Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guangfu Liao | -- | 1257 | 2023-12-06 11:22:49 | | | |
| 2 | Fanny Huang | Meta information modification | 1257 | 2023-12-08 04:06:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Du, C.; Xu, J.; Ding, G.; He, D.; Zhang, H.; Qiu, W.; Li, C.; Liao, G. Advantages of C3N4 with Layered Double Hydroxides Heterojunctions. Encyclopedia. Available online: https://encyclopedia.pub/entry/52430 (accessed on 07 February 2026).
Du C, Xu J, Ding G, He D, Zhang H, Qiu W, et al. Advantages of C3N4 with Layered Double Hydroxides Heterojunctions. Encyclopedia. Available at: https://encyclopedia.pub/entry/52430. Accessed February 07, 2026.
Du, Cheng, Jialin Xu, Guixiang Ding, Dayong He, Hao Zhang, Weibao Qiu, Chunxue Li, Guangfu Liao. "Advantages of C3N4 with Layered Double Hydroxides Heterojunctions" Encyclopedia, https://encyclopedia.pub/entry/52430 (accessed February 07, 2026).
Du, C., Xu, J., Ding, G., He, D., Zhang, H., Qiu, W., Li, C., & Liao, G. (2023, December 06). Advantages of C3N4 with Layered Double Hydroxides Heterojunctions. In Encyclopedia. https://encyclopedia.pub/entry/52430
Du, Cheng, et al. "Advantages of C3N4 with Layered Double Hydroxides Heterojunctions." Encyclopedia. Web. 06 December, 2023.
Copy Citation
Environmental pollution has been decreased by using photocatalytic technology in conjunction with solar energy. An efficient method to obtain highly efficient photocatalysts is to build heterojunction photocatalysts by combining graphitic carbon nitride (g-C3N4) with layered double hydroxides (LDHs).
layered double hydroxides
carbon nitride
heterojunction
1. Introduction
Environmental pollution has made it extremely difficult for humanity to flourish sustainably [1][2][3][4][5]. The use of photocatalytic technology to harness clean and reproducible solar energy is widely considered to be the most effective answer to the issues [6][7][8]. An effective method for reducing pollution is to use photocatalytic technology to degrade organic pollutants by using solar irradiation [9][10]. In principle, photocatalysis is an oxidation-reduction process. The fundamental components of the photocatalytic process, photocatalysts, are crucial in driving the reaction and harvesting light [11][12][13][14][15]. Consequently, research into highly effective photocatalysts is crucial for the advancement of photocatalytic technology.
An organic semiconductor having triazine or heptazine as its main constitutional unit and a layer structure resembling graphite is known as graphitic carbon nitride (g-C3N4) [7][8][16]. Since the use of g-C3N4 in photocatalytic hydrogen synthesis was initially discovered [17], it has garnered considerable interest. Because g-C3N4 possesses a smaller band gap (approximately 2.7 eV) than traditional photocatalysts like TiO2 [18], it can be stimulated by visible light. Furthermore, g-C3N4 is competitive among other photocatalytic materials due to its excellent chemical stability, high thermostability, affordable raw ingredients, and straightforward production procedure [19][20][21][22]. However, there are numerous issues with the industrial uses of g-C3N4. Only blue and purple lights, with a wavelength of 460 nm, may pass through g-C3N4, resulting in a low solar energy utilization rate [23]. The redox capacity of g-C3N4 is reduced because of the rapid recombination of photocarriers. The specific surface area (SSA) of g-C3N4 is relatively tiny because of its bulk characteristics. These flaws prevent g-C3N4 from being developed further for photocatalytic uses. To increase the photocatalytic performance of g-C3N4, various techniques have been used, including decorating co-catalysts, doping elements (such as F, O, Ni, Fe, etc.), building nanostructures, creating heterojunctions [24][25], etc. To boost the separation of charge carriers in g-C3N4, creating heterostructures with different semiconductors is the most important technique. Because of the varying Fermi levels between various photocatalysts, when they come into contact with one another, charge carriers can travel across the semiconductors, which ultimately create an internal electric field (IEF) between them. The electric field allows the photoinduced electrons and holes to flow in a certain direction, thereby separating them [26][27].
A highly efficient method for improving photocatalytic performance has recently been discovered by creating layered double hydroxides (LDHs)/g-C3N4 heterojunctions. Essentially, LDHs are a class of two-dimensional (2D) hydrotalcite-like clay materials made up of exchangeable interlayer anions and positively charged host layers [28][29]. LDHs and their derivatives have been found useful in a variety of sectors, especially photocatalysis, because of their inexpensive features, excellent chemical stability, customizable composition, homogeneous distribution of cations and anions, and interchangeable interlayer anions. Nevertheless, because of the fast recombination of photocarriers, the photocatalytic activity of single LDHs is unsatisfactory [30]. Therefore, by building LDH/g-C3N4 heterojunctions with a smart design, it is possible to obtain optimal photocatalysts with top performance while overcoming the drawbacks of g-C3N4 and LDHs.
2. Advantages of LDH/g-C3N4 Heterojunctions
It has been suggested that one efficient method to enhance the photocatalytic activity of LDH/g-C3N4 composites is to build 2D/2D heterojunctions. In addition to providing a large number of surface active sites to create heterojunctions, the 2D structure of LDHs and g-C3N4 significantly reduces the distance over which photocarriers must move within the 2D/2D heterojunctions, favoring a photocatalytic reaction [31][32]. LDHs are ideal materials for creating 2D/2D heterojunctions with g-C3N4 because of their suitable band structure and variable composition [29]. As is known to all, the band gap of LDHs is about 2.0–3.4 eV, which can be adjusted by changing and regulating the M2+ and M3+, which is advantageous for the capture of visible light [33]. In addition, the materials’ abundance of basic sites makes them useful as heterogeneous photocatalysts for various chemical processes [34]. The active sites of LDH/g-C3N4 2D/2D heterojunctions are also changeable due to the ability to manipulate the cations and anions [35]. Moreover, designing LDHs’ interlayer space, number of layers, and functionalization with g-C3N4 are all rather simple processes (Figure 1). There are several advantages to LDH/g-C3N4 heterojunctions as shown below: (i) Owing to the close contact between LDHs and g-C3N4, photocarriers can conveniently migrate, which is beneficial for photocarrier separation [36]. (ii) The high surface area in LDH/g-C3N4 heterojunctions and their strong light harvesting ability are beneficial for photocatalytic activity [37]. (iii) LDH/g-C3N4 heterojunctions exhibit a shorter migration distance than photocarriers, thus reducing the electron-hole recombination [38]. (iv) The band structure can be simply adjusted [39]. All of these advantages ensure them with huge application potential in organic pollutant removal.
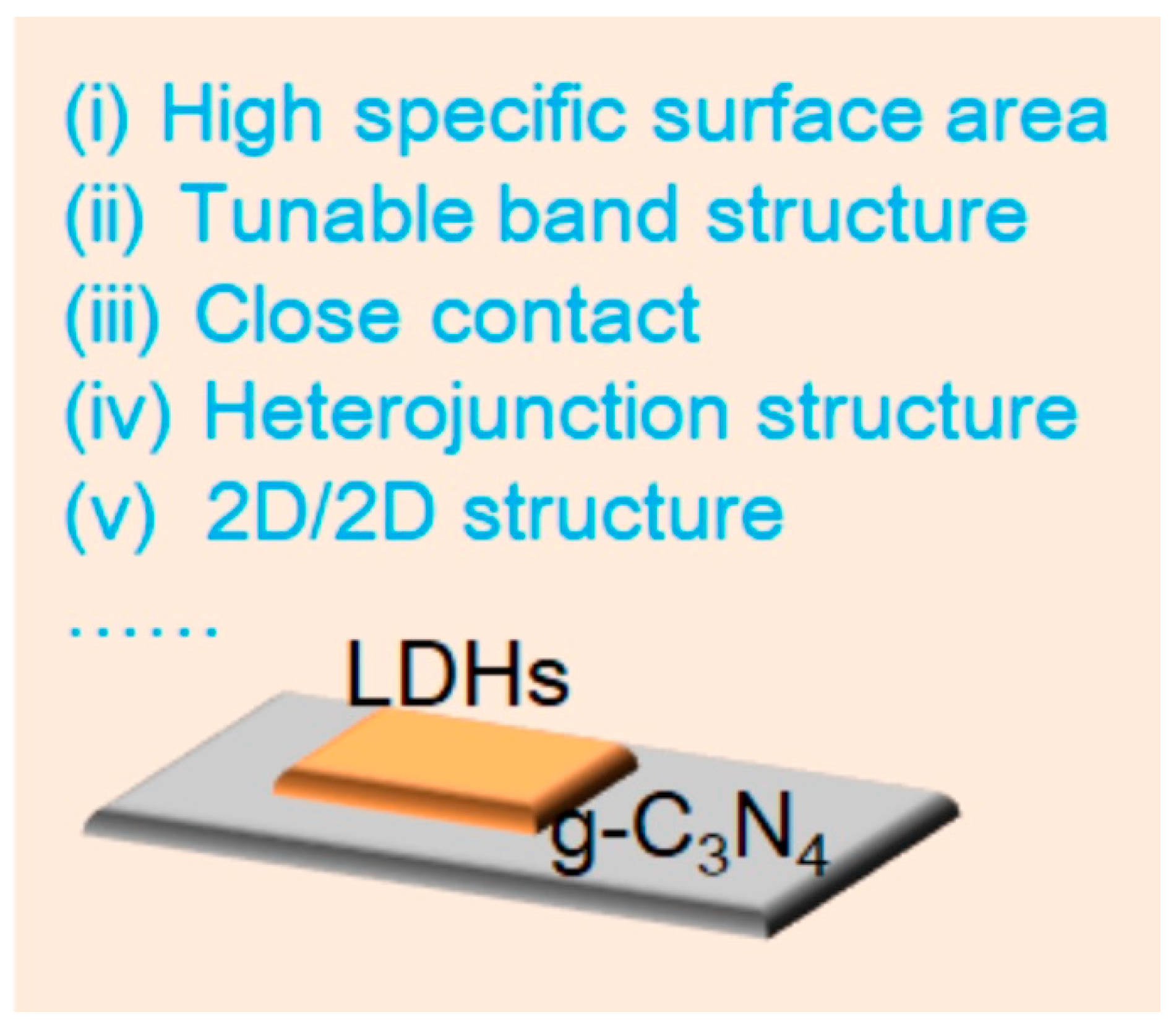
Figure 1. Advantages of LDH/g-C3N4 heterojunctions.
Based on the band alignment between the conduction band (CB) and the valence band (VB) of LDHs and g-C3N4, the heterojunctions formed between them can be classified into three types: straddling-gap junctions (Type I), staggered-gap junctions (Type II), and broken-gap junctions (Type III). When two semiconductors with staggered band alignment are intimately contacted, and there exists a charge migration between the CB in LDHs and the VB in g-C3N4, a Z-scheme system is formed. This Z-scheme system is characterized by an efficient electron-hole separation process, leading to enhanced photocatalytic activity. The charge transfer between the semiconductors results in the formation of a potential difference at the interface, driving the separation of photogenerated charge carriers and reducing their recombination rate. This mechanism significantly improves the overall efficiency and performance of the heterojunction photocatalysts. Therefore, understanding the band alignment and charge transfer processes in heterojunction systems is crucial for the design and optimization of advanced photocatalytic materials with superior performance. Although both the Z-scheme and Type II heterojunctions have the same staggered band alignment, e−-h+ transfer occurs in opposite directions in these two semiconductors. In contrast to Type II heterojunctions, where photogenerated e− and h+ are transferred from high potentials to low potentials, leading to compromised redox capacities, a Z-scheme heterojunction can drive the photogenerated e− in LDHs to migrate and then recombine with the photogenerated h+ in g-C3N4. This process leaves the high energy h+ at the VB of LDHs and the high energy e− at the CB of g-C3N4 for photocatalysis [40].
To confirm the existence of the type of heterojunction, band structure analysis is crucial [41]. The solid UV-vis absorption and the X-ray photoelectron spectroscopy (XPS) valence band spectrum are common techniques for obtaining the band structure. Through the band structure analysis, researchers can determine what kind of heterojunction it is (Type I, Type II, and Type III). Notably, Type II and Z-scheme heterojunctions share a similar staggered band alignment structure. Nevertheless, their charge transfer pathways are significantly different. In order to determine if the charge transfer route is Z-scheme or type II heterojunctions, it is therefore critically necessary to investigate it in detail using some techniques such as (i) self-confirmation by photocatalytic reactions, products, and radical species, (ii) selective photodeposition of a noble metal, (iii) in situ XPS, (iv) femto-second transient absorption spectra (fs-TAS), (v) photoassisted Kelvin probe force microscopy (photo-KPFM), theoretical calculations, etc. For example, the work function (Φ) plays a crucial role in investigating the energy band alignment and charge transfer of heterojunctions, making it an essential physical parameter [42]. Researchers can also determine Φ through theoretical calculations and further determine the charge transfer route.
References
- Liu, Z.; Liu, C.; Chen, Z.; Huang, H.; Liu, Y.; Xue, L.; Sun, J.; Wang, X.; Xiong, P.; Zhu, J. Recent advances in two-dimensional materials for hydrovoltaic energy technology. Exploration 2023, 3, 20220061.
- Jia, R.; He, C.; Li, Q.; Liu, S.-Y.; Liao, G. Renewable plant-derived lignin for electrochemical energy systems. Trends Biotechnol. 2022, 40, 1425–1438.
- Li, C.; Jia, R.; Yang, Y.; Liao, G. A Hierarchical Helical Carbon Nanotube Fiber Artificial Ligament. Adv. Fiber Mater. 2023, 5, 1549–1551.
- Lan, Q.; Jin, S.-J.; Wang, Z.; Li, X.-Y.; Xiong, Y.; Wang, Z.-C.; Liu, S.-S.; Zhang, Z.-M.; Zhao, Q. Design and synthesis of polyoxovanadate-based framework for efficient dye degradation. Tungsten 2023.
- Khan, A.; Nilam, B.; Rukhsar, C.; Sayali, G.; Mandlekar, B.; Kadam, A. A review article based on composite graphene @tungsten oxide thin films for various applications. Tungsten 2023, 5, 391–418.
- Chen, J.; Wang, Y.; Yu, Y.; Wang, J.; Liu, J.; Ihara, H.; Qiu, H. Composite materials based on covalent organic frameworks for multiple advanced applications. Exploration 2023, 3, 20220144.
- Cheng, Q.; Zhang, G.-K. Enhanced photocatalytic performance of tungsten-based photocatalysts for degradation of volatile organic compounds: A review. Tungsten 2020, 2, 240–250.
- Lin, J.-H.; Yan, Y.-T.; Qi, J.-L.; Zha, C.-Y. Atomic tailoring-induced deficiency in tungsten oxides for high-performance energy-related devices. Tungsten 2023.
- Qiao, Y.; Sun, C.; Jian, J.; Zhou, T.; Xue, X.; Shi, J.; Che, G.; Liao, G. Efficient removal of organic pollution via photocatalytic degradation over a TiO2@HKUST-1 yolk-shell nanoreactor. J. Mol. Liq. 2023, 385, 122383.
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Electrospun Bi-decorated BixTiyOz/TiO2 flexible carbon nanofibers and their applications on degradating of organic pollutants under solar radiation. J. Mater. Sci. Technol. 2023, 150, 114–123.
- Liao, G.; Li, C.; Fang, B. Donor-acceptor organic semiconductor heterojunction nanoparticles for efficient photocatalytic H2 evolution. Matter 2022, 5, 1635–1637.
- Liao, G.; Fang, B.; Li, C. A high-pressure-tolerant vapor-fed photocatalytic system for efficient water splitting. Chem Catal. 2023, 3, 100671.
- Ghugal, S.G.; Kumar Tadi, K.; Rani, K.; Mendhe, A.C.; Jeong, Y.; Yoon, J. Improved photocatalytic performance of CdS/α-Fe2O3 heterostructure for anionic dye mineralization and charge storage applications. J. Mol. Liq. 2023, 392, 123384.
- Duta, A.; Andronic, L.; Enesca, A. The influence of low irradiance and electrolytes on the mineralization efficiency of organic pollutants using the Vis-active photocatalytic tandem CuInS2/TiO2/SnO2. Catal. Today 2018, 300, 18–27.
- Tao, X.; Ren, J.; Wang, D.; Huang, H.; Li, Y.; Guo, D.; Shan, B.; Liu, Y.; Wang, J.; Zhen, Y.; et al. Refining the d-band center of S-scheme CdIn2S4/MnZnFe2O4 heterostructures by interfacial electric field with boosting photocatalytic performance. Chem. Eng. J. 2023, 478, 147347.
- Palani, G.; Apsari, R.; Hanafiah, M.M.; Venkateswarlu, K.; Lakkaboyana, S.K.; Kannan, K.; Shivanna, A.T.; Idris, A.M.; Yadav, C.H. Metal-Doped Graphitic Carbon Nitride Nanomaterials for Photocatalytic Environmental Applications-A Review. Nanomaterials 2022, 12, 1754.
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80.
- Liao, G.; Tao, X.; Fang, B. An innovative synthesis strategy for high-efficiency and defects-switchable-hydrogenated TiO2 photocatalysts. Matter 2022, 5, 377–379.
- Li, W.; Sohail, M.; Anwar, U.; Taha, T.A.; Al-Sehemi, A.G.; Muhammad, S.; Al-Ghamdi, A.A.; Amin, M.A.; Palamanit, A.; Ullah, S.; et al. Recent progress in g–C3N4–Based materials for remarkable photocatalytic sustainable energy. Int. J. Hydrog. Energy 2022, 47, 21067–21118.
- Abu-Sari, S.M.; Daud, W.M.A.W.; Patah, M.F.A.; Ang, B.C. A review on synthesis, modification method, and challenges of light-driven H2 evolution using g-C3N4-based photocatalyst. Adv. Colloid Interface Sci. 2022, 307, 102722.
- Zhang, Q.; Liao, G.; Yang, B.; Zhang, Y.; Ge, G.; Lipovka, A.; Liu, J.; Rodriguez, R.D.; Yang, X.; Jia, X. Structural reorganization of ultrathin g-C3N4 nanosheets for significantly boosting wide-spectrum-driven CO2 photoreduction. Appl. Surf. Sci. 2023, 638, 157989.
- Tian, F.; Wu, X.; Chen, J.; Sun, X.; Yan, X.; Liao, G. One-step photodeposition of spatially separated CuOx and MnOx dual cocatalysts on g-C3N4 for enhanced CO2 photoreduction. Dalton Trans. 2023, 52, 11934–11940.
- Tian, F.; Wu, X.; Liu, S.; Gu, Y.; Lin, Z.; Zhang, H.; Yan, X.; Liao, G. Boosting photocatalytic H2 evolution through interfacial manipulation on a lotus seedpod shaped Cu2O/g-C3N4 p-n heterojunction. Sustain. Energy Fuels 2023, 7, 786–796.
- Wudil, Y.S.; Ahmad, U.F.; Gondal, M.A.; Al-Osta, M.A.; Almohammedi, A.; Sa’id, R.S.; Hrahsheh, F.; Haruna, K.; Mohamed, M.J.S. Tuning of graphitic carbon nitride (g-C3N4) for photocatalysis: A critical review. Arabian J. Chem. 2023, 16, 104542.
- Wang, Q.; Fang, Z.; Zhang, W.; Zhang, D. High-Efficiency g-C3N4 Based Photocatalysts for CO2 Reduction: Modification Methods. Adv. Fiber Mater. 2022, 4, 342–360.
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17.
- Liao, G.; Li, C.; Liu, S.-Y.; Fang, B.; Yang, H. Emerging frontiers of Z-scheme photocatalytic systems. Trends Chem. 2022, 4, 111–127.
- Li, C.; Cheng, B.; Lu, H.; Ding, G.; Jiang, Z.; Liao, G. Kinetically and Thermodynamically Favorable Ni–Al Layered Double Hydroxide/Ni-Doped Zn0.5Cd0.5S S-Scheme Heterojunction Triggering Photocatalytic H2 Evolution. Inorg. Chem. 2023, 62, 6843–6850.
- Ding, G.; Li, C.; Ni, Y.; Chen, L.; Shuai, L.; Liao, G. Layered double hydroxides and their composites as high-performance photocatalysts for CO2 reduction. EES Catal. 2023, 1, 369–391.
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766.
- Luo, B.; Liu, G.; Wang, L. Recent advances in 2D materials for photocatalysis. Nanoscale 2016, 8, 6904–6920.
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155.
- Ni, J.; Xue, J.; Xie, L.; Shen, J.; He, G.; Chen, H. Construction of magnetically separable NiAl LDH/Fe3O4–RGO nanocomposites with enhanced photocatalytic performance under visible light. Phys. Chem. Chem. Phys. 2018, 20, 414–421.
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066.
- Xu, Z.P.; Zhang, J.; Adebajo, M.O.; Zhang, H.; Zhou, C. Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 2011, 53, 139–150.
- Tonda, S.; Kumar, S.; Bhardwaj, M.; Yadav, P.; Ogale, S. g-C3N4/NiAl-LDH 2D/2D Hybrid Heterojunction for High-Performance Photocatalytic Reduction of CO2 into Renewable Fuels. ACS Appl. Mater. Interfaces 2018, 10, 2667–2678.
- Abazari, R.; Mahjoub, A.R.; Sanati, S.; Rezvani, Z.; Hou, Z.; Dai, H. Ni–Ti Layered Double Hydroxide@Graphitic Carbon Nitride Nanosheet: A Novel Nanocomposite with High and Ultrafast Sonophotocatalytic Performance for Degradation of Antibiotics. Inorg. Chem. 2019, 58, 1834–1849.
- Han, Y.-Y.; Lu, X.-L.; Tang, S.-F.; Yin, X.-P.; Wei, Z.-W.; Lu, T.-B. Metal-Free 2D/2D Heterojunction of Graphitic Carbon Nitride/Graphdiyne for Improving the Hole Mobility of Graphitic Carbon Nitride. Adv. Energy Mater. 2018, 8, 1702992.
- Li, Y.; Zhang, H.; Liu, P.; Wang, D.; Li, Y.; Zhao, H. Cross-Linked g-C3N4/rGO Nanocomposites with Tunable Band Structure and Enhanced Visible Light Photocatalytic Activity. Small 2013, 9, 3336–3344.
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694.
- Yang, H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 2021, 142, 111406.
- Xue, C.; An, H.; Shao, G.; Yang, G. Accelerating directional charge separation via built-in interfacial electric fields originating from work-function differences. Chin. J. Catal. 2021, 42, 583–594.
More
Information
Subjects:
Materials Science, Composites
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
951
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
08 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




