You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Isabel Pires | -- | 1917 | 2023-12-06 00:35:41 | | | |
| 2 | Peter Tang | Meta information modification | 1917 | 2023-12-06 02:33:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pinheiro, A.V.; Petrucci, G.N.; Dourado, A.; Pires, I. Anaesthesia in Veterinary Oncology. Encyclopedia. Available online: https://encyclopedia.pub/entry/52409 (accessed on 02 January 2026).
Pinheiro AV, Petrucci GN, Dourado A, Pires I. Anaesthesia in Veterinary Oncology. Encyclopedia. Available at: https://encyclopedia.pub/entry/52409. Accessed January 02, 2026.
Pinheiro, Ana Vidal, Gonçalo N. Petrucci, Amândio Dourado, Isabel Pires. "Anaesthesia in Veterinary Oncology" Encyclopedia, https://encyclopedia.pub/entry/52409 (accessed January 02, 2026).
Pinheiro, A.V., Petrucci, G.N., Dourado, A., & Pires, I. (2023, December 06). Anaesthesia in Veterinary Oncology. In Encyclopedia. https://encyclopedia.pub/entry/52409
Pinheiro, Ana Vidal, et al. "Anaesthesia in Veterinary Oncology." Encyclopedia. Web. 06 December, 2023.
Copy Citation
Anaesthesia plays an important role in diagnosis procedures and the treatment and pain management of oncological patients. However, studies suggest that anaesthetic drugs may increase the risk of tumour dissemination in the perioperative period by directly and indirectly suppressing the immune system, which is primarily responsible for controlling tumour growth.
perioperative period
surgery
immune system
tumours
intravenous anaesthetics
volatile anaesthetics
veterinary oncology
1. Introduction
The growing attention paid to animal health [1] and the development of novel diagnostic and therapeutic procedures have significantly impacted the lifespan of companion animals [2]. Malignant tumours tend to be more prevalent in older animals [3][4], which can be linked to various age-related factors such as alterations in cell-mediated immunity and the phenomenon of immunosenescence [5]. The diagnosis of cancer in dogs and cats has increased significantly in recent years, making it one of the most prevalent causes of death. [6][7]. This fact has led to novel therapeutic oncology approaches, which are considered based on the tumour line and the patient’s health [8]. Surgical excision is still considered the gold standard for treating solid tumours [9]; however, even in tumour resections with histologically clean margins, an undetected residual risk of the intra-surgical dissemination of tumour cells still exists [10]. For this reason, it is often required to combine surgical excision with other treatment alternatives, such as chemotherapy, radiation or biological therapies [11][12][13][14].
Considering human and animal studies, the main risk of tumour dissemination occurs in the perioperative period [15][16], during which cancer cells and the immune system are influenced by stress, pain [17][18], anaesthesia [19] and surgical procedures [20]. These factors induce a stress response that starts a cascade of physiological mechanisms beneficial to the restoration of homeostasis but also favourable to tumour growth [20], which include immunomodulatory and pro-tumour effects such as immune suppression and tumour angiogenesis [17][18]. Therefore, the perioperative period can be seen as a window of opportunity to minimise the pro-tumour and anti-immune mechanisms triggered by surgical stress and anaesthetic drugs, suggesting new clinical approaches with neurophysiological and immune preservation. Alternative surgical and anaesthetic procedures, such as minimally invasive surgery [21], regional anaesthesia [22], opioid-free anaesthesia [23], total intravenous anaesthesia [24] and multimodal analgesia [25][26], are gaining significance in clinical practice, with particular emphasis on oncological patients due to their immunoprotective effects.
2. The Immune System as the Main Player in the Tumour’s Defence
The tumour growth and spread process is influenced by the tumour’s characteristics but mainly by the host’s immune system [8]. The scientific community recognises that a high number and high activity of natural killer cells and cytotoxic T lymphocytes correlate with enhanced anti-tumour immune responses and the elimination of neoplastic cells from blood circulation [27][28]. Likewise, dendritic cells (DCs) are an essential subpopulation of leukocytes for inducing and regulating an adaptive anti-tumour immune response [29]. Cancer immunoediting is part of a dynamic concept that explains the role of the immune system and immune cells in malignant transformation, emphasising cancer immunotherapy [30].
In veterinary and human searches, a high count and ratio of circulating leukocytes (CD4+ and CD8+ T cells, dendritic cells, macrophages and regulatory T cells) [31][32], as well as their presence in the tumour microenvironment [33][34], may be associated with the prognosis of certain solid tumours. In felines with mammary carcinoma, the neutrophil-to-lymphocyte ratio has been suggested as a prognostic factor. In contrast, high numbers of total leukocyte counts, neutrophils, and the neutrophil/lymphocyte ratio were associated with an increased risk of tumour-related death [31]. Moreover, the lymphocyte/monocyte ratio can predict the prognosis of newly diagnosed canines with diffuse large B-cell lymphoma in terms of time progression and lymphoma-specific survival [32] and the histopathological grade of canine mast cell tumours [35]. In canine osteosarcoma, monocyte and lymphocyte counts were used as prognostic indicators [36]. Other studies observed that in dogs with oncological disease, the percentage of Th1 was considerably lower and Th2 was significantly higher compared to healthy dogs [37]. In addition, they determined that dogs with metastases had even lower Th1 values than ill dogs without metastases [37]. Researchers observed a correlation between tumour-infiltrating lymphocytes and histopathological characteristics in canines with mammary carcinoma, suggesting that a significant quantity of lymphocytes infiltrating mammary carcinoma is associated with lymphatic invasion and a high histopathological grade [38]. Furthermore, the tumour-infiltrating lymphocytes proved indispensable for tumour growth and their spread potential [38]. In the review “A role for T-lymphocytes in human breast cancer and in canine mammary tumours”, the authors compare tumour T-lymphocyte infiltration and the CD4+/CD8+ T-cell ratio with low survival rates, the action of Th2 cells in the acceleration of tumour progression and a poor prognosis with the presence of large numbers of Treg cells [39]. In human breast cancer, the role of macrophages in tumour progression was observed as a facilitator of the invasion of tumour cells [40] due to their plasticity and ability to adapt to the different physiological conditions that the body presents, which promote the production of cytokines type II, anti-inflammatory responses, and pro-tumour functions [41]. In pancreatic and colorectal human cancers, elevated Th2 levels and an imbalance of the Th1/Th2 ratio lead to an increase in pro-inflammatory interleukins that promote the progression of the disease [42][43].
Considering the immune system as a main player in the body’s defence against tumour growth, some human and animal studies suggest a new approach to minimising the impact on the immune cells of several suppressive factors such as stress, pain, anaesthesia and surgery [19][44][45]. According to studies in dogs, the administration of recombinant canine interferon (rCaIFN-γ) before, during and after anaesthesia may reduce the suppression of natural killer (NK) activity, inhibit intraoperative cancer cell dissemination and prevent postoperative cancer recurrence and metastasis [46][47]. In humans, it has been demonstrated that the administration of nonsteroidal anti-inflammatory agents to oncological patients has anti-tumour properties by inhibiting the pro-tumour activity of the enzyme COX-2 of macrophages and increasing the activity of T regulatory cells [48][49], both of which have been shown to promote tumour progression in humans. Dendritic cells also play an important role in immunotherapies, eliciting Th1 and Th17 cell differentiation [50] and infiltrating solid malignancies [51].
3. Tumour Surgery as a “Starting Point” for Tumour Progression
Excisional tumour surgery is the main approach for treating primary solid tumours [9] and the major facilitator of metastatic cell dissemination [52]. Since the eighteenth century, this condition has been researched as a “starting point” for accelerating tumour progression [53]. Since then, several studies have been published on the relationship between surgery and cancer progression to understand this biological behaviour, including 1. the dissemination of tumour cells via the manipulation of the neoplastic tissue [54]; 2. the effect of surgery as a window of opportunity for the development and spread of tumour cells [55]; 3. the change in the state of tumour dormancy via surgical tumour excision [56] and decreased antiangiogenic factor secretion [57].
First, the surgical manipulation of neoplastic tissue promotes the release of neoplastic cells into the blood and lymphatic system [54], where they seek pre-metastatic niches to initiate a new cell growth cycle [58]. Besides that, before surgery, most oncology patients already have cancer cells in their circulatory system, which does not necessarily indicate the presence of metastases or tumour recurrence [59][60]. In human research, less than 0.01% of circulating tumour cells are successful in tumour metastasis, depending on the immune response and the tumour cells’ ability to avoid the defence cells [61]. Nonetheless, human investigations have demonstrated a correlation between tumour cells in peripheral blood after surgery and poor prognosis prediction [62].
Secondly, the microenvironment of the damaged tissue in the area of the surgical procedure changes, and minutes after the incision, a proportional local and systemic stress response is generated [20]. Physiological mechanisms such as local inflammation, sympathetic nervous system activation, and the hypothalamic–pituitary–adrenal axis (HPA) are activated to restore cellular and tissue homeostasis following tissue injury [20], as demonstrated in Figure 1. In response, immunomodulatory chemicals are released, specifically catecholamines and glucocorticoids, which induce prostaglandins (PGE2), pro-inflammatory cytokines (e.g., IL-10), metalloproteinases and endothelial growth factor secretion [17]. These immunomodulatory effects can result in a suppression of the immune system, producing direct and indirect pro-tumour effects such as tumour angiogenesis, the activation of 2-adrenergic and cyclooxygenase-2 receptors, the release of coagulation factors, and the depletion of natural killer cell (lymphocytes CD3- and CD56+) activity [18], which plays a significant role in anti-tumour immunity by controlling the dissemination of cancer cells [27][28]. Platelets and coagulation factors are released to reestablish haemostasis, forming protective aggregates that shield tumour cells from natural killer cells [63]. In actuality, this biological process, which simultaneously attempts to restore homeostasis, modifies the immunological, metabolic, endocrinological, neuronal, inflammatory and immune microenvironments, thereby fostering tumour growth and dissemination [18].
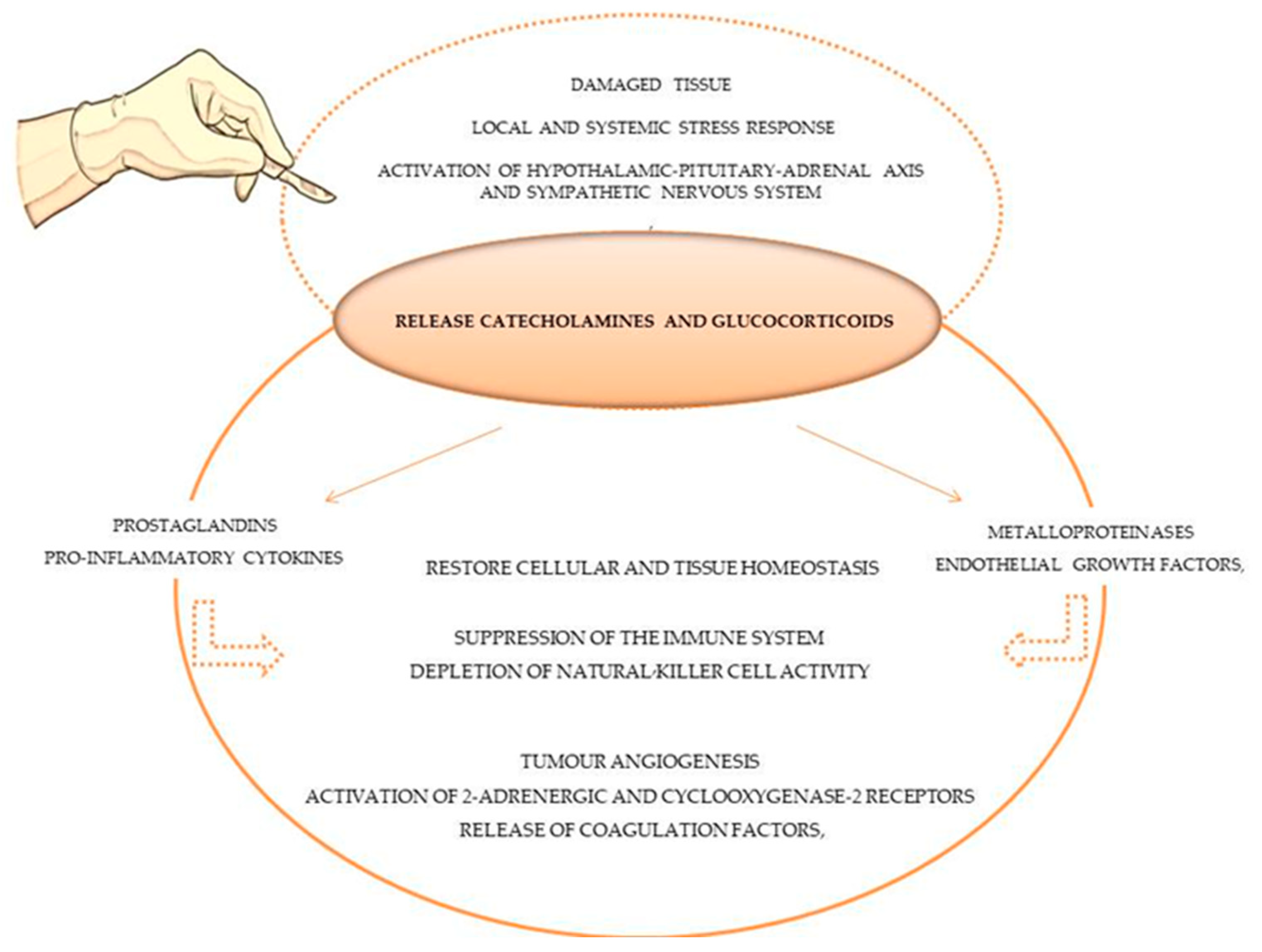
Figure 1. Stress and inflammatory response of tissue surgery damage in immune system and tumour cells.
Considering the inflammatory, immunosuppressive and tumour-spreading effects of surgery trauma, the potential advantages of minimally invasive surgery (MIS) over conventional open surgery have been discussed in human and animal studies, but with inconsistent results [21][64]. MIS seems to offer certain benefits, including reduced tissue inflammation and immune suppression, as well as less blood loss, decreased postoperative pain and reduced hospitalisation [21][64][65]. However, longer surgical and anaesthesia times and an increased risk of technical complications, such as wound dehiscence and local inflammation, could potentially be disadvantages when compared to open surgery [56][66][67].
Lastly, tumour dormancy is a condition characterised by the presence of tumour cells in the absence of evidence of oncological disease [68]. This phenomenon may explain both cancer recurrences after long periods of remission and the maintenance of reduced neoplastic cells after adjuvant oncological therapies [69], as a result of the absence of angiogenic competence, a homeostatic equilibrium between tumour cells and the immune response or the presence of an environment that does not promote tumour growth [68]. Surgical tumour excision helps cancer survive by altering the biological characteristics of neoplastic cells, such as proliferation, apoptosis, metastasis, and dormancy condition [70], and blood levels of tumour growth promoters (e.g., IL-6, TNF-α, VEGF) are higher after surgery, whereas antiangiogenic mediators such as endostatin and angiostatin are undetectable [71]. This finding supports the hypothesis that surgery and neuroendocrine mediators that control angiogenesis and inflammation can “awaken” a dormant tumour condition and dormant micrometastases [72]. As well, tumour angiogenic activity, which is the physiological process of generating new blood vessels from existing ones, is essential for the survival and development of tumour cells, including their growth, invasion and spread [73].
Even though some biological factors may paradoxically enhance the proliferation and dissemination of metastatic cells, surgical tumour removal provides an evident benefit in preventing the growth and spread of cancer [9]. Furthermore, both animal and human studies have demonstrated that the use of a less traumatic surgical technique and pharmaceutical substances, such as COX-2 selective nonsteroidal anti-inflammatory drugs [74][75], antithrombotics [63] and antiangiogenics [76][77], can make these biological effects simpler to control.
4. The Impact of the General Anaesthetics on the Immune System and Their “Anti-” and “Pro-Tumoral” Effects
Oncological diseases often require the use of anaesthetic and analgesic agents during diagnostic [78][79], therapeutic or palliative procedures to control pain [80][81] and minimise the effects of the stress response [76]. General anaesthetics include volatile agents like isoflurane and intravenous agents like propofol, which have been linked to immunomodulatory properties such as the inhibition of NK cell activity [82] and pro-tumour effects such as the inhibition of prostaglandin synthesis by tumour cells [47]. Due to their immunosuppressive effects, volatile anaesthesia may be associated with poorer outcomes than intravenous anaesthesia [83][84], as demonstrated in Figure 2. However, human studies suggest that general anaesthetics in combination with regional anaesthesia can reduce the inflammatory response and endothelial permeability, preventing the spread of cancer cells [65]. Also, studies in dogs and cats suggest that loco-regional therapy serves to reduce the quantity of intra and post-operative opioids, allowing their adverse effects to be reduced [85][86][87].
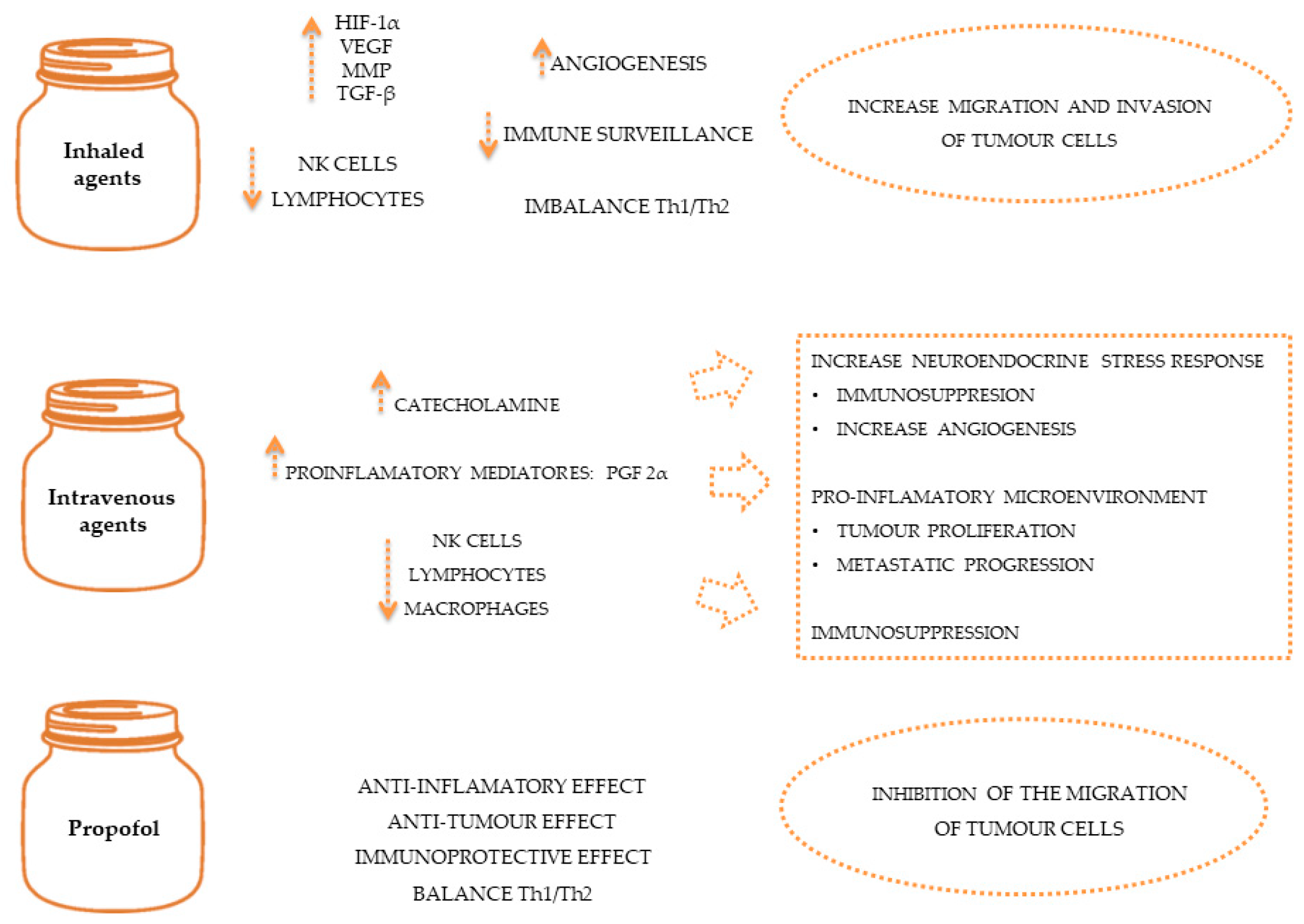
Figure 2. The effect of anaesthetics on immune system and on tumour spread.
References
- Wemelsfelder, F.; Mullan, S. Applying Ethological and Health Indicators to Practical Animal Welfare Assessment. Rev. Sci. Tech. OIE 2014, 33, 111–120.
- Alexander, J.E.; Colyer, A.; Haydock, R.M.; Hayek, M.G.; Park, J. Understanding How Dogs Age: Longitudinal Analysis of Markers of Inflammation, Immune Function, and Oxidative Stress. J. Gerontol. Ser. A 2018, 73, 720–728.
- Pinello, K.; Amorim, I.; Pires, I.; Canadas-Sousa, A.; Catarino, J.; Faísca, P.; Branco, S.; Peleteiro, M.C.; Silva, D.; Severo, M.; et al. Vet-OncoNet: Malignancy Analysis of Neoplasms in Dogs and Cats. Vet. Sci. 2022, 9, 535.
- Rafalko, J.M.; Kruglyak, K.M.; McCleary-Wheeler, A.L.; Goyal, V.; Phelps-Dunn, A.; Wong, L.K.; Warren, C.D.; Brandstetter, G.; Rosentel, M.C.; DiMarzio, L.; et al. Age at Cancer Diagnosis by Breed, Weight, Sex, and Cancer Type in a Cohort of More than 3,000 Dogs: Determining the Optimal Age to Initiate Cancer Screening in Canine Patients. PLoS ONE 2023, 18, e0280795.
- Hong, H.; Wang, Q.; Li, J.; Liu, H.; Meng, X.; Zhang, H. Aging, Cancer and Immunity. J. Cancer 2019, 10, 3021–3027.
- Schwartz, S.M.; Urfer, S.R.; White, M.; Megquier, K.; Shrager, S.; The Dog Aging Project Consortium; Ruple, A. Lifetime Prevalence of Malignant and Benign Tumours in Companion Dogs: Cross-sectional Analysis of Dog Aging Project Baseline Survey. Vet. Comp. Oncol. 2022, 20, 797–804.
- Fleming, J.M.; Creevy, K.E.; Promislow, D.E.L. Mortality in North American Dogs from 1984 to 2004: An Investigation into Age-, Size-, and Breed-Related Causes of Death: Mortality of Dogs in North America. J. Vet. Intern. Med. 2011, 25, 187–198.
- Smith, A.N. Advances in Veterinary Oncology. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, xi–xii.
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247.
- Shibata, J.; Ishihara, S.; Tada, N.; Kawai, K.; Tsuno, N.H.; Yamaguchi, H.; Sunami, E.; Kitayama, J.; Watanabe, T. Surgical Stress Response after Colorectal Resection: A Comparison of Robotic, Laparoscopic, and Open Surgery. Tech. Coloproctol. 2015, 19, 275–280.
- Hume, K.R.; Johnson, J.L.; Williams, L.E. Adverse Effects of Concurrent Carboplatin Chemotherapy and Radiation Therapy in Dogs. J. Vet. Intern. Med. 2009, 23, 24–30.
- Wendelburg, K.M.; Price, L.L.; Burgess, K.E.; Lyons, J.A.; Lew, F.H.; Berg, J. Survival Time of Dogs with Splenic Hemangiosarcoma Treated by Splenectomy with or without Adjuvant Chemotherapy: 208 Cases (2001–2012). J. Am. Vet. Med. Assoc. 2015, 247, 393–403.
- McNally, A.; Rossanese, M.; Suárez-Bonnet, A.; Hardas, A.; Yale, A.D. Urinary Bladder Hemangiosarcoma in a Cat Treated with Partial Cystectomy and Adjuvant Metronomic Cyclophosphamide and Thalidomide. Vet. Intern. Med. 2023, 37, 1488–1492.
- Riggs, J.; Adams, V.J.; Hermer, J.V.; Dobson, J.M.; Murphy, S.; Ladlow, J.F. Outcomes Following Surgical Excision or Surgical Excision Combined with Adjunctive, Hypofractionated Radiotherapy in Dogs with Oral Squamous Cell Carcinoma or Fibrosarcoma. J. Am. Vet. Med. Assoc. 2018, 253, 73–83.
- Inbar, S.; Neeman, E.; Avraham, R.; Benish, M.; Rosenne, E.; Ben-Eliyahu, S. Do Stress Responses Promote Leukemia Progression? An Animal Study Suggesting a Role for Epinephrine and Prostaglandin-E2 through Reduced NK Activity. PLoS ONE 2011, 6, e19246.
- Goldfarb, Y.; Sorski, L.; Benish, M.; Levi, B.; Melamed, R.; Ben-Eliyahu, S. Improving Postoperative Immune Status and Resistance to Cancer Metastasis: A Combined Perioperative Approach of Immunostimulation and Prevention of Excessive Surgical Stress Responses. Ann. Surg. 2011, 253, 798–810.
- Lee, Y.N. Effect of Anesthesia and Surgery on Immunity. J. Surg. Oncol. 1977, 9, 425–430.
- Ogawa, K.; Hirai, M.; Katsube, T.; Murayama, M.; Hamaguchi, K.; Shimakawa, T.; Naritake, Y.; Hosokawa, T.; Kajiwara, T. Suppression of Cellular Immunity by Surgical Stress. Surgery 2000, 127, 329–336.
- Lin, L.; Liu, C.; Tan, H.; Ouyang, H.; Zhang, Y.; Zeng, W. Anaesthetic Technique May Affect Prognosis for Ovarian Serous Adenocarcinoma: A Retrospective Analysis. Br. J. Anaesth. 2011, 106, 814–822.
- Desborough, J.P. The Stress Response to Trauma and Surgery. Br. J. Anaesth. 2000, 85, 109–117.
- Novitsky, Y.W.; Litwin, D.E.M.; Callery, M.P. The Net Immunologic Advantage of Laparoscopic Surgery. Surg. Endosc. 2004, 18, 1411–1419.
- Dourado, A.; Gomes, A.; Teixeira, P.; Lobo, L.; Azevedo, J.T.; Dias, I.R.; Pinelas, R. Antinociceptive Effect of a Sacro-Coccygeal Epidural of Morphine and Lidocaine in Cats Undergoing Ovariohysterectomy. Vet. Sci. 2022, 9, 623.
- White, D.M.; Mair, A.R.; Martinez-Taboada, F. Opioid-Free Anaesthesia in Three Dogs. Open Vet. J. 2017, 7, 104.
- Tomihari, M.; Nishihara, A.; Shimada, T.; Yanagawa, M.; Miyoshi, M.; Miyahara, K.; Oishi, A. A Comparison of the Immunological Effects of Propofol and Isoflurane for Maintenance of Anesthesia in Healthy Dogs. J. Vet. Med. Sci. 2015, 77, 1227–1233.
- Yeo, J.; Park, J.S.; Choi, G.-S.; Kim, H.J.; Kim, J.K.; Oh, J.; Park, S.Y. Comparison of the Analgesic Efficacy of Opioid-Sparing Multimodal Analgesia and Morphine-Based Patient-Controlled Analgesia in Minimally Invasive Surgery for Colorectal Cancer. World J. Surg. 2022, 46, 1788–1795.
- Carvalho, M.I.; Pires, I.; Prada, J.; Ferreira, A.F.; Queiroga, F.L. Positive Interplay Between CD3+ T-Lymphocytes and Concurrent COX-2/EGFR Expression in Canine Malignant Mammary Tumors. Anticancer Res. 2015, 35, 2915–2920.
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of Natural Killer Cells. Nat. Immunol. 2008, 9, 503–510.
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2021, 124, 359–367.
- Lee, Y.S.; Radford, K.J. The Role of Dendritic Cells in Cancer. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 348, pp. 123–178. ISBN 978-0-12-818351-9.
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New Insights into Cancer Immunoediting and Its Three Component Phases—Elimination, Equilibrium and Escape. Curr. Opin. Immunol. 2014, 27, 16–25.
- Petrucci, G.N.; Lobo, L.; Queiroga, F.; Martins, J.; Prada, J.; Pires, I.; Henriques, J. Neutrophil-to-lymphocyte Ratio Is an Independent Prognostic Marker for Feline Mammary Carcinomas. Vet. Comp. Oncol. 2021, 19, 482–491.
- Marconato, L.; Martini, V.; Stefanello, D.; Moretti, P.; Ferrari, R.; Comazzi, S.; Laganga, P.; Riondato, F.; Aresu, L. Peripheral Blood Lymphocyte/Monocyte Ratio as a Useful Prognostic Factor in Dogs with Diffuse Large B-Cell Lymphoma Receiving Chemoimmunotherapy. Vet. J. 2015, 206, 226–230.
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213.
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8 + Tumor-Infiltrating Lymphocytes and a High CD8+/Regulatory T Cell Ratio Are Associated with Favorable Prognosis in Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543.
- Macfarlane, M.J.; Macfarlane, L.L.; Scase, T.; Parkin, T.; Morris, J.S. Use of Neutrophil to Lymphocyte Ratio for Predicting Histopathological Grade of Canine Mast Cell Tumours. Vet. Rec. 2016, 179, 491.
- Sottnik, J.L.; Rao, S.; Lafferty, M.H.; Thamm, D.H.; Morley, P.S.; Withrow, S.J.; Dow, S.W. Association of Blood Monocyte and Lymphocyte Count and Disease-Free Interval in Dogs with Osteosarcoma: CBC Is Prognostic in Osteosarcoma. J. Vet. Intern. Med. 2010, 24, 1439–1444.
- Horiuchi, Y.; Hanazawa, A.; Nakajima, Y.; Nariai, Y.; Asanuma, H.; Kuwabara, M.; Yukawa, M.; Ito, H. T-Helper (Th) 1/Th2 Imbalance in the Peripheral Blood of Dogs with Malignant Tumor. Microbiol. Immunol. 2007, 51, 1135–1138.
- Kim, J.-H.; Chon, S.-K.; Im, K.-S.; Kim, N.-H.; Sur, J.-H. Correlation of Tumor-Infiltrating Lymphocytes to Histopathological Features and Molecular Phenotypes in Canine Mammary Carcinoma: A Morphologic and Immunohistochemical Morphometric Study. Can. J. Vet. Res. 2013, 77, 142–149.
- Carvalho, M.I.; Pires, I.; Prada, J.; Queiroga, F.L. A Role for T-Lymphocytes in Human Breast Cancer and in Canine Mammary Tumors. BioMed Res. Int. 2014, 2014, 130894.
- Goswami, S.; Sahai, E.; Wyckoff, J.B.; Cammer, M.; Cox, D.; Pixley, F.J.; Stanley, E.R.; Segall, J.E.; Condeelis, J.S. Macrophages Promote the Invasion of Breast Carcinoma Cells via a Colony-Stimulating Factor-1/Epidermal Growth Factor Paracrine Loop. Cancer Res. 2005, 65, 5278–5283.
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896.
- De Monte, L.; Reni, M.; Tassi, E.; Clavenna, D.; Papa, I.; Recalde, H.; Braga, M.; Di Carlo, V.; Doglioni, C.; Protti, M.P. Intratumor T Helper Type 2 Cell Infiltrate Correlates with Cancer-Associated Fibroblast Thymic Stromal Lymphopoietin Production and Reduced Survival in Pancreatic Cancer. J. Exp. Med. 2011, 208, 469–478.
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.-H.; Pagès, F.; et al. Clinical Impact of Different Classes of Infiltrating T Cytotoxic and Helper Cells (Th1, Th2, Treg, Th17) in Patients with Colorectal Cancer. Cancer Res. 2011, 71, 1263–1271.
- Gültekin, Ç. Comparison of the Analgesic Effects of Morphine and Tramadol after Tumor Surgery in Dogs. Open Vet. J. 2021, 11, 613.
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic Stress Promotes Tumor Growth and Angiogenesis in a Mouse Model of Ovarian Carcinoma. Nat. Med. 2006, 12, 939–944.
- Miyata, T.; Honma, R.; Sato, A.; Matsumoto, H.; Koyama, H.; Tagawa, M. Effect of rCaIFN-γ Pretreatment on Propofol–Isoflurane Suppression of NK Cytotoxic Activity in the Peripheral Blood of Dogs. Res. Vet. Sci. 2015, 98, 25–29.
- Matsumoto, H.; Miyata, T.; Ohkusa, T.; Teshima, T.; Koyama, H. Effects of Recombinant Canine Interferon-γ Injected before General Anesthesia with Propofol and Isoflurane on Natural Killer Cytotoxic Activity during Anesthesia in Dogs. Res. Vet. Sci. 2019, 125, 416–420.
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in Cancer: A Review. J. Cell. Physiol. 2019, 234, 5683–5699.
- Hashemi, V.; Maleki, L.A.; Esmaily, M.; Masjedi, A.; Ghalamfarsa, G.; Namdar, A.; Yousefi, M.; Yousefi, B.; Jadidi-Niaragh, F. Regulatory T Cells in Breast Cancer as a Potent Anti-Cancer Therapeutic Target. Int. Immunopharmacol. 2020, 78, 106087.
- Terhune, J.; Berk, E.; Czerniecki, B. Dendritic Cell-Induced Th1 and Th17 Cell Differentiation for Cancer Therapy. Vaccines 2013, 1, 527–549.
- Ma, Y.; Shurin, G.V.; Peiyuan, Z.; Shurin, M.R. Dendritic Cells in the Cancer Microenvironment. J. Cancer 2013, 4, 36–44.
- Tvedskov, T.F.; Jensen, M.-B.; Kroman, N.; Balslev, E. Iatrogenic Displacement of Tumor Cells to the Sentinel Node after Surgical Excision in Primary Breast Cancer. Breast Cancer Res. Treat. 2012, 131, 223–229.
- Raven, R.W. Surgical Oncology-Theory and Practice. J. Surg. Oncol. 2006, 30, 145–148.
- Curtin, J.; Thomson, P.; Wong, G.; Lam, A.; Choi, S.-W. The Impact of Surgery on Circulating Malignant Tumour Cells in Oral Squamous Cell Carcinoma. Cancers 2023, 15, 584.
- Bogden, A.; Moreau, J.-P.; Eden, P. Proliferative Response of Human and Animal Tumours to Surgical Wounding of Normal Tissues: Onset, Duration and Inhibition. Br. J. Cancer 1997, 75, 1021–1027.
- Demicheli, R.; Miceli, R.; Moliterni, A.; Zambetti, M.; Hrushesky, W.J.M.; Retsky, M.W.; Valagussa, P.; Bonadonna, G. Breast Cancer Recurrence Dynamics Following Adjuvant CMF Is Consistent with Tumor Dormancy and Mastectomy-Driven Acceleration of the Metastatic Process. Ann. Oncol. 2005, 16, 1449–1457.
- Li, T.-S.; Kaneda, Y.; Ueda, K.; Hamano, K.; Zempo, N.; Esato, K. The Influence of Tumour Resection on Angiostatin Levels and Tumour Growth—An Experimental Study in Tumour-Bearing Mice. Eur. J. Cancer 2001, 37, 2283–2288.
- Shiozawa, Y.; Pedersen, E.A.; Havens, A.M.; Jung, Y.; Mishra, A.; Joseph, J.; Kim, J.K.; Patel, L.R.; Ying, C.; Ziegler, A.M.; et al. Human Prostate Cancer Metastases Target the Hematopoietic Stem Cell Niche to Establish Footholds in Mouse Bone Marrow. J. Clin. Investig. 2011, 121, 1298–1312.
- Patel, H.; Le Marer, N.; Wharton, R.Q.; Khan, Z.A.J.; Araia, R.; Glover, C.; Henry, M.M.; Allen-Mersh, T.G. Clearance of Circulating Tumor Cells after Excision of Primary Colorectal Cancer. Ann. Surg. 2002, 235, 226–231.
- Hüsemann, Y.; Geigl, J.B.; Schubert, F.; Musiani, P.; Meyer, M.; Burghart, E.; Forni, G.; Eils, R.; Fehm, T.; Riethmüller, G.; et al. Systemic Spread Is an Early Step in Breast Cancer. Cancer Cell 2008, 13, 58–68.
- Tremblay, P.-L.; Huot, J.; Auger, F.A. Mechanisms by Which E-Selectin Regulates Diapedesis of Colon Cancer Cells under Flow Conditions. Cancer Res. 2008, 68, 5167–5176.
- Rahbari, N.N.; Aigner, M.; Thorlund, K.; Mollberg, N.; Motschall, E.; Jensen, K.; Diener, M.K.; Büchler, M.W.; Koch, M.; Weitz, J. Meta-Analysis Shows That Detection of Circulating Tumor Cells Indicates Poor Prognosis in Patients with Colorectal Cancer. Gastroenterology 2010, 138, 1714–1726.e13.
- Seth, R.; Tai, L.H.; Falls, T.; De Souza, C.T.; Bell, J.C.; Carrier, M.; Atkins, H.; Boushey, R.; Auer, R.A. Surgical Stress Promotes the Development of Cancer Metastases by a Coagulation-Dependent Mechanism Involving Natural Killer Cells in a Murine Model. Ann. Surg. 2013, 258, 158–168.
- Bachman, S.L.; Hanly, E.J.; Nwanko, J.I.; Lamb, J.; Herring, A.E.; Marohn, M.R.; De Maio, A.; Talamini, M.A. The Effect of Timing of Pneumoperitoneum on the Inflammatory Response. Surg. Endosc. Other Interv. Tech. 2004, 18, 1640–1644.
- Cummings III, K.C.; Zimmerman, N.M.; Maheshwari, K.; Cooper, G.S.; Cummings, L.C. Epidural Compared with Non-Epidural Analgesia and Cardiopulmonary Complications after Colectomy: A Retrospective Cohort Study of 20,880 Patients Using a National Quality Database. J. Clin. Anesth. 2018, 47, 12–18.
- Sakai, E.M.; Connolly, L.A.; Klauck, J.A. Inhalation Anesthesiology and Volatile Liquid Anesthetics: Focus on Isoflurane, Desflurane, and Sevoflurane. Pharmacotherapy 2005, 25, 1773–1788.
- Park, Y.; Ha, J.W. Comparison of One-Level Posterior Lumbar Interbody Fusion Performed With a Minimally Invasive Approach or a Traditional Open Approach. Spine 2007, 32, 537–543.
- Zappalà, G.; McDonald, P.G.; Cole, S.W. Tumor Dormancy and the Neuroendocrine System: An Undisclosed Connection? Cancer Metastasis Rev. 2013, 32, 189–200.
- Aguirre-Ghiso, J.A. Models, Mechanisms and Clinical Evidence for Cancer Dormancy. Nat. Rev. Cancer 2007, 7, 834–846.
- Schmidt-Kittler, O.; Ragg, T.; Daskalakis, A.; Granzow, M.; Ahr, A.; Blankenstein, T.J.F.; Kaufmann, M.; Diebold, J.; Arnholdt, H.; Müller, P.; et al. From Latent Disseminated Cells to Overt Metastasis: Genetic Analysis of Systemic Breast Cancer Progression. Proc. Natl. Acad. Sci. USA 2003, 100, 7737–7742.
- O’Reilly, M.S.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Moses, M.; Lane, W.S.; Cao, Y.; Sage, E.H.; Folkman, J. Angiostatin: A Novel Angiogenesis Inhibitor That Mediates the Suppression of Metastases by a Lewis Lung Carcinoma. Cell 1994, 79, 315–328.
- Varani, J.; Lovett, E.J.; Lundy, J. A Model of Tumor Cell Dormancy: Effects of Anesthesia and Surgery. J. Surg. Oncol. 1981, 17, 9–14.
- Yang, H.; Lee, S.; Lee, S.; Kim, K.; Yang, Y.; Kim, J.H.; Adams, R.H.; Wells, J.M.; Morrison, S.J.; Koh, G.Y.; et al. Sox17 Promotes Tumor Angiogenesis and Destabilizes Tumor Vessels in Mice. J. Clin. Investig. 2013, 123, 418–431.
- Queiroga, F.L.; Pires, I.; Parente, M.; Gregório, H.; Lopes, C.S. COX-2 over-Expression Correlates with VEGF and Tumour Angiogenesis in Canine Mammary Cancer. Vet. J. 2011, 189, 77–82.
- Sui, W.; Zhang, Y.; Wang, Z.; Wang, Z.; Jia, Q.; Wu, L.; Zhang, W. Antitumor Effect of a Selective COX-2 Inhibitor, Celecoxib, May Be Attributed to Angiogenesis Inhibition through Modulating the PTEN/PI3K/Akt/HIF-1 Pathway in an H22 Murine Hepatocarcinoma Model. Oncol. Rep. 2014, 31, 2252–2260.
- Li, Q.; Wang, Y.; Jia, W.; Deng, H.; Li, G.; Deng, W.; Chen, J.; Kim, B.Y.S.; Jiang, W.; Liu, Q.; et al. Low-Dose Anti-Angiogenic Therapy Sensitizes Breast Cancer to PD-1 Blockade. Clin. Cancer Res. 2020, 26, 1712–1724.
- Huang, Y.; Yuan, J.; Righi, E.; Kamoun, W.S.; Ancukiewicz, M.; Nezivar, J.; Santosuosso, M.; Martin, J.D.; Martin, M.R.; Vianello, F.; et al. Vascular Normalizing Doses of Antiangiogenic Treatment Reprogram the Immunosuppressive Tumor Microenvironment and Enhance Immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 17561–17566.
- De Bonis, A.; Collivignarelli, F.; Paolini, A.; Falerno, I.; Rinaldi, V.; Tamburro, R.; Bianchi, A.; Terragni, R.; Gianfelici, J.; Frescura, P.; et al. Sentinel Lymph Node Mapping with Indirect Lymphangiography for Canine Mast Cell Tumour. Vet. Sci. 2022, 9, 484.
- Karayannopoulou, M.; Anagnostou, T.; Margariti, A.; Kritsepi-Konstantinou, M.; Psalla, D.; Savvas, I.; Kazakos, G. Effect of Anaesthesia on Cell-Mediated Immunity in Dogs Undergoing Mastectomy for Mammary Cancer. Vet. Anaesth. Analg. 2022, 49, 265–274.
- Gaynor, J.S. Control of Cancer Pain in Veterinary Patients. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1429–1448.
- Te Boveldt, N.; Vernooij-Dassen, M.; Burger, N.; Vissers, K.; Engels, Y. Pain and Its Interference with Daily Activitiesin Medical Oncology Outpatients. Pain Phys. 2013, 16, 379–389.
- Miyata, T.; Kodama, T.; Honma, R.; Nezu, Y.; Harada, Y.; Yogo, T.; Hara, Y.; Tagawa, M. Influence of General Anesthesia with Isoflurane Following Propofol-Induction on Natural Killer Cell Cytotoxic Activities of Peripheral Blood Lymphocytes in Dogs. J. Vet. Med. Sci. 2013, 75, 917–921.
- Jun, I.-J.; Jo, J.-Y.; Kim, J.-I.; Chin, J.-H.; Kim, W.-J.; Kim, H.R.; Lee, E.-H.; Choi, I.-C. Impact of Anesthetic Agents on Overall and Recurrence-Free Survival in Patients Undergoing Esophageal Cancer Surgery: A Retrospective Observational Study. Sci. Rep. 2017, 7, 14020.
- Wigmore, T.J.; Jhanji, S. Long-Term Survival for Patients Undergoing Volatile versus IV Anesthesia for Cancer Surgery. Retrospective Analysis. Anesthesiology 2016, 124, 69–79.
- Lascelles, B.D.X.; Kirkby Shaw, K. An Extended Release Local Anaesthetic: Potential for Future Use in Veterinary Surgical Patients? Vet. Med. Sci. 2016, 2, 229–238.
- Romano, M.; Portela, D.A.; Breghi, G.; Otero, P.E. Stress-Related Biomarkers in Dogs Administered Regional Anaesthesia or Fentanyl for Analgesia during Stifle Surgery. Vet. Anaesth. Analg. 2016, 43, 44–54.
- Grubb, T.; Lobprise, H. Local and Regional Anaesthesia in Dogs and Cats: Overview of Concepts and Drugs (Part 1). Vet. Med. Sci. 2020, 6, 209–217.
More
Information
Subjects:
Anesthesiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
699
Revisions:
2 times
(View History)
Update Date:
06 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




