang, P.; Li, W.T.; Mao, Z.Y.; Wu, D.M.; Jiang, X.M.; Zhuo, Y.; Fang, Z.F.; et al. Effects of dietary Clostridium butyricum addition to sows in late gestation and lactation on reproductive performance and intestinal microbiota1. J. Anim. Sci. 2019, 97, 3426–3439.
- Collado, M.C.; Gueimonde, M.; Hernandez, M.; Sanz, Y.; Salminen, S. Adhesion of selected Bifidobacterium strains to human intestinal mucus and the role of adhesion in enteropathogen exclusion. J. Food Prot. 2005, 68, 2672–2678.
- Pahumunto, N.; Dahlen, G.; Teanpaisan, R. Evaluation of Potential Probiotic Properties of Lactobacillus and Bacillus Strains Derived from Various Sources for Their Potential Use in Swine Feeding. Probiotics Antimicrob. Proteins 2023, 15, 479–490.
- Šikić Pogačar, M.; Langerholc, T.; Mičetić-Turk, D.; Možina, S.S.; Klančnik, A. Effect of Lactobacillus spp. on adhesion, invasion, and translocation of Campylobacter jejuni in chicken and pig small-intestinal epithelial cell lines. BMC Vet. Res. 2020, 16, 34.
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588.
- Splichal, I.; Donovan, S.M.; Splichalova, Z.; Neuzil Bunesova, V.; Vlkova, E.; Jenistova, V.; Killer, J.; Svejstil, R.; Skrivanova, E.; Splichalova, A. Colonization of Germ-Free Piglets with Commensal Lactobacillus amylovorus, Lactobacillus mucosae, and Probiotic E. coli Nissle 1917 and Their Interference with Salmonella Typhimurium. Microorganisms 2019, 7, 273.
- Deriu, E.; Liu, J.Z.; Pezeshki, M.; Edwards, R.A.; Ochoa, R.J.; Contreras, H.; Libby, S.J.; Fang, F.C.; Raffatellu, M. Probiotic Bacteria Reduce Salmonella Typhimurium Intestinal Colonization by Competing for Iron. Cell Host Microbe 2013, 14, 26–37.
- Choudhury, R.; Middelkoop, A.; de Souza, J.G.; van Veen, L.A.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E.; Kleerebezem, M. Impact of early-life feeding on local intestinal microbiota and digestive system development in piglets. Sci. Rep. 2021, 11, 4213.
- Haindl, R.; Schick, S.; Kulozik, U. Influence of Cultivation pH on Composition, Diversity, and Metabolic Production in an In Vitro Human Intestinal Microbiota. Fermentation 2021, 7, 156.
- Wang, J.; Zeng, Y.; Wang, S.; Liu, H.; Zhang, D.; Zhang, W.; Wang, Y.; Ji, H. Swine-Derived Probiotic Lactobacillus plantarum Inhibits Growth and Adhesion of Enterotoxigenic Escherichia coli and Mediates Host Defense. Front. Microbiol. 2018, 9, 1364.
- Ciurescu, G.; Dumitru, M.; Gheorghe, A.; Untea, A.E.; Drăghici, R. Effect of Bacillus subtilis on growth performance, bone mineralization, and bacterial population of broilers fed with different protein sources. Poult. Sci. 2020, 99, 5960–5971.
- Siahmoshteh, F.; Hamidi-Esfahani, Z.; Spadaro, D.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Control 2018, 89, 300–307.
- Bukhari, S.A.; Salman, M.; Numan, M.; Javed, M.R.; Zubair, M.; Mustafa, G. Characterization of antifungal metabolites produced by Lactobacillus plantarum and Lactobacillus coryniformis isolated from rice rinsed water. Mol. Biol. Rep. 2020, 47, 1871–1881.
- Rattray, J.B.; Thomas, S.A.; Wang, Y.; Molotkova, E.; Gurney, J.; Varga, J.J.; Brown, S.P. Bacterial Quorum Sensing Allows Graded and Bimodal Cellular Responses to Variations in Population Density. mBio 2022, 13, e00745-22.
- Dubern, J.F.; Halliday, N.; Cámara, M.; Winzer, K.; Barrett, D.A.; Hardie, K.R.; Williams, P. Growth rate and nutrient limitation as key drivers of extracellular quorum sensing signal molecule accumulation in Pseudomonas aeruginosa. Microbiology 2023, 169, 001316.
- Dong, Y.-H.; Gusti, A.R.; Zhang, Q.; Xu, J.-L.; Zhang, L.-H. Identification of Quorum-Quenching N-Acyl Homoserine Lactonases from Bacillus Species. Appl. Environ. Microbiol. 2002, 68, 1754–1759.
- Villamil, L.; Reyes, C.; Martínez-Silva, M.A. In vivo and in vitro assessment of Lactobacillus acidophilus as probiotic for tilapia (Oreochromis niloticus, Perciformes: Cichlidae) culture improvement. Aquac. Res. 2014, 45, 1116–1125.
- Chen, F.; Gao, Y.; Chen, X.; Yu, Z.; Li, X. Quorum Quenching Enzymes and Their Application in Degrading Signal Molecules to Block Quorum Sensing-Dependent Infection. Int. J. Mol. Sci. 2013, 14, 17477–17500.
- Kim, J.; Kim, J.; Kim, Y.; Oh, S.; Song, M.; Choe, J.H.; Whang, K.-Y.; Kim, K.H.; Oh, S. Influences of quorum-quenching probiotic bacteria on the gut microbial community and immune function in weaning pigs. Anim. Sci. J. 2018, 89, 412–422.
- Mukiza, C.N.; Dubreuil, J.D. Escherichia coli Heat-Stable Toxin b Impairs Intestinal Epithelial Barrier Function by Altering Tight Junction Proteins. Infect. Immun. 2013, 81, 2819–2827.
- Nassour, H.; Dubreuil, J.D. Escherichia coli STb Enterotoxin Dislodges Claudin-1 from Epithelial Tight Junctions. PLoS ONE 2014, 9, e113273.
- Popoff, M.R. Clostridial pore-forming toxins: Powerful virulence factors. Anaerobe 2014, 30, 220–238.
- Weikel, C.S.; Guerrant, R.L. STb enterotoxin of Escherichia coli: Cyclic nucleotide-independent secretion. Microb. Toxins Diarrhoeal Dis. 1985, 112, 94–115.
- Zhou, M.; Yu, H.; Yin, X.; Sabour, P.M.; Chen, W.; Gong, J. Lactobacillus zeae Protects Caenorhabditis elegans from Enterotoxigenic Escherichia coli-Caused Death by Inhibiting Enterotoxin Gene Expression of the Pathogen. PLoS ONE 2014, 9, e89004.
- Carasi, P.; Trejo, F.M.; Pérez, P.F.; De Antoni, G.L.; Serradell, M.d.l.A. Surface proteins from Lactobacillus kefir antagonize in vitro cytotoxic effect of Clostridium difficile toxins. Anaerobe 2012, 18, 135–142.
- Carey, C.M.; Kostrzynska, M.; Ojha, S.; Thompson, S. The effect of probiotics and organic acids on Shiga-toxin 2 gene expression in enterohemorrhagic Escherichia coli O157:H7. J. Microbiol. Methods 2008, 73, 125–132.
- Jørgensen, H.; Larsen, T.; Zhao, X.-Q.; Eggum, B.O. The energy value of short-chain fatty acids infused into the caecum of pigs. Br. J. Nutr. 1997, 77, 745–756.
- Van Hoffen, E.; Ruiter, B.; Faber, J.; M’Rabet, L.; Knol, E.F.; Stahl, B.; Arslanoglu, S.; Moro, G.; Boehm, G.; Garssen, J. A specific mixture of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at high risk for allergy. Allergy 2009, 64, 484–487.
- Bai, Y.; Zhou, X.; Zhao, J.; Wang, Z.; Ye, H.; Pi, Y.; Che, D.; Han, D.; Zhang, S.; Wang, J. Sources of Dietary Fiber Affect the SCFA Production and Absorption in the Hindgut of Growing Pigs. Front. Nutr. 2022, 8, 719935.
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526.
- Kien, C.L.; Blauwiekel, R.; Bunn, J.Y.; Jetton, T.L.; Frankel, W.L.; Holst, J.J. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J. Nutr. 2007, 137, 916–922.
- Diao, H.; Jiao, A.R.; Yu, B.; Mao, X.B.; Chen, D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr. 2019, 14, 4.
- Zeng, X.; Sunkara, L.T.; Jiang, W.; Bible, M.; Carter, S.; Ma, X.; Qiao, S.; Zhang, G. Induction of porcine host defense peptide gene expression by short-chain fatty acids and their analogs. PLoS ONE 2013, 8, e72922.
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139.
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455.
- Liu, X.-f.; Shao, J.-h.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.-j.; Liu, Z.-z.; He, D.-d.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892.
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252.
- Thomas, S.P.; Denu, J.M. Short-chain fatty acids activate acetyltransferase p300. Elife 2021, 10, e72171.
- Stein, R.A.; Riber, L. Epigenetic effects of short-chain fatty acids from the large intestine on host cells. microLife 2023, 4, uqad032.
- Watt, R.; Parkin, K.; Martino, D. The Potential Effects of Short-Chain Fatty Acids on the Epigenetic Regulation of Innate Immune Memory. Challenges 2020, 11, 25.
- Lamas, B.; Hernandez-Galan, L.; Galipeau, H.J.; Constante, M.; Clarizio, A.; Jury, J.; Breyner, N.M.; Caminero, A.; Rueda, G.; Hayes, C.L.; et al. Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci. Transl. Med. 2020, 12, eaba0624.
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018, 11, 1024–1038.
- Tossou, M.C.B.; Liu, H.; Bai, M.; Chen, S.; Cai, Y.; Duraipandiyan, V.; Liu, H.; Adebowale, T.O.; Al-Dhabi, N.A.; Long, L.; et al. Effect of High Dietary Tryptophan on Intestinal Morphology and Tight Junction Protein of Weaned Pig. BioMed Res. Int. 2016, 2016, 2912418.
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294.
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.-M.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci. Rep. 2019, 9, 643.
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387.
- Yu, K.; Ma, Y.; Zhang, Z.; Fan, X.; Li, T.; Li, L.; Xiao, W.; Cai, Y.; Sun, L.; Xu, P.; et al. AhR activation protects intestinal epithelial barrier function through regulation of Par-6. J. Mol. Histol. 2018, 49, 449–458.
- Meng, D.; Sommella, E.; Salviati, E.; Campiglia, P.; Ganguli, K.; Djebali, K.; Zhu, W.; Walker, W.A. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res. 2020, 88, 209–217.
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 372–385.
- Keir, M.; Yi, Y.; Lu, T.; Ghilardi, N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 2020, 217, e20192195.
- Huang, Z.; de Vries, S.; Fogliano, V.; Wells, J.M.; van der Wielen, N.; Capuano, E. Effect of whole foods on the microbial production of tryptophan-derived aryl hydrocarbon receptor agonists in growing pigs. Food Chem. 2023, 416, 135804.
- Dang, G.; Wen, X.; Zhong, R.; Wu, W.; Tang, S.; Li, C.; Yi, B.; Chen, L.; Zhang, H.; Schroyen, M. Pectin modulates intestinal immunity in a pig model via regulating the gut microbiota-derived tryptophan metabolite-AhR-IL22 pathway. J. Anim. Sci. Biotechnol. 2023, 14, 38.
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.-P.; Michel, M.-L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605.
- Zhang, R.; Huang, G.; Ren, Y.; Wang, H.; Ye, Y.; Guo, J.; Wang, M.; Zhu, W.; Yu, K. Effects of Dietary Indole-3-carboxaldehyde Supplementation on Growth Performance, Intestinal Epithelial Function, and Intestinal Microbial Composition in Weaned Piglets. Front. Nutr. 2022, 9, 896815.
- Xie, Z.; Li, M.; Qian, M.; Yang, Z.; Han, X. Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids. Nutrients 2022, 14, 4475.
- Lessard, M.; Savard, C.; Deschene, K.; Lauzon, K.; Pinilla, V.A.; Gagnon, C.A.; Lapointe, J.; Guay, F.; Chorfi, Y. Impact of deoxynivalenol (DON) contaminated feed on intestinal integrity and immune response in swine. Food Chem. Toxicol. 2015, 80, 7–16.
- Wu, Y.; Zhu, C.; Chen, Z.; Chen, Z.; Zhang, W.; Ma, X.; Wang, L.; Yang, X.; Jiang, Z. Protective effects of Lactobacillus plantarum on epithelial barrier disruption caused by enterotoxigenic Escherichia coli in intestinal porcine epithelial cells. Vet. Immunol. Immunopathol. 2016, 172, 55–63.
- McLamb, B.L.; Gibson, A.J.; Overman, E.L.; Stahl, C.; Moeser, A.J. Early Weaning Stress in Pigs Impairs Innate Mucosal Immune Responses to Enterotoxigenic E. coli Challenge and Exacerbates Intestinal Injury and Clinical Disease. PLoS ONE 2013, 8, e59838.
- Soong, G.; Parker, D.; Magargee, M.; Prince, A.S. The Type III Toxins of Pseudomonas aeruginosa Disrupt Epithelial Barrier Function. J. Bacteriol. 2008, 190, 2814–2821.
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215.
- Boudry, G.; Péron, V.; Le Huërou-Luron, I.; Lallès, J.P.; Sève, B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 2004, 134, 2256–2262.
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953.
- Yang, F.; Wang, A.; Zeng, X.; Hou, C.; Liu, H.; Qiao, S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015, 15, 32.
- Yuying, W.; Xue, Y.; Weiwei, Z.; Yuanyuan, L.; Deping, H.; Kedao, T.; Yunfei, M. Lactobacillus casei Zhang prevents jejunal epithelial damage to early-weaned piglets induced by Escherichia coli K88 via regulation of intestinal mucosal integrity, tight junction proteins and immune factor expression. J. Microbiol. Biotechnol. 2019, 29, 863–876.
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749.
- Sultana, R.; McBain, A.J.; O’Neill, C.A. Strain-Dependent Augmentation of Tight-Junction Barrier Function in Human Primary Epidermal Keratinocytes by Lactobacillus and Bifidobacterium Lysates. Appl. Environ. Microbiol. 2013, 79, 4887–4894.
- Lodemann, U.; Strahlendorf, J.; Schierack, P.; Klingspor, S.; Aschenbach, J.R.; Martens, H. Effects of the probiotic Enterococcus faecium and pathogenic Escherichia coli strains in a pig and human epithelial intestinal cell model. Scientifica 2015, 2015, 235184.
- Bhat, M.I.; Sowmya, K.; Kapila, S.; Kapila, R. Potential Probiotic Lactobacillus rhamnosus (MTCC-5897) Inhibits Escherichia coli Impaired Intestinal Barrier Function by Modulating the Host Tight Junction Gene Response. Probiotics Antimicrob. Proteins 2020, 12, 1149–1160.
- Patel, R.M.; Myers, L.S.; Kurundkar, A.R.; Maheshwari, A.; Nusrat, A.; Lin, P.W. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 2012, 180, 626–635.
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.-J.M.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G851–G859.
- Zhang, G.; Ross, C.R.; Blecha, F. Porcine antimicrobial peptides: New prospects for ancient molecules of host defense. Vet. Res. 2000, 31, 277–296.
- Sang, Y.; Blecha, F. Porcine host defense peptides: Expanding repertoire and functions. Dev. Comp. Immunol. 2009, 33, 334–343.
- Veldhuizen, E.J.A.; Rijnders, M.; Claassen, E.A.; van Dijk, A.; Haagsman, H.P. Porcine β-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol. Immunol. 2008, 45, 386–394.
- Zilbauer, M.; Dorrell, N.; Boughan, P.K.; Harris, A.; Wren, B.W.; Klein, N.J.; Bajaj-Elliott, M. Intestinal Innate Immunity to Campylobacter jejuni Results in Induction of Bactericidal Human Beta-Defensins 2 and 3. Infect. Immun. 2005, 73, 7281–7289.
- Liu, H.; Hou, C.; Wang, G.; Jia, H.; Yu, H.; Zeng, X.; Thacker, P.A.; Zhang, G.; Qiao, S. Lactobacillus reuteri I5007 Modulates Intestinal Host Defense Peptide Expression in the Model of IPEC-J2 Cells and Neonatal Piglets. Nutrients 2017, 9, 559.
- Schlee, M.; Harder, J.; Köten, B.; Stange, E.; Wehkamp, J.; Fellermann, K. Probiotic lactobacilli and VSL# 3 induce enterocyte β-defensin 2. Clin. Exp. Immunol. 2008, 151, 528–535.
- Wang, J.; Zhang, W.; Wang, S.; Liu, H.; Zhang, D.; Wang, Y.; Ji, H. Swine-Derived Probiotic Lactobacillus plantarum Modulates Porcine Intestinal Endogenous Host Defense Peptide Synthesis Through TLR2/MAPK/AP-1 Signaling Pathway. Front. Immunol. 2019, 10, 2691.
- Wang, M.; Wu, H.; Lu, L.; Jiang, L.; Yu, Q. Lactobacillus reuteri Promotes Intestinal Development and Regulates Mucosal Immune Function in Newborn Piglets. Front. Vet. Sci. 2020, 7, 42.
- Thu, T.V.; Loh, T.C.; Foo, H.L.; Yaakub, H.; Bejo, M.H. Effects of liquid metabolite combinations produced by Lactobacillus plantarum on growth performance, faeces characteristics, intestinal morphology and diarrhoea incidence in postweaning piglets. Trop. Anim. Health Prod. 2011, 43, 69–75.
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254.
- Xin, J.; Zeng, D.; Wang, H.; Sun, N.; Zhao, Y.; Dan, Y.; Pan, K.; Jing, B.; Ni, X. Probiotic Lactobacillus johnsonii BS15 Promotes Growth Performance, Intestinal Immunity, and Gut Microbiota in Piglets. Probiotics Antimicrob. Proteins 2020, 12, 184–193.
- Wang, K.; Zhu, Q.; Kong, X.; Song, M.; Azad, M.A.K.; Xiong, L.; Zheng, Y.; He, Q. Dietary Probiotics or Synbiotics Supplementation During Gestation, Lactation, and Nursery Periods Modifies Colonic Microbiota, Antioxidant Capacity, and Immune Function in Weaned Piglets. Front. Vet. Sci. 2020, 7, 597832.
- Qin, D.; Bai, Y.; Li, Y.; Huang, Y.; Li, L.; Wang, G.; Qu, Y.; Wang, J.; Yu, L.-Y.; Hou, X. Changes in Gut Microbiota by the Lactobacillus casei Anchoring the K88 Fimbrial Protein Prevented Newborn Piglets From Clinical Diarrhea. Front. Cell. Infect. Microbiol. 2022, 12, 268.
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069.
- Johansson, M.E.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361.
- Meyer-Hoffert, U.; Hornef, M.W.; Henriques-Normark, B.; Axelsson, L.G.; Midtvedt, T.; Pütsep, K.; Andersson, M. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut 2008, 57, 764.
- Ermund, A.; Schütte, A.; Johansson, M.E.V.; Gustafsson, J.K.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G341–G347.
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.; Hansson, G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151.
- Johansson, M.E.; Gustafsson, J.K.; Sjöberg, K.E.; Petersson, J.; Holm, L.; Sjövall, H.; Hansson, G.C. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 2010, 5, e12238.
- Johansson, M.E.V.; Larsson, J.M.H.; Hansson, G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 4659–4665.
- Gunning, A.P.; Kavanaugh, D.; Thursby, E.; Etzold, S.; MacKenzie, D.A.; Juge, N. Use of Atomic Force Microscopy to Study the Multi-Modular Interaction of Bacterial Adhesins to Mucins. Int. J. Mol. Sci. 2016, 17, 1854.
- Silva, A.J.; Pham, K.; Benitez, J.A. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 2003, 149, 1883–1891.
- Engevik, M.A.; Engevik, A.C.; Engevik, K.A.; Auchtung, J.M.; Chang-Graham, A.L.; Ruan, W.; Luna, R.A.; Hyser, J.M.; Spinler, J.K.; Versalovic, J. Mucin-Degrading Microbes Release Monosaccharides That Chemoattract Clostridioides difficile and Facilitate Colonization of the Human Intestinal Mucus Layer. ACS Infect. Dis. 2021, 7, 1126–1142.
- Yang, H.; Xiong, X.; Wang, X.; Tan, B.; Li, T.; Yin, Y. Effects of Weaning on Intestinal Upper Villus Epithelial Cells of Piglets. PLoS ONE 2016, 11, e0150216.
- Pang, J.; Liu, Y.; Kang, L.; Ye, H.; Zang, J.; Wang, J.; Han, D. Bifidobacterium animalis promotes the growth of weaning piglets by improving intestinal development, enhancing antioxidant capacity, and modulating gut microbiota. Appl. Environ. Microbiol. 2022, 88, e01296-22.
- Zhang, W.; Zhu, Y.-H.; Zhou, D.; Wu, Q.; Song, D.; Dicksved, J.; Wang, J.-F. Oral Administration of a Select Mixture of Bacillus Probiotics Affects the Gut Microbiota and Goblet Cell Function following Escherichia coli Challenge in Newly Weaned Pigs of Genotype MUC4 That Are Supposed To Be Enterotoxigenic E. coli F4ab/ac Receptor Negative. Appl. Environ. Microbiol. 2017, 83, e02747-16.
- Desantis, S.; Mastrodonato, M.; Accogli, G.; Rossi, G.; Crovace, A.M. Effects of a probiotic on the morphology and mucin composition of pig intestine. Histol. Histopathol. 2019, 34, 1037–1050.
- Gāliņa, D.; Ansonska, L.; Valdovska, A. Effect of Probiotics and Herbal Products on Intestinal Histomorphological and Immunological Development in Piglets. Vet. Med. Int. 2020, 2020, 3461768.
- Andrews, C.; McLean, M.H.; Durum, S.K. Cytokine Tuning of Intestinal Epithelial Function. Front. Immunol. 2018, 9, 1270.
- Li, N.; Gu, L.; Qu, L.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine attenuates pro-inflammatory cytokine-induced tight junction disruption in an in vitro model of intestinal epithelial cells. Eur. J. Pharm. Sci. 2010, 40, 1–8.
- Finamore, A.; Roselli, M.; Imbinto, A.; Seeboth, J.; Oswald, I.P.; Mengheri, E. Lactobacillus amylovorus Inhibits the TLR4 Inflammatory Signaling Triggered by Enterotoxigenic Escherichia coli via Modulation of the Negative Regulators and Involvement of TLR2 in Intestinal Caco-2 Cells and Pig Explants. PLoS ONE 2014, 9, e94891.
- Sies, H.; Keaney, J. Oxidative Stress and Vascular Disease; Springer: Berlin/Heidelberg, Germany, 2000.
- Hao, Y.; Xing, M.; Gu, X. Research Progress on Oxidative Stress and Its Nutritional Regulation Strategies in Pigs. Animals 2021, 11, 1384.
- Liu, L.; Wu, C.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; et al. Selenium-Enriched Yeast Alleviates Oxidative Stress-Induced Intestinal Mucosa Disruption in Weaned Pigs. Oxid. Med. Cell Longev. 2020, 2020, 5490743.
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell Longev. 2016, 2016, 5698931.
- Shen, Q.; Shang, N.; Li, P. In Vitro and In Vivo Antioxidant Activity of Bifidobacterium animalis 01 Isolated from Centenarians. Curr. Microbiol. 2011, 62, 1097–1103.
- Sun, Y.; Duarte, M.E.; Kim, S.W. Dietary inclusion of multispecies probiotics to reduce the severity of post-weaning diarrhea caused by Escherichia coli F18+ in pigs. Anim. Nutr. 2021, 7, 326–333.
- Cui, K.; Wang, Q.; Wang, S.; Diao, Q.; Zhang, N. The facilitating effect of tartary buckwheat flavonoids and Lactobacillus plantarum on the growth performance, nutrient digestibility, antioxidant capacity, and fecal microbiota of weaned piglets. Animals 2019, 9, 986.
- Sun, Z.; Li, H.; Li, Y.; Qiao, J. Lactobacillus salivarius, a Potential Probiotic to Improve the Health of LPS-Challenged Piglet Intestine by Alleviating Inflammation as Well as Oxidative Stress in a Dose-Dependent Manner During Weaning Transition. Front. Vet. Sci. 2020, 7, 547425.
- Cao, X.; Tang, L.; Zeng, Z.; Wang, B.; Zhou, Y.; Wang, Q.; Zou, P.; Li, W. Effects of Probiotics BaSC06 on Intestinal Digestion and Absorption, Antioxidant Capacity, Microbiota Composition, and Macrophage Polarization in Pigs for Fattening. Front. Vet. Sci. 2020, 7, 570593.
- Zhao, D.; Wu, T.; Yi, D.; Wang, L.; Li, P.; Zhang, J.; Hou, Y.; Wu, G. Dietary Supplementation with Lactobacillus casei Alleviates Lipopolysaccharide-Induced Liver Injury in a Porcine Model. Int. J. Mol. Sci. 2017, 18, 2535.
- Hill, M. Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 1997, 6, S43–S45.
- Subramanian, V.S.; Marchant, J.S.; Boulware, M.J.; Ma, T.Y.; Said, H.M. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C663–C671.
- Said, H.M.; Ortiz, A.; Subramanian, V.S.; Neufeld, E.J.; Moyer, M.P.; Dudeja, P.K. Mechanism of thiamine uptake by human colonocytes: Studies with cultured colonic epithelial cell line NCM460. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G144–G150.
- Kumar, J.S.; Subramanian, V.S.; Kapadia, R.; Kashyap, M.L.; Said, H.M. Mammalian colonocytes possess a carrier-mediated mechanism for uptake of vitamin B3 (niacin): Studies utilizing human and mouse colonic preparations. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G207–G213.
- Said, H.M.; Ortiz, A.; McCloud, E.; Dyer, D.; Moyer, M.P.; Rubin, S. Biotin uptake by human colonic epithelial NCM460 cells: A carrier-mediated process shared with pantothenic acid. Am. J. Physiol. Cell Physiol. 1998, 275, C1365–C1371.
- Branner, G.R.; Roth-Maier, D.A. Influence of pre-, pro-, and synbiotics on the intestinal availability of different B-vitamins. Arch. Anim. Nutr. 2006, 60, 191–204.
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front. Nutr. 2019, 6, 48.
- Hmani, H.; Daoud, L.; Jlidi, M.; Jalleli, K.; Ben Ali, M.; Hadj Brahim, A.; Bargui, M.; Dammak, A.; Ben Ali, M. A Bacillus subtilis strain as probiotic in poultry: Selection based on in vitro functional properties and enzymatic potentialities. J. Ind. Microbiol. Biotechnol. 2017, 44, 1157–1166.
- Lee, J.; Park, I.; Choi, Y.; Cho, J. Bacillus strains as feed additives: In vitro evaluation of its potential probiotic properties. Rev. Colomb. De Cienc. Pecu. 2012, 25, 577–585.
- Padmavathi, T.; Bhargavi, R.; Priyanka, P.R.; Niranjan, N.R.; Pavitra, P.V. Screening of potential probiotic lactic acid bacteria and production of amylase and its partial purification. J. Genet. Eng. Biotechnol. 2018, 16, 357–362.
- Yan, L.; Kim, I.H. Effect of probiotics supplementation in diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, faecal microbial population and faecal noxious gas content in growing pigs. J. Appl. Anim. Res. 2013, 41, 23–28.




